 Research Article
Research Article
Andrographolide and Melatonin Synergistically Inhibit the Sox Proteins Expression in Colospheroids
Natalie May1, Amber Czinn1, Neha Sharda1, Advaitha Midde1, Sazzad Hassan2, Urs von Holzen2,3,4,5, Hem Sukla6, Jaylyn Waddell1 and Aditi Banerjee1*
1Department of Pediatrics, University of Maryland School of Medicine, Baltimore, Maryland, USA
2Department of Surgery, Indiana University School of Medicine, South Bend, IN, USA
3Harper Cancer Research Institute, South Bend, IN, USA
4Goshen Center for Cancer Care, Goshen, Goshen, IN, USA
5University of Basel, Basel, Switzerland
5Division of Translational Radiation Sciences, Department of Radiation Oncology, University of Maryland School of Medicine, Baltimore, MD, USA
Aditi Banerjee, Department of Pediatrics, University of Maryland School of Medicine, Baltimore, Maryland, USA
Received Date:April 17, 2024; Published Date:April 24, 2024
Abstract
Dysregulation of the Sox protein family is associated with maintenance of colon cancer stem cells (CSCs), a small group of colon cancer cells responsible for colon cancer metastasis, disease recurrence, and drug resistance. However, further research is required to clarify different Sox protein expressions in colon cancer cells and tissues. To evaluate Sox protein expression, our laboratory used colospheroids, which mimic the colonic organization and contain CSCs. We evaluated the effects of a dual compound of andrographolide (AGP) and melatonin (MLT) on colospheroids obtained from a metastatic colon cancer cell (HT-29) to investigate CSCs’ response. Previous studies have shown that this drug combination has anticarcinogenic, antioxidant, and antimetastatic properties and inhibits colospheroids (HT-29s and HCT-15s) proliferation derived from two colon metastatic cell lines. The present study found varying types of Sox protein expression, including Sox-4, Sox-11, and Sox-18, in the colospheroids using immunoblot. Significant protein inhibition was observed in the dual-treatment group compared to the untreated and single-drug-treated groups. In addition, the in vivo study demonstrated the downregulated Sox protein expression (Sox-8 and Sox-9) in the AGP-MLT-treated mouse xenograft tissue. The mechanism of decreased colospheroids growth was identified as the inhibition of the Sox family protein expression. These results provide a rationale for using AGP in combination with MLT to inhibit CSCs.
Keywords:Melatonin; Andrographolide; Colospheroids; Xenograft Tissue; Sox’s Protein
Abbreviations:Sox: Sex Determining region Y-box; CRC: Colorectal cancer; CSCs: Cancer stem cells; EMT: Epithelial mesenchymal Transition; ATP:Adenosine Tri phosphate; ROS: Reactive oxygen species.
Introduction
Cancer metastasis, disease recurrence, and drug resistance are responsible for a subpopulation of cancer cells known as cancer stem cells (CSCs) [1]. Within the tumor, CSCs represent a small subpopulation (1-3%), bestowed with the capacity to selfrenew [2]. The heterogeneity of tumors, CSCs, and progenitors are known obstacles to development of effective cancer therapies [3]. Therefore, CSCs are the leading cancer therapeutic target. The SOX/ Sox (SRY homology box) family of proteins consists of 20 individual members. These proteins can induce the stem-like phenotypes of CSCs and have regulatory functions in development, cell-fate decision, and differentiation [4]. Accumulating evidence suggests that the Sox family plays a pivotal role in carcinogenesis and dysregulation of SOX proteins has been observed in different types of cancers including colon cancer (CRC). For example, Sox 9 (SRYbox transcription factor 9) could regulate the function of cancer stem/initiating cells (CSCs) to further facilitate the progression of colorectal cancer (CRC) [5]. Moreover, aberrant expression of another transcription factor, Sox 2, is in the CSCs of CRC, skin squamous-cell carcinoma (SCC) and breast carcinoma, and bladder carcinoma, but is absent in the normal epidermis [6-9]. Both SOX8 and SOX4 are suspected to play roles in chemo resistant tongue squamous cell carcinoma (TSCC) and are associated with higher lymph node metastasis, advanced tumor stage and shorter overall survival [10]. Over-expression of SOX8 predicts poor prognosis in colorectal cancer [11]. Another important member of Sox family, SOX4, is a tumor promoter which contributes to drug resistance and progression in cervical cancer. It also regulates the Epithelial Mesenchymal Transition (EMT) program in breast cancer [12,13]. Additionally, Sox4 overexpression is associated with lymph node metastasis in Tongue squamous cell carcinoma (TSCC) [14]. While the impact of Sox11 protein expression in cancer progression is controversial, recent research links dysfunctional expression with increased cancer cell survival, inhibited cell differentiation, and tumor progression [15,16], in contrast, a correlation between Sox- 18 and CRC prognosis is reported [17].
