Research Article
Research Article
Physiological And Metabolic Alteration in Renal Cell Carcinoma: Importance for Therapeutic Outcome
Neerja Trivedi1 and Devendra Kumar2*
1Department of Pharmacology and Neuroscience, School of Medicine, Creighton University, United States
2Department of Pediatrics, Division of Hematology/Oncology, University of Nebraska Medical Center, USA
Devendra Kumar, Department of Pediatrics, Hematology and Oncology Division, University of Nebraska Medical Center, USA
Received Date: May 17, 2024; Published Date: June 05, 2024
Abstract
Renal cell carcinoma, the second most common urologic carcinoma, accounts for around 175,000 deaths annually worldwide. Significant advancement has been made in understanding the pathogenesis of the disease with a focus on oncogenic mutations in growth-regulating pathways. However, more work that is contemporary has established metabolic alterations as key drivers of oncogenic transformation and tumor progression in RCC. Recently, robust metabolomics approaches have become indispensable tools in analyzing kidney cancer to identify pathways that impart metabolic vulnerability. Identifying novel clusters of metabolic pathways and providing detailed knowledge of the underlying molecular mechanisms is expected to open new and promising areas of therapeutic intervention to manage the disease. The targeting some of these metabolic pathways with a single or in combination with other anticancer drugs to reduce RCC tumor growth. The review herein elucidates the general metabolic molecular mechanisms involved in RCC, as well as an emerging concept of cancer metabolism in the perspective of therapeutic intervention.
Keywords: Renal cell carcinoma; Kidney cancer; Metabolic pathways; Tumor energetics; Metabolic reprogramming; Metabolic disease
Abbreviations: RCC: Renal Cell Carcinoma; CCRCC: Clear Cell Renal Cell Carcinoma; MRCC: Metastatic Renal Cell Carcinoma; CHRCC: Chromophobe Renal Cell Carcinoma; PRCC: Renal Cell Carcinoma; FGF: Fibroblast Growth Factor; LC-MS: Liquid Chromatography-Mass Spectrometry; NMR: Nuclear Magnetic Resonance; MRI: Magnetic Resonance Imaging; PCT: Proximal Convoluted Tubule; DCT: Distal Convoluted Tubule; ANF: Atrial Natriuretic Factor; ADH: Antidiuretic Hormone; GFR: Glomerular Filtration Rate; CD: Collecting Duct; VHL: Von Hippel-Lindau; TCA: Tricarboxylic Acid; SGLT: Sodium-Glucose Transport Proteins; GLUT: Glucose Transporter Protein; ATP: Adenosine Triphosphate; MCT: Monocarboxylate transporters; HLF: Hypoxia-inducible factor; LDH: Lactate dehydrogenase; PDK: Pyruvate dehydrogenase kinase; PFK: Phosphofructokinase; 2-DG: 2-deoxy-D-glucose; PKM2: Pyruvate kinase M2; PPP: Pentose phosphate pathway; NADPH: Nicotinamide adenine dinucleotide phosphate; G6PDH: Glucose-6-phosphate dehydrogenase; SUCL: Succinate-CoA ligase; mSLP: Mitochondrial substrate-level phosphorylation; IDO: Indoleamine 2,3-dioxygenase; BCAA: Branched-chain amino acid; ASS1: Arginino-succinate-synthase1; ASL: Argininosuccinate lyase; AGAT: L-Arginine-glycine amidino transferase; HADH: L-3-hydroxyacetyl coA dehydrogenase; FABP: Fatty acid-binding proteins; FASN: Fatty acid synthase; VLDL: Very-low-density lipoproteins; LDL: Low-density lipoproteins; HDL: High-density lipoproteins; LH: Luteinizing hormones; FSH: Follicle-stimulating hormones; PRL: Prolactin; HCG: Human chorionic gonadotropin hormone; TSH: Thyroid stimulating hormone (TSH); PTH: Parathyroid hormone; GnRH: Gonadotropin releasing hormon; HMGCR: 3-Hydroxy-3-methylglutary coenzyme A reductase; DON: 6-diazo-5-oxo-1-norleucine; BPTES: Bis-2-(5-phenylacetamido-1,2,4- thiadiazol-2-yl) ethyl sulfide 3
Introduction
Renal cell carcinoma (RCC) is a urological, metabolic metastatic cancer and research is still going to find a better prognosis. RCC is the second-prominent cause of death from urinary cancer worldwide [1], represents over 90% of all kidney-related cancers and shares >2% of all human cancers in adults [2]. Globally, RCC accounts for approximately 175,000 deaths and 400,000 emerging cases annually, according to a report by GLOBOCAN 2018 Research [3]. RCC can arise at any age but more often affects men over 50 [4]. RCC accounts for 5% of all carcinomas in men and 3% in women [5]. The mortality and morbidity of RCC vary among different countries, although it is more common in developed countries [6]. The occurrence of renal cancer in Europe ranks among the world’s greatest and RCC is one of the top 10 metastatic cancers globally [7]. There are several types of kidney cancers, but most research has focused on RCC. Based on morphology, histological findings, genetic characteristics, and clinical behavior RCC is classified into many subtypes including. RCC is classified based on a different spectrum of mutations and clinical behavior. Most kidney cancer patients have clear cell RCC (ccRCC; 75%) followed by nonclear cell RCC (nccRCC; 25%). nccRCC was further classified into several subtypes; chromophobe RCC (chRCC), papillary RCC (pRCC), medullary, translocation and collecting duct RCC, etc. ccRCC, pRCC and chRCC were found generally and well-characterized categories of RCC [8-13]. Furthermore, the clinical grading of renal malignancies is categorized based on spreading tendency, size, metastatic potential, and nodal involvement [4,14]. When RCC is detected early, during the localized stage, it is generally curable through nephrectomy. However, efficient therapeutic options are lacking when it is not detected until advanced, metastatic stages [15,16]. Classical therapies, including some cytokine like interleukin-2 (IL-2) and interferon-α (IFN-α), have been extensively applied as a treatment option for RCC in metastatic stage [17,18]. The introduction of a multikinase inhibitor, sorafenib, transformed the treatment backdrop for RCC patients [19]. Many other therapies (Table 1) like mTOR (mechanistic target of rapamycin) inhibitors (everolimus and temsirolimus), VEGF inhibitors (sunitinib, lenvatinib, sorafenib, pazopanib, cabozantinib, axitinib, and bevacizumab), Immunomodulatory targets inhibitors such as PD-L1 and PDCD-1 (or PD-1) inhibitors (pembrolizumab and nivolumab; atezolizumab and avelumab) have been explored and approved over the years for the management of RCC [20-22]. Several other approaches, such as a combination of immunotherapy and targeted agents, RNA-targeting, peptide-based therapy, fibroblast growth factor (fgf) based therapy [23], immunotoxins, targeting metabolic pathways are being explored and some of them are in advanced developmental stages [9,24].
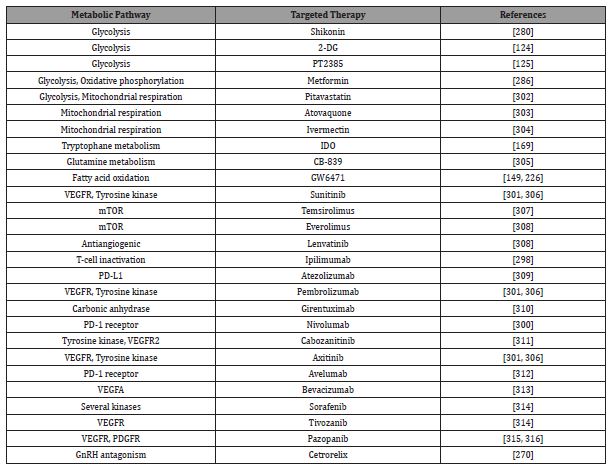
Table 1: Overview of metabolic and signal transduction pathways and their targeted therapy for RCC.
Changes in metabolism indicate early pathophysiology. This altered metabolic state performs a decisive role in cancer progression. Similarly, metabolic reprogramming is regarded as a cancer hallmark [25]. Moreover, there has been a thoughtful growing interest in the field of cancer metabolomics regarding the metabolism in cancer, as a potential therapeutic alternative in cancer management [26]. Interestingly, targeting cancer metabolism to control cancer cell growth in vivo invokes a cancer signaling cascade. Multiple groups are continuously investigating cancer metabolism to find a new potential therapeutic target to cure cancer in near future [26].
The kidney is the most exposed organ to studies involving Liquid chromatography-mass spectrometry (LC-MS) [27-32], nuclear magnetic resonance (NMR) [33-35], and magnetic resonance imaging (MRI). A renal cancer patient’s diagnosis can be complex and challenging [36]. Although there are biomarkersbased detection is being explored for RCC detection, however, it has several limitations and to date, no specific biomarkers can be considered to detect RCC. MRI is the mainstay for the detection of RCC for more than a decade [37,38], but it is not successful in patients with medical implants. Therefore, there is a demand for new tools and techniques for the early detection of RCC.
Metabolomics is a quantitative evaluation of endogenous small-molecule or metabolic intermediates in the biological system [39]. More recently, metabolomics has been used in association with genomics and proteomics approaches to advance the current knowledge and overcome the ambiguous nature of in vitro and in vivo cancer metabolism [40]. Metabolomics has been effectively employed for the discovery of cancer biomarkers [41-42], like breast cancer [43], CRC [44], prostate [45], oral cancer [46], and RCC [41,47]. Metabolites are chemical compounds engaged in various metabolic pathways/regulation; changes in their levels may reflect differences in pathophysiology [39]. Multiple research groups are searching for metabolic biomarkers capable of differentiating RCC patients from healthy individuals. It may also provide more complete information on small-molecule intermediates and help revolutionize disease detection and characterization [48-50].
The expansion of systems biology, and the ‘omics-cascade’ (e.g., genomics, transcriptomics, metabolomics, and proteomics), has started a new era of research into physiology. The latest branch of the ‘omics-cascade’ family is metabolomics, which is based on the identification, quantification, and interaction of metabolites inside biological networks [51-52]. Metabonomics and metabolomics are compatible terms within this branch of science, now emerging to include a comprehensive analysis of the metabolome. The dynamic nature of metabolites places metabolomics as an endpoint of the ‘omics-cascade’. Owing to the vast physical and chemical diversity of metabolites, NMR provides a platform for targeted, structural, and quantitative details for each metabolite [53-54]. NMR-based metabolomics research has become increasingly given complementary features, ease of sample preparation, excellent reproducibility, cost-effectiveness, and simplicity of interpretation by the scientific community. But, LC-MS methods acquire better sensitivity with good dynamic range and detect a wider range of chemical species [35,55]. In particular, metabolomics approach has been more extensively used in cancer research due to its wider scope of applications. Compared to normal cells, cancer cells show signs of distinct metabolic behavior, particularly the impact on systemic metabolism [56-57]. Cancer disrupts the cellular mechanisms of an organism primarily by altering cell growth, differentiation, and tissue integrity [58].
The term biomarker was first devised in 1989 as a Medical Term Heading and defined as “measurable and quantifiable biological parameters which act as indices for health and physiology related assessment, including disease risk” [59]. While this direction offered a clear advancement and paradigm shift in cancer research, the identification of the biomarkers remains challenging. However, vital new technologies in computational metabolomics are simplifying the identification of metabolites. The knack of metabolomics to understand biomarker-discoverybased analysis of metabolites offers significant potential to the field of oncology to describe the metabolism of carcinogenesis. Therefore, biomarkers can confirm the intended metabolic aspects of cancerous cells in general, as well as in specific cancer types like RCC. Further, the identification of alterations in the levels of biomarkers represents a more holistic picture of physiological variations during disease. Similarly, metabolic alterations in the metabolites of renal cancer are considered to be the core promoter of tumorigenesis. Targeting the metabolic pathways with a single or combined with other anticancer drugs would reduce RCC tumor growth. Certainly, evolving data suggests that cancer metabolism is highly heterogenous and dynamic, but no doubt we can get around this obstacle and achieve an even more profound knowledge about the role of metabolism as a diagnostic tool and therapy for RCC. Nonetheless, the better choice of biomarkers that help individualized treatment for RCC still needs to be improved and drive future biomarker-based studies.
This review begins with the metabolic variations in the biological matrix (tissue, urine, and serum) during RCC. Later discussed is a summary of normal kidney function along with cellular bioenergetic pathways, specifically examining the metabolism of glucose, lipids, fatty acids, and amino acids in renal cells at specific locations in nephrons. The purpose and aims of this review are to educate the readers that each part of the nephron plays a dynamic role in controlling renal filtration and reabsorption mechanisms in both healthy individuals and RCC patients and to discuss recent observations on alterations in metabolomics during RCC within the proximal tubule. Third, this review provides examples of unresolved issues meriting future discussion and examination.
In summary, this narrative review is designed to elucidate the present status of the metabolomics of RCC, with the help of NMR, LC-MS, and MRI, as a validated diagnostic implement for the identification, diagnosis, and medication of RCC. A focus is given to kidney function, precisely the function of nephrons. These functional units of the kidney filter and balance biochemicals are present in the bloodstream to attain homeostasis. Further discussed, for comparison, is the physiology of the kidneys under normal conditions as well as during RCC. Each part of the nephron (i.e., renal corpuscle, proximal convoluted tubule [PCT], distal convoluted tubule [DCT], and collecting duct) works distinctively. The stages involved in the normal physiological processes of the kidneys are glomerular filtration, tubular reabsorption, and tubular secretion. These functions are explored further in detail below.
Normal Renal Physiology
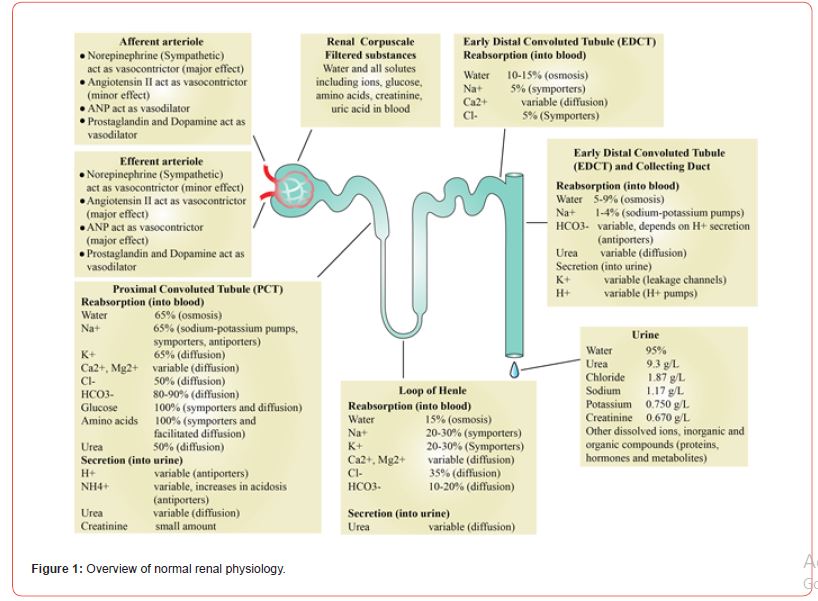
As noted, the nephron is the primary and functional unit of the renal system. At its most basic level, renal filtration occurs through a four-step process in the four specific and unique parts of the nephron, which are the glomerulus, the PCT, the loop of Henle, and the DCT (Figure 1) [60-62].
The afferent and efferent arteriole
Hormonal signals regulate the function of arterioles. Norepinephrine and angiotensin function as vasoconstrictors for afferent and efferent arterioles [63]. Atrial natriuretic factor (ANF) is a cardiac hormone generated by definite cells present in the atrium wall of the heart, which controls electrolyte homeostasis [64]. Like norepinephrine and angiotensin, ANF also functions as a vasodilator and vasoconstrictor. For afferent arterioles, ANF is a vasodilator, and for efferent arterioles, ANF is a vasoconstrictor. Prostaglandin and dopamine vasodilate afferent and efferent arterioles when the filtrate enters the glomerulus of the nephrons [65]. Antidiuretic hormone (ADH) helps in water retention and the excretion of concentrated urine. Aldosterone regulates the salt-water balance by stimulating the secretion of potassium, conservation of sodium, and retention of water to stabilize blood pressure. The hormone, aldosterone, stimulates the distal convoluted tubule of the kidney to reabsorb sodium and chloride ions and water. Aldosterone also promotes the secretion of potassium ions. As such, aldosterone has a net effect on promoting the retention of water and salt. Renin is an enzyme (angiotensinogenase) secreted by the juxtaglomerular cells present in the juxtaglomerular apparatus, which is a structure surrounding afferent arterioles where it enters the glomerulus. Renin secretion is initiated by low sodium ion levels or low blood volume in the afferent arteriole. Similarly, the activation of the reninangiotensin- aldosterone system supports maintaining homeostasis of blood pressure and volume. Through this understanding, hormonal signals play a vital role in kidney functioning. As such, one could posit that during RCC, hormonal signals also fluctuate [66].