Combined AGP and MLT has a synergistic effect in inhibiting various aspects of metastatic colorectal cancer cells, including colospheroids phenotype and patient-derived organoids. This combination also inhibits ATP-level, angiogenesis, invasion, and xenograft tumor formation by inhibiting the β-catenin signal and increasing ROS generation and apoptosis. In addition, there were no observed side effects in a xenograft mouse model. The current study aims to investigate the impact of the AGP-MLT combination on the SOXs family protein.
Material methods
Chemicals
Melatonin (gifted by Russel J Reiter), dimethylsulfoxide (DMSO) from Sigma Aldrich, AGP (L973, CAS 5508-58-7) from A K Scientific.
Cell cultures, colospheroids formation assay
HT29 cells were generously provided by Lin Jiayuh, Ph. D at the University of Maryland School of Medicine. Cells were cultured in 5% complete RPMI media and cell-derived colospheroids formation assay was performed as described earlier [18].
Subcutaneous tumor xenografts
All experiments were performed following a protocol approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Notre Dame and confirmed with NIH guidelines. All animal research used in this study was approved by the University of Notre Dame IACUC under protocol 18-09-4843. This study was conducted using 4 to 6-week-old athymic nude mice that were performed as described previously [6].
SDS-PAGE and immunoblot
SDS-PAGE and immunoblot were performed as published [19,20]. The primary antibodies used were Sox 2 (Cell Signaling; #2748s), Sox 4 (Novus; #NBPI-50776) Sox 11 (Novus; # NBP2- 98872), Sox 18 (Novus# NBP2-41289), and GAPDH (Sigma Aldrich; #G8795). Blots were incubated with HRP-conjugated secondary antibodies followed by enhanced chemiluminescence (ECL) detection. Images were captured using a Syngene G Box digital image (Frederick, MD) and quantified with densitometry as described [21].
Immunohistochemistry
To detect Sox 8, and Sox 9 (Proteinech; #2067-1-AP, Cell Signaling; #82630S) expression in xenograft treated and untreated tissue, immunohistochemistry was performed as described earlier [22].
Statistical Significance
Statistical analysis was performed with Graph Pad Prism for Macintosh 5.0c (Graph Pad Software Inc., San Diego, CA). Data obtained for all in vitro studies were analyzed using one-way ANOVA. In vivo animal tumor studies were analyzed using 2-way ANOVA. Significance between groups was analyzed using the post hoc Tukey’s test and Bonferroni test. P values were considered significant if they were less than 0.05 and are indicated throughout using asterisks: * = P < 0.05, ** = P < 0.01, ***P < 0.001.
Results
Impact of AGP-MLT combination on Sox protein expression of Colospheroids
SOX proteins are transcription factors that play a crucial role in the regulation of stem cell pluripotency and differentiation [23]. Our earlier studies have demonstrated that Sox 2, a known regulator of chemoresistance, was downregulated in the AGP-MLT treated colospheroids [6]. To explore the mechanism by which AGP and MLT exert their synergistic effects on colospheroids inhibition, HT29s lysates were monitored for Sox-4, Sox 11, Sox 18, Sox 8 and Sox 9 along with Sox-2. Dual treatment downregulated Sox-4, Sox- 11, Sox-18 protein expression in colospheroids lysates compared with the untreated or single-drug treatments (Figure. 1, Figure 2A, C, D). However, Sox-2 protein expression is obvious downregulation as our previous and in this study.
Impact of AGP-MLT cotreatment on Sox protein on mice xenograft tissue
In our previous study, we documented that AGP-MLT combination reduced tumor size and volume and the mechanism is inhibition of β-catenin signal, angiogenesis and mitotic index. Here, we determine if xenograft tumor inhibition by dual therapy is mediated by sox protein’s involvement. Tumor tissues were evaluated to monitor the impact of single/dual therapy on Sox protein expression, Sox-8 and Sox-9 by immunohistochemistry. Decreased Sox-8 and Sox-9 positive cells were found in combination of AGP-MLT treatment (Figure. 2B) compared with the untreated and single drug treatment.