The glomerulus
The glomerulus is, in essence, a cluster of nerve endings, capillaries, and blood vessels [60]. The driving mechanism in the function of the glomerulus is filtration pressure, which determines the glomerular filtration rate (GFR). The GFR process defines the amount of filtrate produced in all the renal corpuscles per minute. When properly functioning, the kidneys maintain a relatively constant GFR. The glomerulus adapts based on filtration pressure. For example, if GFR is too high, substances become too rapidly and are not reabsorbed. If GFR is too low, nearly all are reabsorbed, and some waste products are not adequately excreted.
Renal autoregulation
The kidneys themselves maintain GFR by two mechanisms. The myogenic mechanism occurs when stretching triggers, the contraction of smooth muscle cells in afferent arterioles by reducing GFR. The tubule glomerular mechanism occurs when macula densa provides feedback to the glomerulus, which inhibits the afferent arterioles from constricting and thereby decreases GFR.
To better understand these processes, it is also essential to discuss blood pressure, which is, in essence, the pressure exerted on the wall of blood vessels by the circulating blood. This net filtration pressure pushes filtrates through the nephrons. Afferent arterioles cause more significant stress to make filtrates into the renal corpuscle than that caused by efferent arterioles. Low and high blood pressure can affect the rate of glomerular filtration, which is why blood pressure is considered the best measurement of kidney function as such, GFR assists in the detection, evaluation, and management of kidney disease [67-69]. Biochemicals (e.g., glucose, amino acids, urea, and small proteins) and ions (e.g., Na+, K+, Cl-, HCO3-) do not move from the filtrate to go back into the blood circulation. Because renal function is tied to GFR [70], researchers have speculated that GFR also plays a role during RCC. For example, Lowrance and colleagues found impaired GFR to be associated with an increased risk for renal cancer (Medscape Medical News; Impaired Kidney Function Linked to Higher Renal Cancer Risk., May 30, 2014). As such, a potential direction for early identification of RCC patients may be presented through examining GFR. The present review is an important step forward in helping to illuminate the relationship between GFR and RCC and offers a foundation for future studies.
Proximal convoluted tubules (PCT)
The PCT portion of the nephron is present in the cortex of the kidney [71]. PCT comprises cuboidal epithelium containing brush border and has acidophilic cytoplasm due to many mitochondria, which power the cell. PCT is located close to the renal corpuscle, and fluid filtered from the Bowman’s capsule enters the PCT. As such, due to their location immediately after the glomerulus, the PCT performs an incredible amount of work. Approximately 65% of renal reabsorption occurs in the PCT. As such, due to their location immediately after the glomerulus, the PCT performs a great amount of work. Approximately 65% of renal reabsorption occurs in the PCT. Most of the water is reabsorbed. In addition to water, also actively and passively reabsorbed in PCT are ions (e.g., Na+, Cl–, K+, HCO3–, Ca2++). Approximately, 66% of water and salt is reabsorbed in PCT passively by osmosis. Most solutes like glucose, amino acids, and lipid molecules are reabsorbed almost 100% by symporters and facilitated diffusion; urea is absorbed around 50% by diffusion in PCT. Vitamins are also metabolized in PCT. These solutes work via transporters present on the cell membrane. The PCT maintains the pH of the filtrate, and a state of alkaline/acid balance is achieved. In the end, most of the ammonium, which is excreted in the urine, is formed in the PCT [72].
The loop of Henle
After reabsorption and secretion in the PCT, processes continue in the loop of Henle [71]. In the PCT, about two-thirds of the water is reabsorbed; the loop of Henle reabsorbs the rest. In particular, the body reabsorbed Na, Ca, K, and Cl ions in this ascending loop. Filtration of solutes like glucose, amino acids, and lipids only occurs in proximal tubules. As such, in the loop of Henle, only ions and water are reabsorbed. In the ascending loop of Henle, sodium ions are reabsorbed by sodium hydride antiporters and sodiumpotassium- chloride symporters. Some Na is passively diffuse, either in a paracellular or transcellular way. Two limbs of the loop of Henle show different permeability properties, and collectively, with their counterflow, these limbs display countercurrent mechanisms [73- 75].
The distal convoluted tubule
Distal convoluted tubule (DCT) is the final stop for any adjustment in urine after passing through the second convoluted tubule. DCT works for about 10% of the water reabsorption under the control of the hydration signals from the body, where it is a passive process in PCT. Remarkably, the antidiuretic hormone acts on the cells of DCT to boost water uptake. However, the adrenal gland signals the principal cells to secrete aldosterone to increase sodium and water uptake. Arginine vasopressin receptor 2, present on the wall of DCT, stimulates the mechanism to maintain water homeostasis and concentrate the urine. Vasopressin also regulates the homeostasis of water in the body and urine concentration corresponding to the signals accepted by the brain.
The role of DCT in controlling the conservation of ions is considered as important as the role of the loop of Henle in this conversion process [76]. Notable and significant reabsorption of magnesium occurs in DCT [77-78]. Generally, reabsorption of magnesium is load-dependent in the DCT [79-81]. Micro fluorescent studies on mouse DCT cell line (MDCT) show that magnesium uptake was voltage and concentration dependent [82,76]. On average, distal magnesium absorption is less than sodium and calcium [82]. In DCT, sodium is transported against an electrochemical gradient by sodium chloride (Na Cl) symporters. Approximately, 10–15% of water and 5% of calcium/chloride levels are regulated in the early DCT; calcium is reabsorbed in the blood by responding to the secretion of the parathyroid hormone PTH. Finally, the DCT makes its final adjustments of pH by shuttling hydrogen ions and alkaline bicarbonate into or out of the filtrate as required by the peritubular capillaries to regulate pH of the blood. On a side note, little knowledge has been acquired regarding the cellular mechanism involving the transport of cations and anions in the DCT.
Collecting Duct
Collecting duct (CD) is situated in the cortex and runs toward the medulla [83-84]. The lining of the CD wall is made up of epithelium, which can be cuboidal to columnar epithelium. CD cells can be categorized into two types: 1) principal cells, which secrete potassium and reabsorb sodium and water, and 2) intercalated cells, which reabsorb potassium and secrete hydrogen ions. Principal cells in the CD system are sodium-transporting cells. Therefore, each part of the nephron plays a specific function in the reabsorption and secretion of metabolites and ions.
RCC physiology of the nephron changes in reaction to metabolites present in the kidney tissue or biofluids, such as in any other disease. NMR and LC-MS can further detect changes in physiology, given that these instruments can assess sub-picomolar concentrations, demonstrating high sensitivity. Data can be extrapolated to investigate the changes in metabolites and their physiological features of renal cells during cancer and in general. As a result, similar types of reviews, additional research, and clinical studies will work in tandem to expand the diagnosis, care, and treatment of RCC while offering it as a clinical tool. In the future, the potential biomarkers of disease should be further correlated with system physiology, metabolomics, and proteomics.
Altered metabolism in PCT during RCC
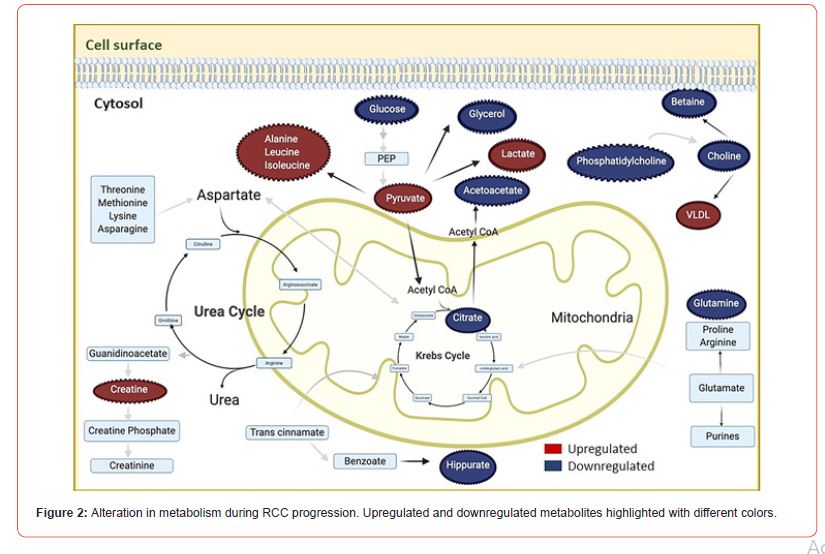
Numerous risk factors play a role in renal cancer. Individuals have a 2.8-fold greater chance to get RCC in their lifetime if they have family history of RCC [85]. Families with inherited RCC have been shown to have the following six identified hereditary syndromes; hereditary papillary RCC, leiomyomatosis, Birt-Hogg- Dube syndrome, von Hippel-Lindau (VHL), tuberous sclerosis complex, and succinate dehydrogenase-associated familial cancer. Many genes are associated with these syndromes [85-86]. However, there are some other factors, also like smoking, hypertension, and obesity, that contribute to RCC [87,15]. An increase in body mass index (BMI) as well as blood pressure could increase the risk of RCC independently, whereas a decrease in blood pressure is linked with a reduced risk of RCC [88]. There are fewer studies on hormonal factors. Pregnancy-associated hormones, particularly high estrogen, may stimulate the proliferation of renal cells directly [89]. In Syrian hamsters, administration of estrogen-induced renal cancer [90]. Studies have shown the presence of steroid hormones like progesterone and estrogen in malignant conditions [89,91,92]. The thyroid hormone also appears to play an important role in the progression and regulation of RCC. The thyroid hormone triiodothyronine was shown to stimulate cell proliferation of RCC cells [93-94]. Cells derived from RCC have also shown disrupted expression of thyroid hormone receptor-β [95]. The activity of the antidiuretic hormone also appears to be altered in RCC [96]. The erythropoietin hormone produced by the kidneys and their receptors has been found in many cancers. Erythropoietin has been revealed to promote proliferation, progression, angiogenesis, and drug resistance in tumors [97]. Also of note, RCC contains the heterogenous group of tumors with different genetic and metabolic alterations. About 60–70% ccRCC originates in PCT with characteristics of clear cytoplasm and sarcomatoid growth pattern. A 5–15% range of papillary RCC also originates from PCT. Oncocytic and chromophobic RCC accounts for about 5–10% of tumors originating in the CD [98]. A comprehensive online search has been made to explore the role of key metabolic components altered during RCC progression (Figure 2).
Carbohydrate/Glucose Metabolism
Over the past two decades, there has been a strong concern about the metabolism of cancer, especially the metabolism of glucose in cancer cells. As a result of subsequent investigations, many cancer biologists have conferred that a major hallmark of cancer is increased cellular glucose metabolism. Many drug candidates based on glycolysis and oxidative phosphorylation and designed to act as anticancer therapy/molecules are in various stages of development [99]. Blood is the major reservoir for glucose, the most abundant substrate in various metabolic pathways governing the growth of cancerous and normal cells. Notably, cancerous cells consume greater levels of glucose compared to normal cells.
Metabolomics studies on renal cancer showed altered levels of biomarkers associated with the process of glycolysis and the tricarboxylic acid (TCA) cycle. During the normal cellular process of glycolysis, the final product of pyruvate is generated from glucose, which can be lowered to lactate or acetyl coenzyme A (acetyl-CoA). Fatty acids and multiple amino acids also generate acetyl-CoA by the oxidation process in mitochondria during the TCA cycle. In renal cancer, it has been shown glucose levels to be downregulated in tissue and serum profiles [48-49], as well as downregulated in urine [50].
For seven decades it has been known that glucose is metabolized in cancer cells through glycolysis, even in the presence of adequate oxygen. This paradox was first explained by Warburg [100]. This phenomenon is also linked to structural and functional impairment in RCC mitochondria [100]. Several changes have happened in response to pseudohypoxia in RCC cell metabolism. The extracellular glucose and its phosphorylated derivative, e.g., glucose-1-phosphate, glucose-6-phosphate) are transported into the cell through apical transporter proteins (i.e., sodium-glucose transport proteins 1 and 2 [SGLT1 and SGLT2] as well as transported to the cell by the family of basolateral transport proteins (e.g., glucose transporter protein type 1 and 2 [GLUT1 and GLUT2]). Higher expression of these facilitative glucose transporters has been stated in RCC. For example, GLUT5 is a well-characterized fructose transporter and is stated in various cell types and body organs (e.g., intestine, kidney brain, testis and skeletal muscle) [101]. Of note, GLUT5 is overexpressed in clear cell RCC [102]. At present, interest in GLUT5 is increasing due to a remarkable increase in the total consumption of fructose in the human diet [103]. Fructose is phosphorylated into fructose 1-phosphate, and then metabolized to glycerol 3-phosphate for the synthesis of glycerol or transformed to acetyl-coA, and finally, integrated into fatty acid metabolism. Only a little fructose is converted into glucose. Increased glycerol is transported to extracellular fluids by the glycerol uptake facilitator protein (glpF) or aquaporin (AQP) transporters [104-105]. Moreover, RCC serum showed an increased level of glycerol [48]. This finding is reliable with the hypothesis that the energy requirement for destructive cell proliferation increases oxidation activity in peroxisomes. This oxidation activates the gluconeogenesis-related transformation of triglycerides.
The final product of glycolysis is pyruvate. Increased pyruvate level is the strong indicator of active glycolysis and glycogenolysis in RCC tumor cells [106]. The increased concentration of pyruvate is likely due to one or more of the following: 1- an increase in enzyme activities involved in glycolysis and reduced utilization of pyruvate during the TCA cycle, 2- the rise of anaerobic cell respiration, and 3- increased production of lactate. Most cancerous cells synthesize lactate independent of hypoxia. An increased lactate metabolic profile has been linked to cancer metastasis in general and an overall poor cancer patient survival rate [107]. In anaerobic glycolysis, the byproduct lactate plays a crucial role in tumorigenesis, maintenance, and drug treatment responses [108]. Moreover, tumor cells utilize lactate to support their growth, development, and mitochondrial metabolism [109]. Lactate is also a substrate in synthesising alanine andglutamate [110]. Like pyruvate, lactate is one of the most promising biomarker metabolites in RCC patients.
Lactate, combined with the decreased levels of glucose in serum or RCC patients, hints at an enhanced activity of anaerobic respiration in RCC. For example, levels of lactate, which is produced by anaerobic glycolysis, were observed to be heightened in tissue, serum and urine profiles of RCC patients [48-50]. The increasing level of lactate in RCC tumors indicates active glycolysis and glycogenolysis. These results further indicate tumor cells switch to glycolysis to offset the failure in synthesizing adenosine triphosphate (ATP) from fatty acid oxidation. The increased pyruvate and lactate flux are consistent while speeding glycolysis in RCC cells, compared with normal renal cells [111].
Lactate efflux is mainly facilitated by monocarboxylate transporters (MCTs), a proton-coupled lactate transporter that exports lactate and H+ ions out from the cells to extracellular environment [112]. MCT4 tends to maintain increased levels of lactate in cancer cells and simultaneously acidifies the extracellular space [113]. Low extracellular pH encourages metastasis [114]. Keshari et al. illustrated that MCT4 expression must be higher in metastatic cells compared with localized cells; they found metastatic cells more rapidly export lactate out of the cells [111]. The hypoxia-inducible factor (HIF-1α) stimulates the transcription of many vital enzymes of the glycolysis and enhance lactate level as the byproduct. In addition, the HIF-1α is controlled by VHL (a tumor suppressor) and VHL/ HIF action extensively improved cell glucose metabolism (anabolism and catabolism). The inactivation of VHL proceeds to the abnormal accumulation of HIF in ccRCC, despite normoxic conditions with the subsequent upregulation of metabolic pathways involved in glucose and fatty acid consumption [100,115]. The responsible enzyme for lactate production is lactate dehydrogenase (LDH), which converts the pyruvate to lactate. It is transported out from the cell by MCT4, and the MCT transporters, particularly MCT1 and MCT4 upregulated in aggressive RCC [116]. It is known that MCT4 and LDH are controlled by HIF-1α [117]. Of note, an increased level of lactate could stimulate a malignant phenotype through the stimulation of several oncogenes and create an acidic condition that safeguards cancer cells from the phagocytic cells of the immune system [113]. The overactivity of HIF-1α also inhibits the oxidative phosphorylation metabolic pathway by targeting pyruvate dehydrogenase kinase-1 (PDK- 1) [118]. Conversely, insufficient oxidative phosphorylation, a characteristic of many cancers, facilitates HIF stability through the inhibition of prolyl hydroxylases [119]. PDK-1 phosphorylates and inhibits pyruvate dehydrogenase activity, rendering it incapable of fulfilling its role in catalyzing pyruvate to convert into acetyl-CoA, which is the initial step for oxidative phosphorylation. Another common molecular event in clear cell RCC is enhanced Akt signaling that leads to speeding up glycolysis and diminished oxidative phosphorylation. The PI3K/Akt signaling pathway is constitutively active in kidney cancer [120]. The Akt hyperactivation regulates signaling cascades, contributing to oncogenic aerobic glycolysis [121]. In addition, these mechanisms induced the expression of glucose transporters, increased expression and activity of hexokinase as well as phosphofructokinase-1(PFK-1); these are highly regulated enzymes in glycolysis. The enzymatic and metabolic cascade of dysregulation stemming from the overactivity of HIF and oncogenes merits greater examination in renal cancers. It has been known that glycolysis enzymes like pyruvate kinase, hexokinase-1, and lactate dehydrogenase-A significantly increased ccRCC patients [122]. The chemically modified substrate 3-bromopyruvate(3- BrPa) acts directly on enzymatic activity of hexokinase [123], and 2-deoxy-D-glucose (2-DG) is believed to inhibit hexokinase [124]. HIF-1α can activate LDH-A, a crucial enzyme in glycolysis. Research has been performed targeting HIF-1α antagonist PT2385 demonstrate inhibition of tumor progression in various RCC cells and is now is under clinical trials (NCT02293980) [125]. An improved understanding of the interrelated processes may help identify potential targets for therapeutic intervention.