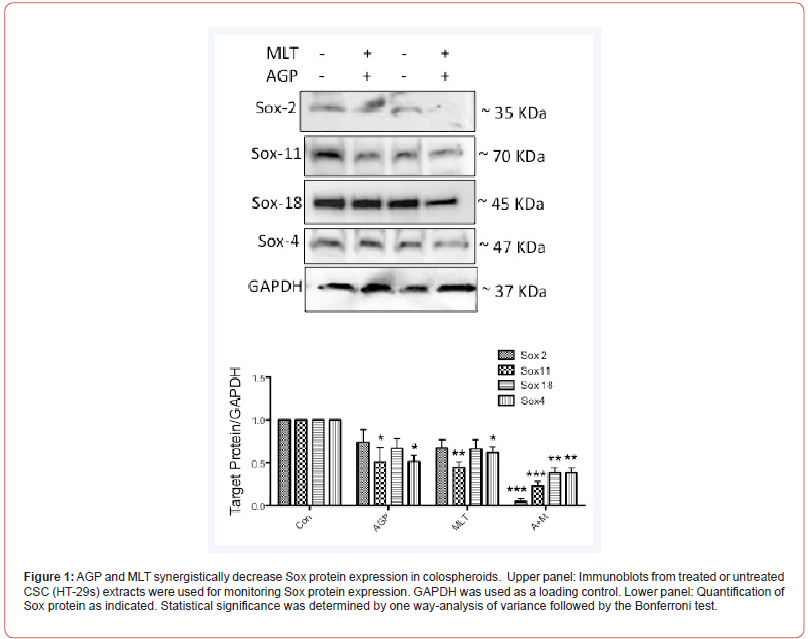
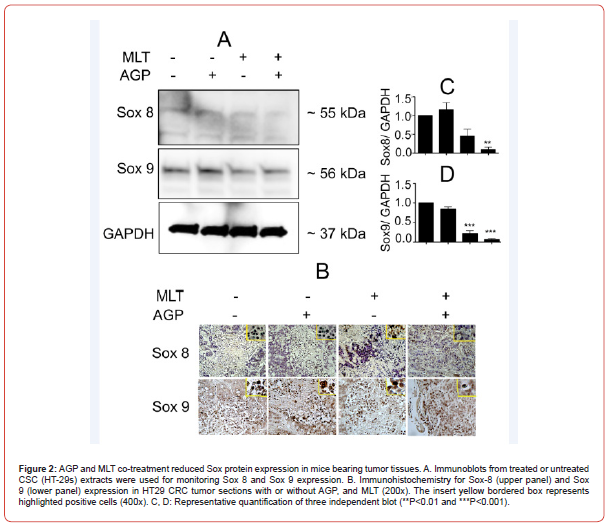
Discussion
Colorectal cancer is one of the most common cancers, with an estimated 152,810 people in the U.S. expected to be diagnosed with the disease in 2024 and 53,010 people expected to die from it. The current treatment for colorectal cancer involves surgical resection and chemotherapy, including 5-FU, depending on the stage of the cancer. However, this treatment does not permanently remove all tumor cells altogether, resulting in possible recurrence, often due to CSCs comprising 20% to 30% of the total CRC cell population. Targeting this specific cell subpopulation could be an effective strategy to eradicate colorectal cancer and increase the survival of metastatic patients. A previous study has shown that combining AGP and MLT can synergistically inhibit the colospheroids phenotype generated from two metastatic cell lines, patient-derived organoids (PDO) and ki 67 expression. This dual drug works by inhibiting angiogenesis, hampering ER stress, and shutting down the β-catenin signal. Furthermore, this treatment reduces mouse tumor growth without causing any changes in body weight.
Sox genes encode transcription factors DNA-binding proteins which contains the high mobility group (HMG) box. The members of this family have shown a critical role in the CSCs maintenance [24]. The key element of CSCs maintenance is pluripotency [25]. Several survival factors including (IGF, EGF, HGF, FGF, and IL-6), survival signal (Hedgehog, Ent, Notch, NF-kβ signal), stemness genes (Nanog, Sox-2, and Oct-4) are associated with CSCs maintenance and expansion [1]. In our previous study and accumulating evidence suggests that transcription factor Sox 2 overexpressed in the CSCs contributes to tumor aggressiveness through major drug resistance mechanisms like epithelial-to- mesenchymal transition, ATP-binding cassette drug transporters, lineage plasticity etc. [26]. Another transcription factor, Sox 4, is associated with the maintenance of colospheroids stemness which contributes to resilience and tumor progression [27]. Additionally, recent studies have characterized Sox 9 as a potential target for suppressing CSCs progression by down-regulating the Sox 9-mediated signaling pathway [28,29]. Moreover, Sox-8 regulates CSC properties and cisplatin-induced EMT in tongue squamous cell carcinoma by acting on the Wnt/β-catenin pathway [10]. In addition, overexpression of Sox11 enhances the CSC phenotype via overexpression of CSC markers in the progression of breast cancer [30].