Pyruvate is another crucial metabolic product to take into account. Pyruvate can be promptly converted into alanine by a transamination process involving alanine aminotransferase or converted into lactate by LDH. Instead, pyruvate can be metabolized to acetyl-CoA and initiate the TCA cycle. The increased levels of pyruvate in RCC serum [126], perhaps due to increase in enzyme activities involving in glycolysis (e.g., PFK and/or pyruvate kinase), that specifies reduced consumption of pyruvate in the TCA cycle and increase in anaerobic cellular respiration due to active glycogenolysis. This hypothesis is supported by the significantly decreased levels of intermediates, such as succinate, malate, and fumarate in TCA cycle, that altered glycolytic metabolism in RCC and may imitate a catabolic state caused by neoplasia and could be partially considered an adaptive mechanism for sustaining energy homeostasis following the diminishing of TCA cycle. In RCC tissue, TCA intermediates fumarate, malate, and succinate were reported to be downregulated [127]. Conversely, upregulation of the oncometabolite 2-hydroxyglutarate, an analog of α-ketoglutarate (intermediate of TCA). 2-hydroxyglutarate can prevent 2-oxoglutrerate-dependendet dioxygenase that facilitates epigenetic modification, including DNA and histone demethylation and has been linked to the cancerous phenotype progression [128]. Considering the TCA cycle is an aerobic respiration creates the energy following oxidative phosphorylation, but an anaerobic energy metabolism and reduced respiration happen, even in the presence of oxygen in the cancer cell, which is also referred to as aerobic glycolysis or the Warburg effect [129]. It is essential to mention, however, that the glycolytic pyruvate kinase M2 isoform (PKM2) is more abundant than the pyruvate kinase isozymes M1 isoform in RCC, indicating that little ATP synthesis would be generated through the glycolytic pathway in RCC [130-131]. Also recently discovered in RCC, both in vitro and in vivo was the under expression of fumarate hydratase [132], a crucial enzyme in the TCA cycle that catalyzed the fumarate to malate [133]. It has been recognized that fumarate hydratase impairment reduces the TCA cycle activity and, consequently, the accumulation of fumarate which induces many metabolites that are involved in the cancer progression. Zheng et al demonstrated how interrupted metabolism triggered by the deficiency of fumarate hydratase that leads to unique metabolic function and induces the production of stable metabolites they can serve as the specific and unique biomarker for the early diagnosis of RCC [134-135].
The pentose phosphate pathway (PPP) generates reducing equivalents in the form of nicotinamide adenine dinucleotide phosphate (NADPH, a reducing agent) and ribose-5-phosphate. Ribose-5-phosphate is a precursor molecule for nucleotide synthesis in malignant cells. Clear cell RCC is conferred with enhanced activity of the PPP [136]. RCC cells possess chemo and radio resistance as well as resistance to apoptosis. This high level of resistance is most likely due to overexpression of the p-glycoprotein, thereby the Warburg effect [137]. The RCC cells utilize these unique properties to generate huge amounts of NADPH via the PPP, which plays an important role against oxidative stress. NADPH is a reducing agent involved in the glutathione pathway and reduces the glutathione disulfide to produce glutathione. Then, hydrogen peroxide is reduced by involving glutathione to maintain a low level of oxidative stress in cells [138]. The ratio of NADPH/NADP+ production in the PPP is typically increased in promptly proliferating cancer cells [139]. Therefore, PPP appears to be important to support the prompt growth of ccRCC. The two key enzymes of PPP, glucose-6-phosphate dehydrogenase (G6PDH) and transketolase have revealed the overexpression and activity in RCC [136]. Dysregulation of G6PDH has been reported in RCC and elevated level of glucose-6-phosphate is correlated with the high level of intermediates derived from PPP and proposed the major role in RCC [140-141]. The overexpression of these enzyme was demonstrated to associate with the malignant RCC phenotype, which illustrated the key function of the PPP in RCC progression [136].
Amino Acid Metabolism
The amino acid production is primarily initiated by the TCA cycle intermediates and furnished the major pathways. Of note, RCC patients exhibit relatively decreased glutamine (nonessential amino acid) and increased glutamate, valine, and isoleucine [142]. Both substrates’ levels are increased in RCC tissue [49]. However, a significant feature of cancer cells is the consumption and metabolism of glutamine [143,144]. As such, glutamine is considered a clinical nutrient given that it plays a central character in nucleotide biosynthesis as well as cell proliferation, one of the hallmarks of cancer [144]. Interestingly, it has been seen that cancerous cells synthesize glutamine via the de novo pathway [143,144]. Hypoxic cells or hypoxia-inducible factor-1 activating cells utilize glutamine as the primary substrate for the synthesis of fatty acids [145]. Eventually, cancerous cells utilize glutamine as a key player in the synthesis of abundant antioxidants such as glutathione, which is ultimately crucial for redox homeostasis and the survival of cancerous cells in response to oxidative stress [146]. The glutamine consumption is also raised in ccRCC compared to normal renal function [27,29,147]. So, targeting the enzymes involved in glutamine metabolism, like glutaminase (GLS) by the drug CB3940, is being tested in clinical research and observed to be able to follow the response of RCC patients to such therapies [27, 29]. In other clinical study, CB-839 an inhibitor of glutaminase, is used with mTOR inhibitor everolimus and promising results are found for RCC patients [148]. A study on mouse models revealed that inhibitors of NADPH synthesis like KPT-9274 can decrease tumor burden in RCC [149]. Furthermore, increased concentrations of glutamine in the blood have been observed in late-stage cancer patients [150]. In 2016, Hosios et al. showed that glutamine and glucose are the two nutrients that multiple types of cultured cancerous cells mostly consume for their proliferation [151].
Glutamine is the nonessential amino acid and most abundantly present in the blood [152], comprising 50% of free amino acids. The free glutamine in the blood is absorbed in the renal system [72,153]. Renal absorption of glutamine in humans ranges between 7–10 g/d, an amount that equals 10–15% of whole-body glutamine flux [153]. After uptake, glutamine is predominantly metabolized by the glutaminase present in the intra-mitochondrial membrane. Only 10% of glutamine is metabolized by membrane-bound-glutamyl transferase in the DCT and PCT [154-155]. Enhanced glutaminase activity decreases the level of glutamine and other side glutamate is consistently increased. Plasma glutamine reduction has also been stated in, gastrointestinal, breast, and head and neck cancers [156].
Glutamate is a key amino acid for the synthesis of nucleotide in most growing cells. It is derived from glutamine by the catalytic activity of glutaminase. Glutamate can be released into the systemic circulation to formed α-ketoglutarate by glutamate dehydrogenase and produced ammonia as a byproduct. Then α-ketoglutarate would be involved in TCA cycle [157]. The produced ammonia by the action of glutaminase could be excreted in the urine or recirculated to the renal vein [158]. It was observed that increased level of glutamate in RCC tumors is linked to enhanced glutaminase activity and also observed higher levels of glutamate in the plasma samples of RCC patients [142]. Recent studies have linked excessive glutamine consumption in glioblastoma (cancer in the brain or spine cord) to form ATP through the succinate-CoA ligase (SUCL) activity in the mitochondrial matrix [159]. When the SUCL continues to work towards ATP production, it is referred to as ‘mitochondrial substrate-level phosphorylation’ (mSLP), a process that produces high-energy phosphates in the presence or absence of oxygen in cells with insufficient oxidative phosphorylation [159]. Therefore, mSLP could compensate for insufficient ATP synthesis in RCC cells that overexpress the PKM2 isoform and have recognized defects in mitochondria structure and function.
Ornithine itself is a non-protein amino acid formed mainly from glutamate and synthesized from the urea cycle. That is a precursor molecule for polyamines. As pointed out, polyamine metabolism was differentially controlled in aggressive ccRCC. Inhibitor such as 2-difluromethylornithine (DFMO) that targets polyamine metabolism and have been recommended for the treatment of neuroblastoma [160-162], and might be represent a curious therapeutic option for RCC as well. Indeed, the treatment of DFMO significantly diminished the cell viability and reduced mitochondrial activity in spheroid culture of RCC cell line 786-O. In this perspective strategies combining DFMO with other drugs (like proteosome inhibitor bortezomab) are currently studied in neuroblastoma and should be considered a rational therapeutic option for RCC [162].
The decrease of tyrosine, nonessential amino acid and apparent increase of acetyl phenylalanine suggested alteration in phenylalanine metabolism in RCC patients. The formation of acetyl phenylalanine from the acetyl phenylalanine and increased levels of it caused renal impairment. It appears in large amounts in the genetic disease phenylketonuria due to a deficiency of phenylalanine hydroxylase, necessary for metabolizing phenylalanine to tyrosine [163]. Like phenylketonuria, phenylalanine metabolism disorders have been found in RCC, which indicates the inhibition of phenylalanine hydroxylase. However, decades ago, Lichter-Konecki and colleagues clearly described phenylalanine 4-hydroxylase activity in the kidney [164]. The significant role of the kidney is to convert the phenylalanine to tyrosine by the hydroxylation of phenylalanine [165-166]. It has been suggested that tyrosine deficiency by inadequate hydroxylation of phenylalanine contributes to net protein catabolism and muscle deterioration in persons with chronic renal dysfunction; therefore, tyrosine as a dietary supplement could be considered to provide under these conditions [167]. Apart from tyrosine, tryptophan is an essential amino acid that can only be acquired from dietary supplements and cannot be synthesized in the body. A lower level of tryptophan is also found in RCC patients. It should be noted that both tyrosine and tryptophan are physiologically very important, they are precursors of several neurotransmitters and hormones. The deficiency of tryptophan could lead to endocrine dysfunction, often found in RCC [168]. Tryptophan is catabolized by the rate-limited enzyme indoleamine 2,3-dioxygenase (IDO) [169]. Several ongoing clinical research on RCC using IDO inhibitors in combination or singly (NCT02178722; NCT02318277; NCT02559492; NCT02048709) are recruiting. However, these studies are still incomplete and do not utilize interferon (IFN) in combination with IDO inhibitors because INF-α can induce IDO levels [170], and INFα therapy with IDO inhibitors can perform significant role for the treatment of RCC.
In humans, kidneys take up glycine and release serine [153,171,172,173]. This has been explained as support for the transformation of glycine to serine. This transformation is mediated by a pathway concerning glycine modification enzyme and serine hydroxyl methyltransferase [153] in the proximal tubule [172]. Another pathway that involves phosphorylating intermediates for the transformation of glutamine, glutamate, and aspartate to phosphoserine and consequently to serine.
Protein breakdown in muscle provides substrates like valine and isoleucine for enhanced oxidation of branched-chain amino acids (BCAA) in muscle tissue. Therefore, it has been posited that increased protein breakdown in muscle is the initial process behind the enhancement of BCAA levels in the early stages of cancer [174]. In renal cancer patients, alanine concentrations increased in serum and urine but decreased in renal tissue [48-50]. The increased levels of alanine might be linked with the alteration of pyrimidine synthesis [175]. The observation showed an increase in proline as well as a decrease or reduced activity and expression of proline oxidase in tumor tissue could demonstrated the RCC tumor growth [176-177]. Apart from proline, arginine played a crucial role in RCC. Two enzymes, arginino-succinate-synthase1 (ASS1) and arginosuccinate lyase (ASL) are involved in the synthesis of arginine [178]. Arginine is involved in the formation of urea as a waste nitrogen carrier and spread into the renal vein by the synthesis from citrulline [179]. Additionally, arginine itself accelerates the synthesis of hepatic N-acetyl glutamate, an essential allosteric activator of carbamoyl phosphate synthetase, this is one of the important enzymes in the urea cycle [177]. Ultimately, concerning the relationship between the renal production of arginine [180], it has been demonstrated that arginine is metabolized into urea by the arginase, the enzymes heterogeneously distributed along the kidney tubules [102], and the activity of arginase improved toward the renal medulla [178,180].
This compartmentalization of arginine formation, primarily in the cortex, and breakdown, mainly in the medulla [178], permits the kidney to export arginine to the bloodstream and metabolize it into urea [181]. Likewise, arginine is a precursor of creatine and then creatinine in the renal system [181]. Arginine may be related to enhancing the synthesis of fundamental building blocks in parallel to Warburg metabolism [182]. In the kidney, the ammonia detoxification process is performed by forming arginine through the urea cycle. Here ASS1 acts as a rate-limiting enzyme. In ccRCC, ASS1 has been shown to be downregulated in tumor cells, causing cancer to be auxotrophic for arginine [183]. These studies are consistent with RENCA mouse model study, where arginine deprivation inhibited the tumor growth of RCC [183]. The reduced contribution of ASS1 and ASL leads toward the RCC progression [184]. The pegylated arginine deiminase or iminohydrolase, also known as ADI-PEG20 must be utilized to break down the arginine in cancer cells [185]. Given that 90% of the creatine in the human body is contained in muscle, a constant percentage of this pool is converted into creatinine daily by a non-enzymatic mechanism. Creatine metabolism principally occurs in the kidney [186]. L-Arginine-glycine amidinotransferase (AGAT), an enzyme that catalyzes transfer of an amidino group from L-arginine to glycine, involved in creatine and creatinine metabolism, has been found to exhibit low levels of activity during chronic renal failure in rabbits [187]. Remarkably, AGAT expression was downregulated in Wilms’ tumor, which is a rare kidney cancer [188]. In rat nephrons, AGAT immunoreactivity and its activity are limited to the cells of the PCT and almost negligible in other parts of nephrons [189,190]. Patients with RCC are known to have reduced the activity of AGAT as well as creatinine levels [50].
In addition, amino acid transporters perform a decisive role in RCC metabolism. For the export of neutral amino acids, a large amino acid transporter (LAT) [191-192] and involved in the absorption of amino acids from the small intestine as well as in the PCT [193- 194]. Accordingly, the four LATs (LAT1, LAT2, LAT3, and LAT4) have been investigated in RCC patients. In these, LAT1 is associated with cancer [195-198]. On the other hand, LATs 1–4 appear to have an important influence on normal kidney function. Findings revealed that on the basis of mRNA expression, LAT1 was increased in tumor tissue compared with healthy tissue. In addition, the LAT1 mRNA level was significantly greater in less-differentiated primary tumors as well as metastatic tumors. These findings suggest LAT1 can influence the proliferation and invasion of ccRCC [199].
Lipid Metabolism
One of the characteristic features of cancer cells is an increased prevalence of lipogenesis. The biosynthesis of lipids is progressively seen as a possible target for anti-cancer therapy, given its importance as a metabolic process in cancer cells. The proliferating cancer cells create a high requirement of phospholipids and cholesterol as membrane building blocks. Fatty acids are saturated or unsaturated carboxylic acids with an unbranched aliphatic chain and are fundamental molecules for energy production and storage, building blocks of cells, signaling molecules, as well as important components of cellular homeostasis and physiology. Lipidomics is an emerging area of research in cancer biology [200]. In recent years, it has been shown that fatty acid metabolism plays an important role in several types of cancer. Dietary (exogenous) fat is the main source for lipids synthesis in normal cells, while cancer cells both actively synthesize lipids and use exogenous lipids [201]. Therefore, cancer cells have higher expression and activity of key enzymes involved in lipid metabolism. The overexpressed enzymes can serve as potential therapeutic targets [201]. Because normal mitochondrial function is essential to produce ATP from fatty acids, the mitochondrial abnormalities documented in RCC can prevent energy generation from fatty acids. The clear cell appearance of RCC could result, in part, from decreased triglycerides (a storage fatty acid) as seen in other cancers [202]. Of note, the storage of triglycerides is assumed to be a protective approach against the toxicity of free fatty acids [203]. RCC may be the result of enhancement in fatty acid oxidation, it was noticed in other cancer, as prostate cancer in particular [204-205]. The L-3-hydroxyacetyl coA dehydrogenase (HADH) is involved in the β-oxidation of medium chain length fatty acid. A reduced expression of HADH was associated with immune infiltration and poor clinical outcomes in RCC patients [206]. In the scientific literature, tumor markers of RCC can be found in fatty acid-binding proteins (FABP), involved in the transport of fatty acids [207-208] and key enzyme fatty acid synthase (FASN) apparently associated with the initiation and progression of cancer [209], which emphasizes the prominence of fatty acid metabolism in RCC. Remarkably, the upregulation of fatty acids appears to be associated with metastasis in particular [210]. This association indicates the increased fatty acid synthesis or uptake and decrease the mitochondrial oxidation of fatty acid may be rather late events in the development of RCC to an invasive and metastatic stage [211-212].
The occurrence of uncontrolled metabolism and accumulation of cholesterol in solid tumors has been recognized very well [213]. This uncontrolled condition is associated with the accelerated cellular growth and divisions of tumor cells, which require higher levels of lipids or cholesterols and their derivative precursors. Approximately 50% part of the cell membrane is organized by the various lipids and cholesterol. The membrane cholesterols are generated by the uptake of circulating serum lipoproteins. The extreme uptake of lipoprotein cholesterol from serum is a prospective source of cholesteryl esters in RCC [214]. The LC-MS and NMR based analysis of kidney biopsy samples have revealed the increased levels of lipid in RCC [215-216]. The final product of lipid metabolism is the ketone bodies was shown to have concomitant decrease that can be correlated to decrease lipid consumption due to the higher need in cell proliferation in RCC [142].
NADPH performs a typical role in histopathological complications in RCC by facilitating metabolism and accumulation of lipids in the cells. The increased rate of NADPH production may be due to the excess activation of the PPP in cancer cells. Nonetheless, in many tumor cells was revealed altered lipid metabolism and this alternation supports the notion that lipogenesis is involved in the malignant progression in RCC [217]. FASN is a crucial enzyme that is able to synthesize fatty acids de novo [218]. Overexpression of FASN is a common phenomenon in many cancer types [219-222]. For example, Horiguchi et al. described that FASN overexpression is a marker of tumor violence and inadequate prognosis in RCC [209]. Of note, in their experimental design using immunohistochemical analysis, only 15% of the tumors revealed positive stains for FASN expression [209]. RCC cells comprise the elevated levels of esterified form and normal cholesterol [223]. However, the level of free cholesterol is several folds less than that of esterified cholesterol [223]. Free cholesterol accumulation in RCC cells is toxic and is protected by elevated levels of esterified cholesterol [224]. The metabolomics studies on RCC showed an increase in fatty acid utilization by the tumor cells [225]. In a xenograft study, peroxisome proliferator activator receptor-α antagonist ‘GW6471’ an inhibitor of fatty acid oxidation, was tested in various ccRCC models and demonstrated to inhibit ccRCC tumor growth [149, 226]. Besides, FASN involved in the synthesis of fatty acids and cholesterol, are over-expressed in ccRCC [227]. By inhibiting FASN via cerulin or its derivative C75 can reduce the lipogenesis process in different cancer models including RCC cells [227,228].
Glunde and colleagues found choline and phosphocholine synthesis to be connected with modifications in cell membrane structure that accompany the expansion of cancer [229]. They have shown elevated levels of choline in RCC tumors. Choline can only be obtained through diet. It is speculated that the increased levels of choline may be caused by the impairment in the phospholipidlipid pool or maybe due to a higher demand for phospholipids during the uncontrolled proliferation of malignant cells [48, 230]. Additionally, the sharp reduction of glycerophosphorylcholine might be revealed to be a disorder in the function of osmolarity in kidney cells in RCC patients. Sizeland and colleagues found that glycerophosphorylcholine is one of the key organic osmolytes in renal medullary cells, [231] and it can counteract the effects of urea on other macromolecules and enzymes as well. The accretion of the organic osmolytes in the renal medullary cells, the kidneys can respond to hypertonic stress (i.e., abnormally high osmotic pressure) caused by the fluctuation in extracellular solutes [232]. In eukaryotic cell membranes, phospholipid choline (i.e., phosphocholine) plays major structural and functional roles. The significant rise in the serum lipid concentration and consequent decrease in the choline/phosphocholine levels examined in RCC patients are consistent with those described in an earlier study [215].
In RCC patients, studies have shown the sphingolipid class of lipids to be abnormal compared to controls [233]. In RCC, the sphingolipid sphingomyelin, which is found in cell membranes, is downregulated, while sphingolipids, ganglioside, and sphinganin are upregulated. Ganglioside is a sialic acid-containing glycosphingolipid, and sphinganin is a precursor to ceramide (N-acyl sphingosine). Ceramide is a hydrolysis product of sphingomyelin, but it can also be synthesized from palmitate and serine. Other complex sphingolipids (e.g., glucosylceramide, lactosylceramide and gangliosides) originated from ceramide. Of note, gangliosides have attracted considerable interest for nearly two decades as prospective targets for cancer treatment and diagnosis [234,37]. It is sufficient to consider that more gangliosides originated from ceramide to inhibit the proliferation of renal carcinoma cells and this production results in a reduction of the sphingomyelin. Sphingolipids perform a significant role in cell migration and proliferation. Moreover, several drugs are considered in cancer therapy to target the enzymes involve in sphingolipids metabolism [235] and may improve RCC therapy in combination with TKI or mTOR inhibitors [236-237]. RCC patients show lipids profiles of tumors appearing, who could benefit from such a therapeutic option.
Very-low-density lipoproteins (VLDL) are synthesized in the liver and degraded by lipoprotein lipase. VLDL, intermediatedensity lipoproteins (IDL), and low-density lipoproteins (LDL) are interrelated. IDL and LDL appear in the bloodstream as remnants of VLDL, which is converted to LDL by the removal of proteins, except apolipoprotein B100, and by the esterification of most of the cholesterol by lecithin-cholesterol acyl transferase that is associated with high-density lipoproteins (HDL). The esterification process ensues by the transfer of a fatty acid from lecithin to cholesterol, which forms lysolecithin. A decrease in LDL/VLDL signaling is associated with hypolipidemia examined in various cancers [238-239] and the well-described the increase of lipids of kidney specimens with RCC [223]. In ccRCC tissues, the receptor of VLDL is controlled and associated with the lower survival rate of the RCC patients [240].
Vitamin Metabolism
In addition to macronutrients, ongoing investigations in cancer metabolism are broadening to include micronutrients, such as vitamins, which have been largely underexplored in cancer metabolism. However, researchers are examining this domain as an emerging key player in cancer metabolism, and as such, vitamins are attracting considerable interest as therapeutic options [241]. The metabolism of vitamins like E, B, and D also plays a vital role in RCC. For example, based on a case study by Li and colleagues found to reduce the risk of RCC by nearly 25% by taking vitamin E compared with minimal intake. This study occurred at the Third Military Medical University in China and involved 5,789 PCC cases and 14,866 controls [242]. Vitamin E is an antioxidant reported to inhibit cancer development, tissue lipid peroxidation, and the formation of 8-hydroxydeoxyguanosine, as well as protect DNA from fragmentation [243-244]. α-tocopherol is the primary form of vitamin E and is involved in the antioxidant system of animals [245]. During RCC, α-tocopherol is upregulated [246], which may, in turn, indicate increased uptake of fatty acids and lipids [247]. Some studies showed inverse associations between α-tocopherol and RCC risk [248-251], while some studies reported marginal or no significant association between RCC risk and tocopherol intake [252-254]. Similarly, Vitamin B6 and Vitamin D deficiency was related to increased risks of cancer [255]. Vitamin D is found in food in the form of vitamin D2 and D3 and is also produced in the body after exposure to ultraviolet (UV) rays. Both the forms are processed in the liver and afterward in the kidney to form functionally active vitamin D i.e., calcitriol. Interestingly, calcitriol has been displayed to inhibit the progression of tumors. The exact anti-cancer mechanism of vitamin D is not well explored; however, it is speculated that vitamin D and its metabolites abrogate the oncogenesis by inhibiting proliferation, invasion angiogenesis, and metastasis. It is also determined that vitamin D and its metabolites may stimulate cell differentiation and apoptosis [256]. Recently it has been indicated that calcitriol prevents infiltration and migration of RCC cells by repressing Smad2/3-, STAT3- and β-cateninmediated epithelial-mesenchymal transition (EMT) [257]. In addition, the availability of folate, among the vitamins B complex, has been shown to increase the risk of RCC [258]. Decreased folate concentration can be indicated by increased levels of serum homocysteine. Of note, these elevated homocysteine levels may indicate folate deficiency and influence other metabolic pathways. The association between serum folate and serum seems modest [259]. Recently, it has been shown that oral intake of folate can reduce mucositis in metastatic RCC [260]. Furthermore, circulating vitamin B6 has also been shown as an additional prognostic marker for RCC and higher circulating levels of vitamin B6 were noticed to be related with better survival, independently of disease stage [261]. Vitamins ingested directly through a diet of fruits and vegetables have also revealed promising results. For example, a 2009 cohort study by Lin observed the relationship between increased fruit and vegetable intake, including Alpha-Tocopherol and Beta-Carotene (ATBC), to provide a significant protective effect against cancer [262]. Similar results were also shown by Bock and colleagues (2018) [251].
Vegetables and fruits are the primary sources for folate, specifically, it was demonstrated in the studies conducted for fortified and non-fortified folic acid in the population [263-264]. As such, these monitored relationships support the role of folate ingestion. Nevertheless, fruits and vegetables are also sources of various other factors presented to decrease carcinogenesis. Taking this into account, there are possibly other non-folate explanations for the associations underlying these findings. Interestingly, the role of one-carbon metabolism in the risk for developing RCC was supported by a candidate gene study. Here, researchers reported substantial relationships between single-nucleotide polymorphisms (SNP) in one-carbon metabolism genes and the susceptibility to RCC [258]. Among all metabolites examined by Mao and colleagues, vitamins emerged as an important classifier of tumor-versus-normal tissue. Therefore, this data potentially underscores the importance of vitamins in the pathology of RCC, and additional and focused studies may be merited to show vitamins as novel therapeutic strategies.
Hormonal Imbalance
Research revealed that hormones are also one of the attributions of the cause of RCC. Uncontrolled endocrine activation plays a considerable role in RCC pathology. Alteration in paracrine and autocrine signaling might involve cell migration, proliferation angiogenesis, and drug resistance in RCC. The clinical biochemical research shows that serum levels of luteinizing hormones (LH), follicle-stimulating hormones (FSH), prolactin (PRL), human chorionic gonadotropin hormone (HCG), thyroid stimulating hormone (TSH), and parathyroid hormone (PTH) were significantly altered in urogenital cancer patients including RCC [265]. PTH levels were found to be downregulated or suppressed in RCC patients after surgical removal of the kidney (Nephrectomy) [266]. The elevated frequency occurrence of PRL was found in 45% ccRCC patient regardless of their stage of the disease [267]. FSH and TSH were found to be very abnormal during metastatic conditions in RCC patients. The role of hormonal imbalance in RCC pathophysiology is still being explicated to require details of hormonal interactions with oncogenesis.
The gonadotropin releasing hormone (GnRH) and follicle stimulating hormone-releasing hormone (FSH-RH) mediates the release of LH and FSH from the anterior pituitary. The appearance of GnRH receptors was examined in specimens of RCC and surgically removed human cell lines. The positive expression was found in both cases, wherein tumor samples GnRH receptor expression was too high [268]. Therefore, it was assumed that inhibitors of GnRH receptors (for example, AEZS-108 or AN-201) can be targeted against ccRCC tumors during the overexpressed condition of these receptors [268-269]. Jungwirth in 1998 demonstrated in Caki-1 cell line-based xenograft models that GnRH antagonists (Cetrorelix named; SB-75) were tested and displayed to reduce the progression of RCC and latter, this group of compounds proposed to be considered in therapies for ccRCC patients [270].
The FSH receptors (FSHR) are present in solid tumors, including RCC, after forming angiogenic blood vessels [271-272]. The FSHR expression in neo-vascular tumors is observed solely in the boundaries of the tumor but not revealed in the blood vessels of nonmalignant tissue [273]. FSHR may be involved in neo-angiogenesis, making it a possible therapeutic target. The application of FSH-neutralizing antibodies could result in a greater reduction in FSH levels. Recently, a negative allosteric modulator of FSHR “ADX61623” was investigated; it can inhibit the FSHR signaling [273]. FSHR-antagonizing molecules need to be investigated for their role in targeting FSHR in RCC.
The receptors of estrogen (ER) and progesterone (PR) are found in the cytosols of the RCC kidneys compared to normal kidneys. The expression of both receptors (ER and PR) in RCC tumors has suggested that they are associated with excessive exogenous estrogens, as it is usually thought that human kidneys are unresponsive to estrogen. The frequency of expression of ER in human RCC was different in various studies [274, 275]. Estrogen treatment can significantly decrease cell migration, proliferation, invasion, and apoptosis in different cell lines with high endogenous ER-β. ER-β has a cancer-suppressive role, and it was indicated that ER-β could be used as a diagnostic marker for RCC [274]. PRs were also found in ccRCC and serve as a specific and sensitive diagnostic marker for oncocytomas and RCC, which could be used to distinguish between these two subtypes. Patients who were positive for ER/PR or both had positive results from pregestational therapy.
The steroid hormone metabolism is considerably different in RCC patients for benign and malignant distinction. Metabolites of testosterone and cortolone were raised in RCC patients [41]. The receptors of these hormones, e.g., glucocorticoid and androgen receptors, are the prominent markers designated tumor suppressive activity in RCC [276], However, overexpression of mineralocorticoid receptors encourages cell survival and enhances RCC progression [265]. It is advised that the proficiency of these hormones could process the response of RCC patients against the high dose of hormone administration to prevent the activity of glucocorticoid receptors, which may be engaged in tumor growth [277]. It may be instigated by differential expression levels of steroid receptors [278]. The above-stated examples may demonstrate an alternative pathway activation in RCC via hormones. Studies showed the possibility of RCC induction through hormone-activated pathways. Inhibition of hormonal signaling can be crucial in supportive therapies against RCC.
Therapeutic Opportunities Targeting Altered Metabolism During RCC
Understanding the dysregulation of RCC metabolism and the ability to translate these findings into actionable anticancer targets are new opportunities for cancer therapeutics. Different promising strategies that target altered metabolic pathways in combination or alone with anticancer therapies are highlighted in Table 1. Additional research provides important examples to support the idea that targeting metabolism can be used by clinicians for rehabilitation.
For instance, pyruvate kinase plays a pivotal role in regulating cancer metabolism. It regulates the rate-limiting step of glycolysis that shifts the glucose metabolism from the normal respiratory chain to lactate production in renal cancerous cells. As a metabolic regulator simultaneously, it acts like a protein kinase contributing to renal tumorigenesis. Immunohistochemical staining of the RCC tumor showed the presence of the PKM2 [279]. Huang et al. demonstrated that inhibition of 3-Hydroxy-3-methylglutary coenzyme A reductase (HMGCR) led to an increase in glycolysis, so maintaining the level of PKM2 or suppressing the glycolysis can reverse the HMGCR inhibition-induced tumor growth acceleration in RCC mice. The coadministration of PKM2 inhibitor Shikonin can revert the tumor development induced by HMGCR pathway. So, based on the findings, Shikonin could be used as a possible clinical medication for the prevention or treatment of tumor corrosion related to glycolysis or HMGCR abnormalities [280].
In a different context of RCC, metformin acts as an inhibitor of fatty acid oxidation in mitochondrial metabolism and also shows an antitumor effect [281-284]. Metformin induces the activation of AMP-activated protein kinase (AMPK) and inhibits the mTOR [285], a central regulator of protein synthesis and cell growth [286]. Liu and colleagues evaluated that daily treatment of mice with metformin prevented RCC tumor growth and may be used for the treatment of RCC [286]. Fiala et al. evaluate the use of metformin on the survival of metastatic RCC (mRCC) patients treated with pazopanib or sunitinib and found favorable outcomes [283]. Hamieh et al. also investigated the survival benefit of metformin use in mRCC patients [287]. Whereas Miskimins et al. showed more cytotoxic towards cancer cells compared to metformin [288].