In this study to evaluate the Sox protein expression, our laboratory used settings 3D cell model derived from a metastatic colon cancer cells HT29 and dual treatment response. We have already reported that bicyclic diterpenoid andrographolide (AGP) has a synergistic effect and inhibited Wnt/β-catenin to reduce spheroid survival derived from metastatic cancer cells when combined with the neurohormone melatonin (MLT) (nonprovisional patent; #PCT/US2021/030084). In this study, we first reported that AGP-MLT combination inhibits sox’s protein expression including Sox-4, Sox-2, Sox-8, Sox-9, Sox-11 and Sox 18 on CSCs. However, additional research is needed to explore the Sox transcription factors in CSCs maintenance in colospheroids derived from other metastatic colon cancer cells and patient-derived organoids.
Conclusion
Conventional chemotherapy and radiotherapy are limited by adverse side effects. Therefore, personalized cancer treatment is needed to overcome this situation. Sox molecules are associated with the CSC maintenance which could be a new therapeutic regimen. AGP-MLT combination has shown the downregulation of Sox family protein in the CSCs.
Data availability
The data that support the findings of this study are available upon request from the corresponding author.
Author Contributions
Natalie May, and Amber Czinn: Data curation, formal analysis, performed the experiments, analyzed the data. Md Sazzad Hassan: performed in vivo experiments and provided the treated and untreated tissues. Urs von Holzen and Jaylyn Waddell review, and editing. Aditi Banerjee: conceptualization, analyzed the data, writing original draft, supervision.
Acknowledgement
The authors thank Pathology shared Service of the University of Maryland Marlene and Stewart Greenebaum Comprehensive Cancer Center for histology sections from xenograft tissues.
Conflict of Interest
No Conflict of Interest.
References
- Pouremamali F, Vahedian V, Hassani N, Mirzaei S, Pouremamali A, et al. (2022) The role of SOX family in cancer stem cell maintenance: With a focus on SOX2. Pathol Res Pract 231: 153783.
- Koren E, Fuchs Y (2016) The bad seed: Cancer stem cells in tumor development and resistance. Drug Resist Updat 28: 1-12.
- Kusoglu A, Biray Avci C (2019) Cancer stem cells: A brief review of the current status. Gene 681: 80-85.
- Grimm D, Bauer J, Wise P, Kruger M, Simonsen U, et al. (2020) The role of SOX family members in solid tumours and metastasis. Semin Cancer Biol 67(Pt 1): 122-153.
- Zhou T, Wu L, Ma N, Tang F, Yu Z, et al. (2020) SOX9-activated FARSA-AS1 predetermines cell growth, stemness, and metastasis in colorectal cancer through upregulating FARSA and SOX9. Cell Death Dis 11(12): 1071.
- Sokolov D, Sharda N, Giri B, Hassan MS, Singh D, et al (2022) Melatonin and andrographolide synergize to inhibit the colospheroid phenotype by targeting Wnt/beta-catenin signaling. J Pineal Res 73(1): e12808.
- Boumahdi S, Driessens G, Lapouge G, Rorive S, Nassar D, et al (2014) SOX2 controls tumour initiation and cancer stem-cell functions in squamous-cell carcinoma. Nature 511(7508): 246-250.
- Siegle JM, Basin A, Sastre-Perona A, Yonekubo Y, Brown J, et al. (2014) SOX2 is a cancer-specific regulator of tumour initiating potential in cutaneous squamous cell carcinoma. Nat Commun 5: 4511.
- Zhu F, Qian W, Zhang H, Liang Y, Wu M, et al. (2017) SOX2 Is a Marker for Stem-like Tumor Cells in Bladder Cancer. Stem Cell Reports 9(2): 429-437.
- Xie SL, Fan S, Zhang SY, Chen WX, Li QX, et al (2018) SOX8 regulates cancer stem-like properties and cisplatin-induced EMT in tongue squamous cell carcinoma by acting on the Wnt/beta-catenin pathway. Int J Cancer 142(6): 1252-1265.
- Wang Y, Yang W, Liu T, Bai G, Liu M, et al. (2019) Over-expression of SOX8 predicts poor prognosis in colorectal cancer: A retrospective study. Medicine (Baltimore) 98(27): e16237.
- Sun R, Jiang B, Qi H, Zhang X, Yang J, et al. (2015) SOX4 contributes to the progression of cervical cancer and the resistance to the chemotherapeutic drug through ABCG2. Cell Death Dis 6(11): e1990.