Energy production and glucose and glutamine utilization in RCC cells enhance glutamine supply to support cell growth and proliferation. Inhibitors of glutaminase (GLS) target glutamine addiction as a possible treatment approach in mRCC [289]. The 6-diazo-5-oxo-1-norleucine (DON) was first described as GLS inhibitor and suggested to be helpful in chemotherapy [289]. Another example of an allosteric inhibitor of GLS is a Bis-2-(5- phenylacetamido-1,2,4-thiadiazol-2-yl) ethyl sulfide 3 (BPTES) [290]. CB-839 is a novel glutamine inhibitor of kidney-type glutaminase. CB-839 is under clinical trials for several tumors including ccRCC, alone or in combination with cabozantinib, everolimus, and nivolumab [22]. The gaining of drug resistance is a decisive factor against the long-term drug effects for the treatment of RCC and is accountable for the poor clinical outcomes [291]. Recently, tyrosine kinase inhibitors (TKI), mTOR inhibitors [292- 293], and immune checkpoint inhibitors [294-296] were used as the first-line therapy for the treatment of mRCC [297-301]. According to the completed or ongoing clinical trials everolimus, lenvatinib/ nivolumab, and cabozantinib changed the treatment landscape for RCC. So, the comprehensive understanding of metabolic alterations and the efficacy of different therapies to target glycolysis, mitochondrial respiration, and various metabolic pathways must be therapeutically exploited in RCC. For the prediction of drug efficacy, metabolic biomarker detection is the way to overcome the problem, reduce cost, and improve patient survival rate.
Conclusions and Future Perspectives
Undoubtedly, research in cancer metabolism is rapidly advancing. The application of multi-omics interventions to investigate the origins of disease has strengthened our grasp on the complexity of these disease networks. The latest discoveries in cancer biology and biochemistry bear witness to the need for a major update of biochemistry textbooks and a targeted biochemical review of the field of cancer, with a more holistic interpretation of data. To explain the cellular regulation of metabolism, the emphasis in the 21st century has dramatically changed from biochemical networks to protein networks. Apart from progress in basic science, there are essential concerns about whether metabolism may be the heel for combatting cancer.
Interestingly, an abundance of metabolic proteins, enzymes, and their biochemical pathways are still waiting to be investigated as novel potential targets in cancer therapy. Considering and examining metabolism as a central player is expected to reveal several novel therapeutic targets to bring new hope in cancer treatment and recovery. The synthesis and removal of reactive oxygen species are closely related to respiration and the synthesis of glucose and glutamine. There are many worthy and thought-provoking topics to examine in more detail to propel research forward, including the roles of reactive oxygen species, redox metabolism, and metabolic/ biochemical changes in the tumor stroma. Lastly, one could speculate that targeting cancer energetics and metabolic signaling pathways and cascades will bring new promise in cancer-specific therapy for multiple cancer types, particularly considering that healthy cells’ energy dependence differs significantly from cancer metabolism. The review reveals the metabolic molecular processes involved in RCC, as well as the promising concept of cancer metabolism in the context of therapeutic intervention.
Competing interests
Authors declare that they have no competing interest that could have appeared to influence the work reported in this article.
Acknowledgment
None.
- X Liu, M Zhang, X Liu, H Sun, Z Guo, et al. (2019) Urine Metabolomics for Renal Cell Carcinoma (RCC) Prediction: Tryptophan Metabolism as an Important Pathway in RCC. Front Oncol 9: 663.
- J Oto, A Fernandez-Pardo, M Roca, E Plana, MJ Solmoirago, et al. (2020) Urine metabolomic analysis in clear cell and papillary renal cell carcinoma: A pilot study. J Proteomics 218: 103723.
- F Bray, J Ferlay, I Soerjomataram, RL Siegel, LA Torre, et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6): 394-424.
- B Ljungberg, NC Cowan, DCHanbury, M Hora, MA Kuczyk, et al. (2010) G European Association of Urology Guideline, EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol 58(3): 398-406.
- A Znaor, J Lortet-Tieulent, M Laversanne, A Jemal, F Bray, et al. (2015) International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 67(3): 519-530.
- M Medina-Rico, HL Ramos, M Lobo, J Romo, JG Prada, et al. (2018) Epidemiology of renal cancer in developing countries: Review of the literature. Can Urol Assoc J 12(3): E154-E162.
- RL Siegel, KD Miller, HE Fuchs, A Jemal (2022) Cancer statistics, 2022. CA Cancer J Clin 72(1): 7-33.
- MH Ather, N Masood, T Siddiqui (2010) Current management of advanced and metastatic renal cell carcinoma. Urol J 7(1): 1-9.
- M Santoni, F Massari, V Di Nunno, A Conti, A Cimadamore, et al. (2018) Immunotherapy in renal cell carcinoma: latest evidence and clinical implications. Drugs Context 5(7): 212528.
- H Moch, AL Cubilla, PA Humphrey, VE Reuter, TM Ulbright (2016) The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol 70(1): 93-105.
- H Moch (2013) An overview of renal cell cancer: pathology and genetics. Semin Cancer Biol 23(1): 3-9.
- F Algaba, H Akaza, A Lopez-Beltran, G Martignoni, H Moch, et al. (2011) Current pathology keys of renal cell carcinoma. Eur Urol 60(4): 634-643.
- M Basso, A Cassano, C Barone (2010) A survey of therapy for advanced renal cell carcinoma. Urol Oncol 28(2): 121-133.
- A Adam, AK Dixon, JH Gillard, C Schaefer-Prokop, RG Grainger (2014) Grainger & Allison's Diagnostic Radiology, Elsevier Health Sciences.
- P Cairns (2010) Renal cell carcinoma. Cancer Biomark: section A of Disease markers 9(1-6): 461-473.
- N Vasudev, R Banks, C Edelstein (2011) Biomarkers of renal cancer. Biomarkers in kidney disease 8: 313-344.
- DF McDermott, MB Atkins (2004) Application of IL-2 and other cytokines in renal cancer. Expert Opin Biol Ther 4(4): 455-468.
- TE Hutson, DI Quinn (2005) Cytokine therapy: a standard of care for metastatic renal cell carcinoma?. Clin Genitourin Cancer 4(3): 181-186.
- A Ben Mousa (2008) Sorafenib in the treatment of advanced hepatocellular carcinoma. Saudi J Gastroenterol 14(1): 40-42.
- B Escudier, C Porta, M Schmidinger, N Rioux-Leclercq, A Bex, et al. (2019) Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann Oncol 30(50): 706-720.
- MB Atkins, NM Tannir (2018) Current and emerging therapies for first-line treatment of metastatic clear cell renal cell carcinoma. Cancer Treat Rev 70: 127-137.
- PC Barata, BI Rini (2017) Treatment of renal cell carcinoma: Current status and future directions. CA Cancer J Clin 67(6): 507-524.
- N Trivedi, D Kumar (2021). Fibroblast growth factor and kidney disease: Updates for emerging novel therapeutics. J Cell Physiol 236(12): 7906-7925.
- DC Yang, CH Chen (2020) Potential New Therapeutic Approaches for Renal Cell Carcinoma. Semin Nephrol 40(1): 86-97.
- NN Pavlova (2016) CB Thompson, The Emerging Hallmarks of Cancer Metabolism. Cell Metab 23(1): 27-47.
- UE Martinez-Outschoorn, M Peiris-Pages, RG Pestell, F Sotgia, MP Lisanti (2017) Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol 14(1): 11-31.
- AA Hakimi, E Reznik, CH Lee, CJ Creighton, AR Brannon, et al. (2016) An Integrated Metabolic Atlas of Clear Cell Renal Cell Carcinoma. Cancer Cell 29(1): 104-116.
- J Reigle, D Secic, J Biesiada, C Wetzel, B Shamsaei, et al. (2021) Tobacco smoking induces metabolic reprogramming of renal cell carcinoma. J Clin Invest 131(1): e 140522.
- HI Wettersten, AA Hakimi, D Morin, C Bianchi, ME Johnstone, et al. (2015) Grade-Dependent Metabolic Reprogramming in Kidney Cancer Revealed by Combined Proteomics and Metabolomics Analysis. Cancer Res 75(12): 2541-2552.
- J Niziol, V Bonifay, K Ossolinski, T Ossolinski, A Ossolinska, et al. (2018) Metabolomic study of human tissue and urine in clear cell renal carcinoma by LC-HRMS and PLS-DA. Anal Bioanal Chem 410(16): 3859-3869.
- L Jing, JM Guigonis, D Borchiellini, M Durand, T Pourcher, et al. (2019) LC-MS based metabolomic profiling for renal cell carcinoma histologic subtypes. Sci Rep 9(1): 15635.
- MR Martinefski, B Elguero, ME Knott, D Gonilski, L Tedesco, et al. (2021) Mass Spectrometry-Based Metabolic Fingerprinting Contributes to Unveil the Role of RSUME in Renal Cell Carcinoma Cell Metabolism. J Proteome Res 20(1): 786-803.
- R Ragone, F Sallustio, S Piccinonna, M Rutigliano, G Vanessa, et al. (2016) Renal Cell Carcinoma: A Study through NMR-Based Metabolomics Combined with Transcriptomics. Diseases 4(1): 7.
- OS Falegan, SA Arnold Egloff, A Zijlstra, ME Hyndman, HJ Vogel (2019) Urinary Metabolomics Validates Metabolic Differentiation Between Renal Cell Carcinoma Stages and Reveals a Unique Metabolic Profile for Oncocytomas. Metabolites 9(8): 155.
- OS Falegan, MW Ball, RA Shaykhutdinov, PM Pieroraio, F Farshidfar, et al. (2017) Urine and Serum Metabolomics Analyses May Distinguish between Stages of Renal Cell Carcinoma. Metabolites 7(1): 6.
- Y Zhou, M van Melle, H Singh, W Hamilton, G Lyratzopoulos, et al. (2019) Quality of the diagnostic process in patients presenting with symptoms suggestive of bladder or kidney cancer: a systematic review. BMJ Open 9(10): e029143.
- MR Oliva, JN Glickman, KH Zou, SY Teo, KJ Mortele, et al. (2009) Renal cell carcinoma: t1 and t2 signal intensity characteristics of papillary and clear cell types correlated with pathology. AJR Am J roentgenolog 192(6): 1524-1530.
- AB Rosenkrantz, N Hindman, EF Fitzgerald, BE Niver, J Melamed, et al. (2010) MRI features of renal oncocytoma and chromophobe renal cell carcinoma. AJR Am J roentgenology 195(6): W421-427.
- JK Nicholson, JC Lindon (2008) Systems biology: Metabonomics. Nature 455(7216): 1054-1056.
- M Momcilovic, DB Shackelford (2018) Imaging Cancer Metabolism. Biomol Ther (Seoul) 26(1): 81-92.
- M Zhang, X Liu, X Liu, H Li, W Sun, et al. (2020) A pilot investigation of a urinary metabolic biomarker discovery in renal cell carcinoma. Int Urol Nephrol 52(3); 437-446.
- JL Spratlin, NJ Serkova, SG Eckhardt (2009) Clinical applications of metabolomics in oncology: a review, Clinical cancer research: an official journal of the American Association for Cancer Research 15(2): 431-440.
- WM Claudino, A Quattrone, L Biganzoli, M Pestrin, I Bertini, et al. (2007) Metabolomics: available results, current research projects in breast cancer, and future applications, Journal of clinical oncology: official journal of the American Society of Clinical Oncology 25(19); 2840-2846.
- Y Qiu, G Cai, M Su, T Chen, X Zheng, et al. (2009) Serum metabolite profiling of human colorectal cancer using GC-TOFMS and UPLC-QTOFMS. J Proteome Res 8(10): 4844-4850.
- D Kumar, A Gupta, K Nath, (2016) NMR-based metabolomics of prostate cancer: a protagonist in clinical diagnostics. Expert Rev Mol Diagn 16(6): 651-661.
- J Zhou, B Xu, J Huang, X Jia, J Xue, et al. (2009) 1H NMR-based metabonomic and pattern recognition analysis for detection of oral squamous cell carcinoma, Clin Chim Acta; international journal of clinical chemistry 401(1-2): 8-13.
- KA Lippa, JJ Aristizabal-Henao, RD Beger, JA Bowden, C Broeckling, et al. (2022) Reference materials for MS-based untargeted metabolomics and lipidomics: a review by the metabolomics quality assurance and quality control consortium (mQACC). Metabolomics 18(4): 24.
- H Gao, B Dong, X Liu, H Xuan, Y Huang, et al. (2008) Metabonomic profiling of renal cell carcinoma: high-resolution proton nuclear magnetic resonance spectroscopy of human serum with multivariate data analysis. Anal Chim Acta 624(2): 269-277.
- H Gao, B Dong, J Jia, H Zhu, C Diao, et al. (2012) Application of ex vivo (1)H NMR metabonomics to the characterization and possible detection of renal cell carcinoma metastases. J Cancer Res Clin Oncol 138(5): 753-761.
- R Ragone, F Sallustio, S Piccinonna, M Rutigliano, G Vanessa, et al. (2016) Renal Cell Carcinoma: A Study through NMR-Based Metabolomics Combined with Transcriptomics. Diseases 4(1): 7.
- A Gupta, N Bansal, B Houston (2012) Metabolomics of urinary tract infection a new uroscopy in town. Expert review of molecular diagnostics 12(4): 361-369.
- M Cuperlovic-Culf, D Ferguson, A Calf, P Morin, M Toubia (2012)1H NMR metabolomics analysis of glioblastoma subtypes correlation between metabolomics and gene expression characteristics. The Journal of biological chemistry 287(24): 20164-20175.
- WB Dunn, DI Broadhurst, HJ Atherton, R Goodacre, JL Griffin (2011) Systems level studies of mammalian metabolomes the roles of mass spectrometry and nuclear magnetic resonance spectroscopy. Chemical Society reviews 40(1): 387-426.
- L Cheng, U Pohl (2007) The handbook of metabolomics and metabolomics.
- AH Emwas (2015) The strengths and weaknesses of NMR spectroscopy and mass spectrometry with particular focus on metabolomics research. Methods Mol Biol 1277: 161-193.
- D Hanahan, RA Weinberg (2011) Hallmarks of cancer the next generation. Cell 144(5) 646-674.
- JR Cantor, DM Sabatini (2012) Cancer cell metabolism one hallmark many faces. Cancer discovery 2(10): 881-898.
- M Mareel, A Leroy (2003) Clinical cellular and molecular aspects of cancer invasion. Physiol Rev 83(2): 337-376.
- S Gutman, LG Kessler (2006) The US Food and Drug Administration perspective on cancer biomarker development. Nature reviews Cancer 6(7): 565-571.
- F Kiil, J Ostensen (1989) Essentials of glomerulotubular balance. Acta Physiol Pharmacal Bulg 15(1): 3-12.
- WJO Connor (1984) Tubular reabsorption in normal renal function. Ren Physiol 7(4): 193-204.
- BM Wilkes, LU Mailloux (1986) Acute renal failure. Pathogenesis and prevention. The American Journal of Medicine 80(6): 1129-1136.
- RM Edwards (1983) Segmental effects of norepinephrine and angiotensin II on isolated renal micro vessels. Am J Physiol 244(5): F526-534.
- LR Potter, AR Yoder, DR Flora, LK Antos, DM Dickey (2009) Natriuretic peptides their structures, receptors, physiologic functions and therapeutic applications, Handb Exp Pharmacol 191: 341-366.
- Tang, K. Loutzenhiser, R. Loutzenhiser (2000) Biphasic actions of prostaglandin E (2) on the renal afferent arteriole: role of EP (3) and EP (4) receptors. Circ Res 86(6): 663-670.
- R Bellomo, JA Kellum, C Ronco (2012) Acute kidney injury. Lancet 380(9843): 756-766.
- L Wesson (1969) Physiology of the Human Kidney Grune and Stratton, New York, USA pp. 431.
- TH Mathew, G Australasian (2005) Creatinine Consensus Working, Chronic kidney disease and automatic reporting of estimated glomerular filtration rate a position statement. The Medical journal of Australia 183(3): 138-141.
- LA Stevens, AS Levey (2005) Measurement of kidney function. The Medical clinics of North America 89(3): 457-473.
- D Chapman, R Moore, S Kaltenbach, B Braam (2010) Residual renal function after partial or radical nephrectomy for renal cell carcinoma. Canadian Urological Association journal = Journal de l Association des urologues du Canada 4(5): 337-343.
- CP Jaya Pandian, Y Chen, AR Janowczyk, MB Palmer, CA Cassol, et al. (2021) Development and evaluation of deep learning-based segmentation of histologic structures in the kidney cortex with multiple histologic stains. Kidney Int 99(1): 86-101.
- ID Weiner, JW Verlander (2013) Renal ammonia metabolism and transport. Comprehensive Physiology 3(1): 201-220.
- MB Burg (1982) Thick ascending limb of Henle s loop. Kidney Int 22(5): 454-464.
- DB Mount (2014) Thick ascending limb of the loop of Henle. Clin J Am Soc Nephrol 9(11): 1974-1986.
- TL Pannabecker (2012) Structure and function of the thin limbs of the loop of Henle. Compr Physiol 2(3): 2063-2086.
- LJ Dai, G Ritchie, D Kerstan, HS Kang, DE Cole, et al. (2001) Magnesium transport in the renal distal convoluted tubule. Physiol Rev 81(1): 51-84.
- GA Quamme (1981) Effect of furosemide on calcium and magnesium transport in the rat nephron. The American journal of physiology 241(4): F340-347.
- IM Shafik, GA Quamme (1989) Early adaptation of renal magnesium reabsorption in response to magnesium restriction. The American journal of physiology 257(6 Pt 2): F974-977.
- GA Quamme (1989) Control of magnesium transport in the thick ascending limb. The American journal of physiology 256(2 Pt 2): F197-210.
- GA Quamme (1997) Renal magnesium handling: new insights in understanding old problems. Kidney international 52(5): 1180-1195.
- C de Rouffignac, G Quamme (1994) Quamme Renal magnesium handling and its hormonal control. Physiol Rev 74(2): 305-322.
- GA Quamme, JH Dirks (1980) Intraluminal and contraluminal magnesium on magnesium and calcium transfer in the rat nephron. The American journal of physiology 238(3): F187-198.
- D Pearce, R Soundararajan, C Trimpert, OB Kashlan, PM Deen, et al. (2015) Collecting duct principal cell transport processes and their regulation. Clin J Am Soc Nephrol 10(1): 135-146.
- R Lashhab, A Ullah, E Cordat (2019) Renal collecting duct physiology and pathophysiology (1). Biochem Cell Biol 97(3): 234-242.
- J Clague, J Lin, A Cassidy, S Matin, NM Tannir, et al. (2009) Family history and risk of renal cell carcinoma: results from a case-control study and systematic meta-analysis. Cancer epidemiology, biomarkers & prevention 18(3): 801-807.
- WM Linehan, R Srinivasan, LS Schmid (2010) The genetic basis of kidney cancer: a metabolic disease. Nature reviews urology 7(5): 277-285.
- JK McLaughlin, L Lipworth, RE Tarone (2006) Epidemiologic aspects of renal cell carcinoma. Seminars in oncology 33(5): 527-533.
- WH Chow, G Gridley, JF Fraumeni, B Jarvholm (2000) Obesity, hypertension, and the risk of kidney cancer in men. The New England journal of medicine 343(18): 1305-1311.
- G Concolino, C Lubrano, M Ombres, A Santonati, GP Flammia, et al. (1993) Acquired cystic kidney disease. the hormonal hypothesis Urology 41(2): 170-175.
- EL Cavalieri, S Kumar, R Todorovic, S Higginbotham, AF Badawi, et al. (2001) Imbalance of estrogen homeostasis in kidney and liver of hamsters treated with estradiol: implications for estrogen-induced initiation of renal tumors. Chemical research in toxicology 14(8): 1041-1050.
- G Concolino, A Marocchi, F Concolino, F Sciarra, F Di Silverio, et al. (1976) Human kidney steroid receptors. Journal of steroid biochemistry 7(10): 831-835.
- E Ronchi, G Pizzocaro, P Miodini, L Piva, R Salvioni, et al. (1984) Steroid hormone receptors in normal and malignant human renal tissue: relationship with progestin therapy. Journal of steroid biochemistry 21(3): 329-335.
- P Poplawski, A Nauman (2008) Thyroid hormone - triiodothyronine - has contrary effect on proliferation of human proximal tubules cell line (HK2) and renal cancer cell lines (Caki-2, Caki-1) - role of E2F4, E2F5 and p107, p130. Thyroid research 1(1): 5.
- L Szymanski, D Matak, E Bartnik, C Szczylik, AM Czarnecka (2016) Thyroid Hormones as Renal Cell Cancer Regulators. Journal of signal transduction 2016: 1362407.
- A Master, A Wojcicka, A Piekielko-Witkowska, J Boguslawska, P Poplawski, et al. (2010) Untranslated regions of thyroid hormone receptor beta 1 mRNA are impaired in human clear cell renal cell carcinoma. Biochimica et biophysica acta 1802(11): 995-1005.
- K Orywal, W Jelski, T Werel, M Szmitkowski (2016) The diagnostic significance of serum alcohol dehydrogenase isoenzymes and aldehyde dehydrogenase activity in renal cell cancer patients. Experimental and molecular pathology 100(3): 416-420.
- C Morais, DW Johnson, DA Vesey, GC Gobe (2013) Functional significance of erythropoietin in renal cell carcinoma. BMC cancer 13: 14.
- BI Rini, SC Campbell, B Escudier (2009) Renal cell carcinoma. Lancet 373: 1119-1132.
- L Wilde, M Roche, M Domingo-Vidal, K Tanson, N Philp, et al. (2017) Metabolic coupling and the Reverse Warburg Effect in cancer: Implications for novel biomarker and anticancer agent development. Semin Oncol 44(3): 198-203.
- O Warburg (1956) On the origin of cancer cells. Science 123(3191): 309-314.
- Veronique Douard, Ronaldo P Ferraris (2008) Regulation of the fructose transporter GLUT5 in health and disease. Am J Physiol Endocrinol Metab 295(2): E227-E237.
- Luis M Antón Aparicio, Vanessa Medina Villaamil, Moisés Blanco Calvo, Luis Valbuena Rubira, José Manuel Rois et al. (2010) Glucose transporter expression and the potential role of fructose in renal cell carcinoma: A correlation with pathological parameters. Mol Med Rep 3(4): 575-580.
- Annette Schürmann (2008) Insight into the "odd" hexose transporters GLUT3, GLUT5, and GLUT7. Am J Physiol Endocrinol Metab 295(2): E225-226.
- Gordon J Cooper, Yuehan Zhou, Patrice Bouyer, Irina I Grichtchenko, Walter F Boron (2002) Transport of volatile solutes through AQP1. J Physiol 542(Pt 1): 17-29.
- D Gomes, A Agasse, P Thiebaud, S Delrot, H Geros, et al. (2009) Aquaporins are multifunctional water and solute transporters highly divergent in living organisms. Biochim Biophys Acta 1788(6): 1213-1228.
- Takatsugu Okegawa, Megumi Morimoto, Satoru Nishizawa, Satoshi Kitazawa, Kohei Honda, et al. (2017) Intratumor Heterogeneity in Primary Kidney Cancer Revealed by Metabolic Profiling of Multiple Spatially Separated Samples within Tumors. EBioMedicine 19: 31-38.
- F Hirschhaeuser, U G Sattler, W Mueller Klieser (2011) Lactate: a metabolic key player in cancer. Cancer Res 71(22): 6921-6925.
- Michelangelo Certo, Chin-Hsien Tsai, Valentina Pucino, Ping-Chih Ho, Claudio Mauro (2021) Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat Rev Immunol 21(3): 151-161.
- Carmela Guido, Diana Whitaker-Menezes, Zhao Lin, Richard G Pestell, Anthony Howell, et al. (2012) Mitochondrial fission induces glycolytic reprogramming in cancer-associated myofibroblasts, driving stromal lactate production, and early tumor growth. Oncotarget 3(8): 798-810.
- Kelly M Kennedy, Peter M Scarbrough, Anthony Ribeiro, Rachel Richardson, Hong Yuan, et al. (2013) Catabolism of exogenous lactate reveals it as a legitimate metabolic substrate in breast cancer. PLoS One 8(9): e75154.
- Kayvan R Keshari, Renuka Sriram, Bertram L Koelsch, Mark Van Criekinge, David M Wilson, et al. (2013) Hyperpolarized 13C-pyruvate magnetic resonance reveals rapid lactate export in metastatic renal cell carcinomas. Cancer Res 73(2): 529-38.
- Guido Kroemer, Jacques Pouyssegur (2008) Tumor cell metabolism: cancer's Achilles' Cancer cell 13 13(6): 472-82.
- Robert A Gatenby, Robert J Gillies (2004) Why do cancers have high aerobic glycolysis?. Nat Rev Cancer 4(11): 891-894.
- Pawel Swietach, Richard D Vaughan-Jones, Adrian L Harris (2007) Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev 26(2): 299-310.
- Joanne R Doherty, John L Cleveland (2013) Targeting lactate metabolism for cancer therapeutics. J Clin Invest 123(9): 3685-3692.
- Ara Yoo, Hyeonhee Lee, Jinyoung Jung, Sang Seok Koh, Soojin Lee (2021) Monocarboxylate transporter 9 (MCT9) is down-regulated in renal cell carcinoma. Genes Genomics 43(4): 351-359.
- Mohammed S Ullah, Andrew J Davies, Andrew P Halestrap (2006) The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem 281(14): 9030-9037.
- Ioanna Papandreou, Rob A Cairns, Lucrezia Fontana, Ai Lin Lim, Nicholas C Denko (2006) HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab (3): 187-197.
- Moritz J, Strowitzki, Eoin P. Cummins, Cormac T. Taylor (2019) Protein Hydroxylation by Hypoxia-Inducible Factor (HIF) Hydroxylases: Unique or Ubiquitous?. Cells 8(5): 384.
- Jin Young Park, Pei-yin Lin, Robert H Weiss (2007) Targeting the PI3K–Akt pathway in kidney cancer. Expert Rev Anticancer Ther 7(6): 863-870.
- R Brooks Robey, Nissim Hay (2009) Is Akt the "Warburg kinase"-Akt-energy metabolism interactions and oncogenesis. Semin Cancer Biol 19(1): 25-31.
- Bertrand Perroud, Tatz Ishimaru, Alexander D Borowsky, Robert H Weiss (2009) Grade-dependent proteomics characterization of kidney cancer. Mol Cell Proteomics 8(5): 971-985.
- Zhao Chen, Hui Zhang, Weiqin Lu, Peng Huang (2009) Role of mitochondria-associated hexokinase II in cancer cell death induced by 3-bromopyruvate. Biochim Biophys Acta 1787(5): 553-560.
- Luis E Raez, Kyriakos Papadopoulos, Alejandro D Ricart, E Gabriella Chiorean, Robert S Dipaola, et al. (2013) A phase I dose-escalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol 71(2): 523-530.
- Wenfang Chen, Haley Hill, Alana Christie, Min Soo Kim, Eboni Holloman, et al. (2016) Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 539(7627): 112-117.
- Jie Shen, Zhen Chen, Qianfeng Zhuang, Min Fan, Tao Ding, et al. (2016) Prognostic Value of Serum Lactate Dehydrogenase in Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. PLoS One 11(11): e0166482.
- Gareth Catchpole, Alexander Platzer, Cornelia Weikert, Carsten Kempkensteffen, Manfred Johannsen, et al. (2011) Metabolic profiling reveals key metabolic features of renal cell carcinoma. Cell Mol Med 15(1): 109-118.
- Eun Hee Shim, Carolina B Livi, Dinesh Rakheja, Jubilee Tan, Daniel Benson, et al. (2014) L-2-Hydroxyglutarate: an epigenetic modifier and putative oncometabolite in renal cancer. Cancer Discov 4(11): 1290-1298.
- Robert Jan Raterink, Yoeri Witkam, Rob J Vreeken, Rawi Ramautar, Thomas Hankemeier (2014) Gas pressure assisted microliquid-liquid extraction coupled online to direct infusion mass spectrometry: a new automated screening platform for bioanalysis. Anal Chem 86(20): 10323-10330.
- Prasanta Dey, Ji Yeon Son, Amit Kundu, Kyeong Seok Kim, Yura Lee, et al. (2019) Knockdown of Pyruvate Kinase M2 Inhibits Cell Proliferation, Metabolism, and Migration in Renal Cell Carcinoma. Int J Mol Sci 20(22): 5622.
- Min Yu, Shengying Chen, Weifeng Hong, Yujun Gu, Bowen Huang, et al. (2019) Prognostic role of glycolysis for cancer outcome: evidence from 86 studies. J Cancer Res Clin Oncol 145(4): 967-999.
- Han Xie, Vladimir A. Valera, Maria J. Merino, Angela M. Amato, Sabina Signoretti, et al. (2009) LDH-A inhibition, a therapeutic strategy for treatment of hereditary leiomyomatosis and renal cell cancer (HLRCC). Mol Cancer Ther 8(3): 626-635.
- Jennifer S Isaacs, Yun Jin Jung, David R Mole, Sunmin Lee, Carlos Torres-Cabala, et al. (2005) HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell 8(2): 143-153.
- Liang Zheng, Zi-Ran Zhu, Tal Sneh, Wei-Tuo Zhang, Zao-Yu Wang, et al. (2023) Circulating succinate-modifying metabolites accurately classify and reflect the status of fumarate hydratase-deficient renal cell carcinoma. J Clin Invest 133(11): e165028.
- Divya Bezwada, James Brugarolas (2023) Reporting on FH-deficient renal cell carcinoma using circulating succinylated metabolites. J Clin Invest 133(11): e170195.
- Sigrun Langbein, Wilma M Frederiks, Axel zur Hausen, Juljane Popa, Jan Lehmann, et al. (2008) Metastasis is promoted by a bioenergetic switch: new targets for progressive renal cell cancer. Int J Cancer 122(11): 2422-2428.
- Rui Hua Xu, Helene Pelicano, Yan Zhou, Jennifer S Carew, Li Feng, et al. (2005) Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res 65(2): 613-621.
- D J Spitz, V Reddy, S M Selvaggi, L Kluskens, L Green, et al. (2000) Fine-needle aspiration of scalp lesions. Diagn Cytopathol 23(1): 35-38.
- Krushna C Patra, Nissim Hay (2014) The pentose phosphate pathway and cancer. Trends Biochem Sci 39(8): 347-354.
- Qiao Zhang, Qiaoqiao Han, Zhe Yang, Yueli Ni, Yannick Luther Agbana, et al. (2020) G6PD facilitates clear cell renal cell carcinoma invasion by enhancing MMP2 expression through ROS‑MAPK axis pathway. Int J Oncol 57(1): 197-212.
- Qiao Zhang, Yueli Ni, Shujie Wang, Yannick Luther Agbana, Qiaoqiao Han, et al. (2022) G6PD upregulates Cyclin E1 and MMP9 to promote clear cell renal cell carcinoma progression. Int J Med Sci 19(1): 47-64.
- Athina N Zira, Stamatios E Theocharis, Dionisios Mitropoulos, Vasilios Migdalis, Emmanuel Mikros (2010) (1)H NMR metabonomic analysis in renal cell carcinoma: a possible diagnostic tool. J Proteome Res9(8): 4038-4044.
- Yeon-Kyung Choi, Keun-Gyu Park (2018) Targeting Glutamine Metabolism for Cancer Treatment. Biomol Ther (Seoul) 26(1): 19-28.
- Ahmad A Cluntun, Michael J Lukey, Richard A Cerione, Jason W Locasale (2017) Glutamine Metabolism in Cancer: Understanding the Heterogeneity. Trends Cancer 3(3): 169-180.
- Christian M. Metallo, Paulo A. Gameiro, Eric L. Bell, Katherine R. Mattaini, Juanjuan Yang, et al. (2011) Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 481(7381): 380-384.
- Maximilian Diehn, Robert W Cho, Neethan A Lobo, Tomer Kalisky, Mary Jo Dorie, et al. (2009) Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 458(7239): 780-783.
- Paulo A Gameiro, Juanjuan Yang, Ana M Metelo, Rocio Pérez-Carro, Rania Baker, et al. (2013) In vivo HIF-mediated reductive carboxylation is regulated by citrate levels and sensitizes VHL-deficient cells to glutamine deprivation. Cell Metab 17(3): 372-385.
- Meric Bernstam F, Tannir NMHJJ (2016) B MK Phase 1 study of CB-839, a small molecule inhibitor of glutaminase (GLS), alone and in combination with everolimus (E) in patients (pts) with renal cell cancer (RCC). J Clin Oncol 34: 4568-4568.
- Omran Abu Aboud, Ching Hsien Chen, William Senapedis, Erkan Baloglu, Christian Argueta, et al. (2016) Dual and Specific Inhibition of NAMPT and PAK4 By KPT-9274 Decreases Kidney Cancer Growth. Mol Cancer Ther 15(9): 2119-2129.
- Hong Shiee Lai, Jenq Chang Lee, Po Huang Lee, Shan Tair Wang, Wei Jao Chen (2005) Plasma free amino acid profile in cancer patients. Semin Cancer Biol 15(4): 267-276.
- AM Hosios, VC Hecht, LV Danai, MO Johnson, JC Rathmell, et al. (2016) Amino Acids Rather than Glucose Account for the Majority of Cell Mass in Proliferating Mammalian Cells. Dev Cell 36(5): 540-549.