- Parvani JG, Schiemann WP (2013) Sox4, EMT programs, and the metastatic progression of breast cancers: mastering the masters of EMT. Breast Cancer Res 15(4): R72.
- Watanabe M, Ohnishi Y, Wato M, Tanaka A, Kakudo K (2014) SOX4 expression is closely associated with differentiation and lymph node metastasis in oral squamous cell carcinoma. Med Mol Morphol 47(3): 150-155.
- Yang Z, Jiang S, Lu C, Ji T, Yang W, et al. (2019) SOX11: friend or foe in tumor prevention and carcinogenesis? Ther Adv Med Oncol 11: 1-25.
- Sun Q, Du J, Dong J, Pan S, Jin H, et al. (2022) Systematic Investigation of the Multifaceted Role of SOX11 in Cancer. Cancers (Basel) 14(24): 6103.
- Miao Z, Deng X, Shuai P, Zeng J (2018) Upregulation of SOX18 in colorectal cancer cells promotes proliferation and correlates with colorectal cancer risk. Onco Targets Ther 11: 8481-8490.
- Banerjee V, Sharda N, Huse J, Singh D, Sokolov D, et al. (2021) Synergistic potential of dual andrographolide and melatonin targeting of metastatic colon cancer cells: Using the Chou-Talalay combination index method. Eur J Pharmacol 897: 173919.
- Banerjee A, Basu M, Blanchard TG, Chintalacharuvu SR, Guang W, et al. (2016) Early Molecular Events in Murine Gastric Epithelial Cells Mediated by Helicobacter pylori CagA. Helicobacter 21(5): 395-404.
- Banerjee A, Lang JY, Hung MC, Sengupta K, Banerjee SK, et al. (2011) Unfolded protein response is required in nu/nu mice microvasculature for treating breast tumor with tunicamycin. J Biol Chem 286(33): 29127-29138.
- Blanchard TG, Lapidus R, Banerjee V, Bafford AC, Czinn SJ, et al. (2018) Upregulation of RASSF1A in Colon Cancer by Suppression of Angiogenesis Signaling and Akt Activation. Cell Physiol Biochem 48(3): 1259-1273.
- Blanchard TG, Czinn SJ, Banerjee V, Sharda N, Bafford AC, et al. (2019) Identification of Cross Talk between FoxM1 and RASSF1A as a Therapeutic Target of Colon Cancer. Cancers (Basel) 11(2): 199.
- Weina K, Utikal J (2014) SOX2 and cancer: current research and its implications in the clinic. Clin Transl Med 3: 19.
- Chen Y, Shi L, Zhang L, Li R, Liang J, et al (2008) The molecular mechanism governing the oncogenic potential of SOX2 in breast cancer. J Biol Chem 283(26): 17969-17978.
- Smits P, Li P, Mandel J, Zhang Z, Deng JM, et al. (2001) The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev Cell 1(2): 277-290.
- Mamun MA, Mannoor K, Cao J, Qadri F, Song X (2020) SOX2 in cancer stemness: tumor malignancy and therapeutic potentials. J Mol Cell Biol 12(2): 85-98.
- Liu J, Qiu J, Zhang Z, Zhou L, Li Y, et al (2021) SOX4 maintains the stemness of cancer cells via transcriptionally enhancing HDAC1 revealed by comparative proteomics study. Cell Biosci 11(1): 23.
- Zhao J, Li H, Yuan M (2021) EGR1 promotes stemness and predicts a poor outcome of uterine cervical cancer by inducing SOX9 expression. Genes Genomics 43(5): 459-470.
- Aguilar-Medina M, Avendano-Felix M, Lizarraga-Verdugo E, Bermudez M, Romero-Quintana JG, et al. (2019) SOX9 Stem-Cell Factor: Clinical and Functional Relevance in Cancer. J Oncol 2019: 6754040.
- Oliemuller E, Kogata N, Bland P, Kriplani D, Daley F, et al. (2017) SOX11 promotes invasive growth and ductal carcinoma in situ progression. J Pathol 243(2): 193-207.
-
Natalie May, Amber Czinn, Neha Sharda, Advaitha Midde, Sazzad Hassan, Urs von Holzen, Hem Sukla, Jaylyn Waddell and Aditi Banerjee*. Andrographolide and Melatonin Synergistically Inhibit the Sox Proteins Expression in Colospheroids. Arch Phar & Pharmacol Res. 4(2): 2024. APPR.MS.ID.000585.
-
Andrographolide, Melatonin, Colospheroids, Sox’s Protein, Xenograft Tissue, Cancer Metastasis, Drug Treatment
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