- JM Lacey, DW Wilmore (1990) Is glutamine a conditionally essential amino acid?. Nutrition reviews 48(8): 297-309.
- A Tizianello, G De Ferrari, G Garibotto, G Gurreri, C Robaudo (1980) Renal metabolism of amino acids and ammonia in subjects with normal renal function and in patients with chronic renal insufficiency. The Journal of clinical investigation 65(5): 1162-1173.
- GT Nagami (1989) Ammonia production and secretion by the proximal tubule, American journal of kidney diseases: the official journal of the National Kidney Foundation 14(4): 258-261.
- TC Welbourne (1990) Glucocorticoid control of ammonia genesis in the proximal tubule. Seminars in nephrology 10(4): 339-349.
- A Kubota, MM Meguid, DC Hitch (1992) Amino acid profiles correlate diagnostically with organ site in three kinds of malignant tumors. Cancer 69(9): 2343-2348.
- TC Welbourne, PD Dass (1988) Gamma glutamyl transferase contribution to renal ammonia genesis in vivo. Pflueger’s Archiv: European journal of physiology 411(5): 573-578.
- ML Halperin, JH Ethier, KS Kamel (1990) The excretion of ammonium ions and acid base balance, Clinical biochemistry 23(3): 185-188.
- C Chino Poulos, TN Seyfried (2018) Mitochondrial Substrate-Level Phosphorylation as Energy Source for Glioblastoma: Review and Hypothesis. ASN Neuro 10: 1759091418818261.
- RA Casero Jr, LJ Marton (2007) Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov 6(5): 373-390.
- DJ Kim, E Roh, MH Lee, N Oi, DY Lim, et al. (2016) Herbacetin Is a Novel Allosteric Inhibitor of Ornithine Decarboxylase with Antitumor Activity. Cancer Res 76(5): 1146-1157.
- TR Murray-Stewart, PM Woster, RA Casero Jr (2016) Targeting polyamine metabolism for cancer therapy and prevention. Biochem J 473(19): 2937-2953.
- E Gerlo, R Van Coster, W Lissens, G Winckelmans, L De Meirleir, et al. (2006) Gas chromatographic-mass spectrometric analysis of N-acetylated amino acids: the first case of aminoacylase I deficiency. Analytica chimica acta 571(2): 191-199.
- U Lichter-Konecki, CM Hipke, DS Konecki (1999) Human phenylalanine hydroxylase gene expression in kidney and other nonhepatic tissues. Molecular genetics and metabolism 67(4): 308-316.
- P Tessari, G Deferrari, C Robaudo, M Vettore, N Pastorino, et al. (1999) Phenylalanine hydroxylation across the kidney in humans’ rapid communication. Kidney international 56(6): 2168-2172.
- N Moller, S Meek, M Bigelow, J Andrews, KS Nair (2000) The kidney is an important site for in vivo phenylalanine-to-tyrosine conversion in adult humans: A metabolic role of the kidney. Proceedings of the National Academy of Sciences of the United States of America 97(3): 1242-1246.
- G Garibotto, P Tessari, D Verzola, L Dertenois (2002) The metabolic conversion of phenylalanine into tyrosine in the human kidney: does it have nutritional implications in renal patients?. Journal of renal nutrition: the official journal of the Council on Renal Nutrition of the National Kidney Foundation 12(1): 8-16.
- GS Palapattu, B Kristo, J Rajfer (2002) Paraneoplastic syndromes in urologic malignancy: the many faces of renal cell carcinoma. Rev Urol 4(4): 163-170.
- T Shimizu, S Nomiyama, F Hirata, O Hayaishi (1978) Indoleamine 2,3-dioxygenase. Purification and some properties. J Biol Chem 253(13): 4700-4706.
- JF Trott, J Kim, O Abu Aboud, H Wettersten, B Stewart, et al. (2016) inhibiting tryptophan metabolism enhances interferon therapy in kidney cancer. Oncotarget 7(41): 66540-66557.
- A Tizianello, G Deferrari, G Garibotto, C Robaudo, G Salvidio,et al. (1985) Renal ammoniagenesis in the postprandial period. Contributions to nephrology 47: 44-57.
- RF Pitts, AC Damian, MB MacLeod (1970) Synthesis of serine by rat kidney in vivo and in vitro. The American journal of physiology 219(3): 584-589.
- RF Pitts, MB MacLeod (1972) Synthesis of serine by the dog kidney in vivo. The American journal of physiology 222(2): 394-398.
- M Nogueira, HL Kim (2008) Molecular markers for predicting prognosis of renal cell carcinoma. Urologic oncology 26(2): 113-124.
- SM Sanins, JA Timbrell, C Elcombe, JK Nicholson (1992) Proton NMR spectroscopic studies on the metabolism and biochemical effects of hydrazine in vivo. Archives of toxicology 66(7): 489-495.
- JM Phang, J Pandhare, Y Liu (2008) The metabolism of proline as microenvironmental stress substrate. The Journal of nutrition 138(10): 2008S-2015S.
- L Cynober, J Le Boucher, MP Vasson (1995) Arginine metabolism in mammals, The Journal of Nutritional Biochemistry. 6(8): 402-413.
- F Morel, A Hus-Citharel, O Levillain (1996) Biochemical heterogeneity of arginine metabolism along kidney proximal tubules. Kidney international 49(6): 1608-1610.
- SN Dhanakoti, JT Brosnan, GR Herzberg, ME Brosnan (1990) Renal arginine synthesis: studies in vitro and in vivo. The American journal of physiology 259(3 Pt 1): E437-442.
- D Promeneur, G Rousselet, L Bankir, P Bailly, JP Cartron, et al. (1996) Evidence for distinct vascular and tubular urea transporters in the rat kidney. Journal of the American Society of Nephrology: JASN 7(6): 852-860.
- GO Perez, M Epstein, B Rietberg, R Loutzenhiser (1978) Metabolism of arginine by the isolated perfused rat kidney. The American journal of physiology 235(4): F376-380.
- S Rabinovich, L Adler, K Yizhak, A Sarver, A Silberman, et al. (2015) Diversion of aspartate in ASS1-deficient tumors fosters de novo pyrimidine synthesis. Nature 527(7578): 379-383.
- CY Yoon, YJ Shim, EH Kim, JH Lee, NH Won, et al. (2007) Renal cell carcinoma does not express argininosuccinate synthetase and is highly sensitive to arginine deprivation via arginine deiminase. Int J Cancer 120(4): 897-905.
- S Khare, LC Kim, G Lobel, PT Doulias, H Ischiropoulos, et al. (2021) ASS1 and ASL suppress growth in clear cell renal cell carcinoma via altered nitrogen metabolism. Cancer Metab 9(1): 40.
- MM Phillips, MT Sheaff, PW Szlosarek (2013) Targeting arginine-dependent cancers with arginine-degrading enzymes: opportunities and challenges. Cancer Res Treat 45(4): 251-262.
- G Ogimoto, SOzawa, T Maeba, S Owada, M Ishida (1992) 31P-NMR study for the energy metabolism of skeletal muscle in chronic haemodialysis patients. Guanidino Compounds in Biology and Medicine 281-285.
- M Kuroda (1993) Study on impaired metabolism of guanidinoacetic acid in chronic renal failure rabbits with special reference to impaired conversion of arginine to guanidinoacetic acid. Nephron 65(4): 605-611.
- E Austruy, M Cohen Salmon, C Antignac, C Beroud, et al. (1993) Isolation of kidney complementary DNAs down-expressed in Wilms' tumor by a subtractive hybridization approach. Cancer research 53(12): 2888-2894.
- M Takeda, H Koide, KY Jung, H Endou (1992) Intranephron distribution of glycine-amidinotransferase activity in rats. Renal physiology and biochemistry 15(3-4): 113-118.
- M Takeda, S Nakayama, Y Tomino, K Jung, H Endou,et al. (1992) Heterogeneity of transamidinase activity and creatine content along the rat nephron. Guanidino compounds in biology and medicine pp. 153-157.
- Y Kanai, H Segawa, K Miyamoto, H Uchino, E Takeda, et al. (1998) Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98). The Journal of biological chemistry 273(37): 23629-23632.
- O Yanagida, Y Kanai, A Chairoungdua, DK Kim, H Segawa, et al. (2001) Human L-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochimica et biophysica acta 1514(2): 291-302.
- HN Christensen (1990) Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev 70(1): 43-77.
- JD McGivan, M Pastor Anglada (1994) Regulatory and molecular aspects of mammalian amino acid transport. The Biochemical journal 299(Pt 2): 321-334.
- Nawashiro, N. Otani, N. Shinomiya, S. Fukui, H. Ooigawa, et al. (2006) L-type amino acid transporter 1 as a potential molecular target in human astrocytic tumors. international journal of cancer 119(3): 484-492.
- K Kaira, N Oriuchi, H Imai, K Shimizu, N Yanagitani, et al. (2008) Prognostic significance of L-type amino acid transporter 1 expression in resectable stage I-III nonsmall cell lung cancer. British journal of cancer 98(4): 742-748.
- K Nakanishi, S Ogata, H Matsuo, Y Kanai, H Endou, et al. (2007) Expression of LAT1 predicts risk of progression of transitional cell carcinoma of the upper urinary tract. Virchows Archiv : an international journal of pathology 451(3): 681-690.
- T Sakata, G Ferdous, T Tsuruta, T Satoh, S Baba, et al. (2009) L-type amino-acid transporter 1 as a novel biomarker for high-grade malignancy in prostate cancer. Pathology international 59(1): 7-18.
- H Betsunoh, T Fukuda, N Anzai, D Nishihara, T Mizuno, et al. (2013) Increased expression of system large amino acid transporter (LAT)-1 mRNA is associated with invasive potential and unfavorable prognosis of human clear cell renal cell carcinoma. BMC cancer 13: 509.
- X Liu, M Zhang, X Cheng, X Liu, H Sun, et al. (2020) LC-MS-Based Plasma Metabolomics and Lipidomics Analyses for Differential Diagnosis of Bladder Cancer and Renal Cell Carcinoma. Front Oncol 10: 717.
- T Mashima, H Seimiya, T Tsuruo (2009) De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. British journal of cancer 100(9): 1369-1372.
- B Qiu, D Ackerman, DJ Sanchez, B Li, JD Ochocki, et al. (2015) HIF2alpha-Dependent Lipid Storage Promotes Endoplasmic Reticulum Homeostasis in Clear-Cell Renal Cell Carcinoma. Cancer Discov 5(6): 652-667.
- J Sim, RS Johnson (2015) Through a Clear Cell, darkly: HIF2alpha/PLIN2-Maintained Fat Droplets Protect ccRCCs from ER Stress. Cancer Discov 5(6): 584-585.
- Y Liu (2006) Fatty acid oxidation is a dominant bioenergetic pathway in prostate cancer. Prostate cancer and prostatic diseases 9(3): 230-234.
- S Zha, S Ferdinandusse, JL Hicks, S Denis, TA Dunn, et al. (2005) Peroxisomal branched chain fatty acid beta-oxidation pathway is upregulated in prostate cancer. The Prostate 63(4): 316-323.
- H Jiang, H Chen, P Wan, N Chen (2021) Decreased expression of HADH is related to poor prognosis and immune infiltration in kidney renal clear cell carcinoma. Genomics 113(6): 3556-3564.
- B Seliger, R Lichtenfels, D Atkins, J Bukur, T Halder, et al. (2005) Identification of fatty acid binding proteins as markers associated with the initiation and/or progression of renal cell carcinoma. Proteomics 5(10): 2631-2640.
- T Teratani, T Domoto, K Kuriki, T Kageyama, T Takayama, et al. (2007) Detection of transcript for brain-type fatty Acid-binding protein in tumor and urine of patients with renal cell carcinoma. Urology 69(2): 236-240.
- A Horiguchi, T Asano, T Asano, K Ito, M Sumitomo, et al. (2008) Fatty acid synthase over expression is an indicator of tumor aggressiveness and poor prognosis in renal cell carcinoma. J Urol 180(3): 1137-1140.
- JH Pinthus, KF Whelan, D Gallino, JP Lu, N Rothschild (2011) Metabolic features of clear-cell renal cell carcinoma: mechanisms and clinical implications. Can Urol Assoc J 5(4): 274-282.
- JC van der Mijn, L Fu, F Khani, T Zhang, AM Molina, et al. (2020) Combined Metabolomics and Genome-Wide Transcriptomics Analyses Show Multiple HIF1alpha-Induced Changes in Lipid Metabolism in Early-Stage Clear Cell Renal Cell Carcinoma. Transl Oncol 13(2): 177-185.
- H Yang, H Zhao, Z Ren, X Yi, Q Zhang, et al. (2022) Overexpression CPT1A reduces lipid accumulation via PPARalpha/CD36 axis to suppress the cell proliferation in ccRCC. Acta Biochim Biophys Sin (Shanghai) 54(2): 220-231.
- X Ding, W Zhang, S Li, H Yang (2019) The role of cholesterol metabolism in cancer. Am J Cancer Res 9(2): 219-227.
- M Rudling, VP Collins (1996) Low density lipoprotein receptor and 3-hydroxy-3-methylglutaryl coenzyme A reductase mRNA levels are coordinately reduced in human renal cell carcinoma. Biochimica et biophysica acta 1299(1): 75-79.
- AR Tate, PJ Foxall, E Holmes, D Moka, M Spraul, et al. (2000) Distinction between normal and renal cell carcinoma kidney cortical biopsy samples using pattern recognition of 1H magic angle spinning (MAS) NMR spectra. NMR in Biomedicine 13(2): 64-71.
- MR Tosi, V Tugnoli (2005) Cholesteryl esters in malignancy. Clinica chimica acta; international journal of clinical chemistry 359(1-2): 27-45.
- HW Tun, LA Marlow, CA von Roemeling, SJ Cooper, P Kreinest, et al. (2010) Pathway signature and cellular differentiation in clear cell renal cell carcinoma. PloS one 5(5): e10696.
- SJ Kridel, WT Lowther, CWt Pemble (2007) Fatty acid synthase inhibitors: new directions for oncology. Expert opinion on investigational drugs 16(11): 1817-1829.
- PL Alo, P Visca, G Trombetta, A Mangoni, L Lenti, et al. (1999) Fatty acid synthase (FAS) predictive strength in poorly differentiated early breast carcinomas. Tumori 85(1): 35-40.
- S Tadros, SK Shukla, RJ King, V Gunda, E Vernucci, et al. (2017) De Novo Lipid Synthesis Facilitates Gemcitabine Resistance through Endoplasmic Reticulum Stress in Pancreatic Cancer. Cancer Res 77(20): 5503-5517.
- P Visca, V Sebastiani, C Botti, MG Diodoro, RP Lasagni, et al. (2004) Fatty acid synthase (FAS) is a marker of increased risk of recurrence in lung carcinoma. Anticancer Res 24(6): 4169-4173.
- US Shah, R Dhir, SM Gollin, UR Chandran, D Lewis, et al. (2006) Fatty acid synthase gene overexpression and copy number gain in prostate adenocarcinoma. Hum Pathol 37(4): 401-409.
- RL Gebhard, RV Clayman, WF Prigge, R Figenshau, NA Staley, et al. (1987) Abnormal cholesterol metabolism in renal clear cell carcinoma. Journal of lipid research 28(10): 1177-1184.
- K Matsumoto, Y Fujiwara, R Nagai, M Yoshida, S Ueda (2008) Expression of two isozymes of acyl-coenzyme A: cholesterol acyltransferase-1 and -2 in clear cell type renal cell carcinoma. international journal of urology: official journal of the Japanese Urological Association 15(2): 166-170.
- S Ganti, SL Taylor, O Abu Aboud, J Yang, C Evans, et al. (2012) Kidney tumor biomarkers revealed by simultaneous multiple matrix metabolomics analysis. Cancer Res 72(14): 3471-3479.
- O Abu Aboud, HI Wettersten, RH Weiss (2013) Inhibition of PPARalpha induces cell cycle arrest and apoptosis, and synergizes with glycolysis inhibition in kidney cancer cells. PLoS One 8(8): e71115.
- A Horiguchi, T Asano, T Asano, K Ito, M Sumitomo, et al. (2008) Pharmacological inhibitor of fatty acid synthase suppresses growth and invasiveness of renal cancer cells. J Urol 180(2): 729-736.
- ES Pizer, J Thupari, WF Han, ML Pinn, FJ Chrest, et al. (2000) Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced by fatty-acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res 60(2): 213-218.
- K Glunde, MA Jacobs, ZM Bhujwalla (2006) Choline metabolism in cancer: implications for diagnosis and therapy. Expert review of molecular diagnostics 6(6): 821-829.
- C Kent, GM Carman (1999) Interactions among pathways for phosphatidylcholine metabolism, CTP synthesis and secretion through the Golgi apparatus. Trends in biochemical sciences 24(4): 146-150.
- PC Sizeland, ST Chambers, M Lever, LM Bason, RA Robson (1993) Organic osmolytes in human and other mammalian kidneys. Kidney international 43(2): 448-453.
- MB Burg (1995) Molecular basis of osmotic regulation. The American journal of physiology 268(6 Pt 2): F983-996.
- WK Lee, RN Kolesnick (2017) Sphingolipid abnormalities in cancer multidrug resistance: Chicken or egg?. Cell Signal 38: 134-145.
- H Zeng, H Li, Z Shi (2000) [Infusion chemotherapy and chemoembolization of liver metastases from cancer of the alimentary tract]. Zhonghua zhong liu za zhi [Chinese journal of oncology] 22(5): 422-424.
- E Schaeffeler, F Buttner, A Reustle, V Klumpp, S Winter, et al. (2019) Metabolic and Lipidomic Reprogramming in Renal Cell Carcinoma Subtypes Reflects Regions of Tumor Origin. Eur Urol Focus 5(4): 608-618.
- S Beloribi Djefaflia, S Vasseur, F Guillaumond (2016) Lipid metabolic reprogramming in cancer cells. Oncogenesis 5(1): e189.
- B Ogretmen (2018) Sphingolipid metabolism in cancer signalling and therapy. Nat Rev Cancer 18(1): 33-50.
- S Vitols, K Soderberg Reid, M Masquelier, B Sjostrom, C Peterson (1990) Low density lipoprotein for delivery of a water-insoluble alkylating agent to malignant cells. In vitro and in vivo studies of a drug-lipoprotein complex. British journal of cancer 62(5): 724-729.
- AM Fiorenza, A Branchi, D Sommariva (2000) Serum lipoprotein profile in patients with cancer. A comparison with non-cancer subjects. international journal of clinical & laboratory research 30(3): 141-145.
- JL Goldstein, MS Brown (1990) Regulation of the mevalonate pathway. Nature 343(6257): 425-430.
- PB Calderon, J Cadrobbi, C Marques, N Hong Ngoc, JM Jamison, et al. (2002) Potential therapeutic application of the association of vitamins C and K3 in cancer treatment. Curr Med Chem 9(24): 2271-2285.
- L Jia, Q Jia, Y Shang, X Dong, L Li (2015) Vitamin C intake and risk of renal cell carcinoma: a meta-analysis. Sci Rep 5: 17921.
- AJ Ham, DC Liebler (1997) Antioxidant reactions of vitamin E in the perfused rat liver: product distribution and effect of dietary vitamin E supplementation. Archives of biochemistry and biophysics 339(1): 157-164.
- A Azzi, R Gysin, P Kempna, A Munteanu, Y Negis, et al. (2004) Vitamin E mediates cell signaling and regulation of gene expression. Annals of the New York Academy of Sciences 1031: 86-95.
- CS Yang, P Luo, Z Zeng, H Wang, M Malafa, et al. (2020) Vitamin E and cancer prevention: Studies with different forms of tocopherols and tocotrienols. Mol Carcinog 59(4): 365-389.
- MR Tosi, MT Rodriguez Estrada, G Lercker, A Poerio, A Trinchero, et al. (2004) Magnetic resonance spectroscopy and chromatographic methods identify altered lipid composition in human renal neoplasms. international journal of molecular medicine 14(1): 93-100.
- P Mardones, A Rigotti (2004) Cellular mechanisms of vitamin E uptake: relevance in alpha-tocopherol metabolism and potential implications for disease. J Nutr Biochem 15(5): 252-260.
- KK Nicodemus, C Sweeney, AR Folsom (2004) Evaluation of dietary, medical and lifestyle risk factors for incident kidney cancer in postmenopausal women. Int J Cancer 108(1): 115-121.
- J Hu, Y Mao, K White (2003) G. Canadian Cancer Registries Epidemiology Research, Diet and vitamin or mineral supplements and risk of renal cell carcinoma in Canada. Cancer Causes Control 14(8): 705-714.
- C Bosetti, L Scotti, LD Maso, R Talamini, M Montella, et al. (2007) Micronutrients and the risk of renal cell cancer: a case-control study from Italy. Int J Cancer 120(4): 892-896.
- CH Bock, JJ Ruterbusch, AN Holowatyj, SE Steck, AL Van Dyke, et al. (2018) Renal cell carcinoma risk associated with lower intake of micronutrients. Cancer Med 7(8): 4087-4097.
- RJ Prineas, AR Folsom, ZM Zhang, TA Sellers, J Potter (1997) Nutrition and other risk factors for renal cell carcinoma in postmenopausal women. Epidemiology 8(1): 31-36.
- JE Lee, E Giovannucci, SA Smith Warner, D Spiegelman, WC Willett, et al. (2006) Intakes of fruits, vegetables, vitamins A, C, and E, and carotenoids and risk of renal cell cancer. Cancer Epidemiol Biomarkers Prev 15(12): 2445-2452.
- M Bertoia, D Albanes, ST Mayne, S Mannisto, J Virtamo, et al. (2010) No association between fruit, vegetables, antioxidant nutrients and risk of renal cell carcinoma. Int J Cancer 126(6): 1504-1512.
- M Johansson, A Fanidi, DC Muller, JK Bassett, O Midttun, SE Vollset, et al. (2014) Circulating biomarkers of one-carbon metabolism in relation to renal cell carcinoma incidence and survival. J Natl Cancer Inst 106(12): dju327.
- S Karami, P Brennan, M Navratilova, D Mates, D Zaridze, et al. (2010) Vitamin d pathway genes, diet, and risk of renal cell carcinoma. Int J Endocrinol 2010: 879362.
- S Xu, ZH Zhang, L Fu, J Song, DD Xie, et al. (2020) Calcitriol inhibits migration and invasion of renal cell carcinoma cells by suppressing Smad2/3-, STAT3- and beta-catenin-mediated epithelial-mesenchymal transition. Cancer Sci 111(1): 59-71.
- B Mao, Y Li, Z Zhang, C Chen, Y Chen, et al. (2015) One-Carbon Metabolic Factors and Risk of Renal Cell Cancer: A Meta-Analysis. PloS one 10(10): e0141762.
- MA Beydoun, MR Shroff, HA Beydoun, AB Zonderman (2010) Serum folate vitamin B-12, and homocysteine and their association with depressive symptoms among U.S. adults. Psychosom Med 72(9): 862-873.
- N Fristrup, F Donskov (2019) Folic Acid Reduces Mucositis in Metastatic Renal Cell Carcinoma Patients: A Retrospective Study. Clin Genitourin Cancer 17(4): 254-259.
- DC Muller, M Johansson, D Zaridze, A Moukeria, V Janout, et al. (2015) Circulating Concentrations of Vitamin B6 and Kidney Cancer Prognosis: A Prospective Case-Cohort Study. PLoS One 10(10): e0140677.
- J Lin, NR Cook, C Albert, E Zaharris, JM Gaziano, et al. (2009) Vitamins C and E and beta carotene supplementation and cancer risk: a randomized controlled trial. J Natl Cancer Inst 101(1): 14-23.
- G Alfthan, MS Laurinen, LM Valsta, T Pastinen, A Aro (2003) Folate intake, plasma folate and homocysteine status in a random Finnish population. European journal of clinical nutrition 57(1): 81-88.
- A Brevik, SE Vollset, GS Tell, H Refsum, PM Ueland, et al. (2005) Plasma concentration of folate as a biomarker for the intake of fruit and vegetables: the Hordaland Homocysteine Study. The American journal of clinical nutrition 81(2): 434-439.
- AM Czarnecka, M Niedzwiedzka, C Porta, C Szczylik (2016) Hormone signaling pathways as treatment targets in renal cell cancer (Review). Int J Oncol 48(6): 2221-2235.
- U Dunzendorfer, D Drahovsky, H Schmidt Gayk (1981) [Peptide hormones LH, FSH, TSH, prolactin, beta-HCG and PTH in patients with urogenital tumors]. Onkologie 4(4): 188-192.
- K Melkersson, K Berinder, AL Hulting (2011) Effect of antipsychotic-induced hyperprolactinemia on anthropometric measures, insulin sensitivity and lipid profile in patients with schizophrenia or related psychoses. Neuro Endocrinol Lett 32(4): 428-436.
- G Keller, AV Schally, T Gaiser, A Nagy, B Baker, et al. (2005) Receptors for luteinizing hormone releasing hormone expressed on human renal cell carcinomas can be used for targeted chemotherapy with cytotoxic luteinizing hormone releasing hormone analogues. Clin Cancer Res 11(15): 5549-5557.
- J Engel, G Emons, J Pinski, AV Schally (2012) AEZS-108: a targeted cytotoxic analog of LHRH for the treatment of cancers positive for LHRH receptors. Expert Opin Investig Drugs 21(6): 891-899.
- A Jungwirth, AV Schally, G Halmos, K Groot, K Szepeshazi, et al. (1998) Inhibition of the growth of Caki-I human renal adenocarcinoma in vivo by luteinizing hormone-releasing hormone antagonist Cetrorelix, somatostatin analog RC-160, and bombesin antagonist RC-3940-II. Cancer 82: 909-917.
- A Siraj, V Desestret, M Antoine, G Fromont, M Huerre, et al. (2013) Expression of follicle-stimulating hormone receptor by the vascular endothelium in tumor metastases. BMC Cancer 13: 246.
- A Radu, C Pichon, P Camparo, M Antoine, Y Allory, et al. (2010) Expression of follicle-stimulating hormone receptor in tumor blood vessels. N Engl J Med 363(17): 1621-1630.
- BA Gartrell, CK Tsao, MD Galsky (2013) The follicle-stimulating hormone receptor: a novel target in genitourinary malignancies. Urol Oncol 31(8): 1403-1407.
- L Sun, Z Gao, L Luo, H Tan, G Zhang (2018) Estrogen affects cell growth and IGF-1 receptor expression in renal cell carcinoma. Onco Targets Ther 11: 5873-5878.
- HEM El Deek, AM Ahmed, TS Hassan, AM Morsy (2018) Expression and localization of estrogen receptors in human renal cell carcinoma and their clinical significance.Int J Clin Exp Pathol 11(6): 3176-3185.
- E Yakirevich, A Matoso, E Sabo, LJ Wang, R Tavares, et al. (2011) Expression of the glucocorticoid receptor in renal cell neoplasms: an immunohistochemical and quantitative reverse transcriptase polymerase chain reaction study. Hum Pathol 42(11): 1684-1692.
- L Chen, FR Weiss, S Chaichik, I Keydar (1980) Steroid receptors in human renal carcinoma. Isr J Med Sci 16: 756-760.
- G Jakse, E Muller Holzner (1988) Hormone receptors in renal cancer: an overview. Semin Surg Oncol 4(3): 161-164.
- R Weinberger, B Appel, A Stein, Y Metz, A Neheman, et al. (2007) The pyruvate kinase isoenzyme M2 (Tu M2-PK) as a tumour marker for renal cell carcinoma. Eur J Cancer Care (Engl) 16(4): 333-337.
- J Huang, X Zhao, X Li, J Peng, W Yang, et al. (2021) HMGCR inhibition stabilizes the glycolytic enzyme PKM2 to support the growth of renal cell carcinoma. PLoS Biol 19(4): e3001197.
- F Coperchini, P Leporati, M Rotondi, L Chiovato (2015) Expanding the therapeutic spectrum of metformin: from diabetes to cancer. J Endocrinol Invest 38(10): 1047-1055.
- M Wei, S Mao, G Lu, L Li, X Lan, et al. (2018) Valproic acid sensitizes metformin-resistant human renal cell carcinoma cells by upregulating H3 acetylation and EMT reversal. BMC Cancer 18(1): 434.
- O Fiala, P Ostasov, A Rozsypalova, M Hora, O Sorejs, et al. (2021) Metformin Use and the Outcome of Metastatic Renal Cell Carcinoma Treated with Sunitinib or Pazopanib. Cancer Manag Res 13: 4077-4086.
- A Song, C Zhang, X Meng (2021) Mechanism and application of metformin in kidney diseases: An update. Biomed Pharmacother 138:111454.
- MJ Goldman, B Craft, M Hastie, K Repecka, F McDade, et al. (2020) Visualizing and interpreting cancer genomics data via the Xena platform. Nat Biotechnol 38(6): 675-678.
- J Liu, M Li, B Song, C Jia, L Zhang, et al. (2013) Metformin inhibits renal cell carcinoma in vitro and in vivo Urol Oncol 31(2): 264-270.
- L Hamieh, RR McKay, X Lin, RB Moreira, R Simantov, et al. (2017) Effect of Metformin Use on Survival Outcomes in Patients with Metastatic Renal Cell Carcinoma. Clin Genitourin Cancer 15(2): 221-229.
- WK Miskimins, HJ Ahn, JY Kim, S Ryu, YS Jung, et al. (2014) Synergistic anti-cancer effect of phenformin and oxamate. PLoS One 9(1): e85576.
- MJ Lukey, KF Wilson, RA Cerione (2013) Therapeutic strategies impacting cancer cell glutamine metabolism. Future Med Chem 5(14): 1685-1700.
- B DeLaBarre, S Gross, C Fang, Y Gao, A Jha, et al. (2011) Full-length human glutaminase in complex with an allosteric inhibitor. Biochemistry 50(50): 10764-10770.
- R Sharma, E Kadife, M Myers, G Kannourakis, P Prithviraj, et al. (2021) Determinants of resistance to VEGF-TKI and immune checkpoint inhibitors in metastatic renal cell carcinoma. J Exp Clin Cancer Res 40(1): 186.
- A Kapoor (2016) First-line treatment options in metastatic renal cell cancer. Can Urol Assoc J 10: S236-S238.
- T Powles (2021) Recent eUpdate to the ESMO Clinical Practice Guidelines on renal cell carcinoma on cabozantinib and nivolumab for first-line clear cell renal cancer: Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 32(3): 422-423.
- M Gigante, P Pontrelli, W Herr, M Gigante, M D'Avenia ,et al. (2016) miR-29b and miR-198 overexpression in CD8+ T cells of renal cell carcinoma patients down-modulates JAK3 and MCL-1 leading to immune dysfunction. J Transl Med 14: 84.
- GS Netti, G Lucarelli, F Spadaccino, G Castellano, M Gigante, et al. (2020) PTX3 modulates the immunoflogosis in tumor microenvironment and is a prognostic factor for patients with clear cell renal cell carcinoma, Aging (Albany NY). 12(8): 7585-7602.
- S De Marco, B Torsello, E Minutiello, I Morabito, C Grasselli, et al. (2023) The cross-talk between Abl2 tyrosine kinase and TGFbeta1 signalling modulates the invasion of clear cell Renal Cell Carcinoma cells. FEBS Lett 597(8): 1098-1113.
- JX Xu, VE Maher, L Zhang, S Tang, R Sridhara, et al. (2017) FDA Approval Summary: Nivolumab in Advanced Renal Cell Carcinoma After Anti-Angiogenic Therapy and Exploratory Predictive Biomarker Analysis, Oncologist 22(3): 311-317.
- RJ Motzer, NM Tannir, DF McDermott, O Aren Frontera, B Melichar, et al. (2018) Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med 378(14): 1277-1290.
- RJ Motzer, K Penkov, J Haanen, B Rini, L Albiges, et al. (2019) Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 380(12): 1103-1115.
- RJ Motzer, BI Rini, DF McDermott, O Aren Frontera, HJ Hammers, et al. (2019) Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol 20(10): 1370-1385.
- T Powles, ER Plimack, D Soulieres, T Waddell, V Stus, et al. (2020) Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol 21(12): 1563-1573.
- J Huang, X Yang, X Peng, W Huang (2017) Inhibiting prenylation augments chemotherapy efficacy in renal cell carcinoma through dual inhibition on mitochondrial respiration and glycolysis. Biochem Biophys Res Commun 493(2): 921-927.
- D Chen, X Sun, X Zhang, J Cao (2018) Targeting mitochondria by anthelmintic drug atovaquone sensitizes renal cell carcinoma to chemotherapy and immunotherapy. J Biochem Mol Toxicol 32(9): e22195.
- M Zhu, Y Li, Z Zhou (2017) Antibiotic ivermectin preferentially targets renal cancer through inducing mitochondrial dysfunction and oxidative damage. Biochem Biophys Res Commun 492(3): 373-378.
- RJL Funda Meric Bernstam, Bradley Curtis Carthon, Othon Iliopoulos, James Walter Mier, Manish R Patel, et al. (2019) CB-839, a glutaminase inhibitor, in combination with cabozantinib in patients with clear cell and papillary metastatic renal cell cancer (mRCC): Results of a phase I study. Journal of Clinical Oncology 37(7): 549-549.
- BI Rini, ER Plimack, V Stus, R Gafanov, R Hawkins, et al. (2019) Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 380(12): 1116-1127.
- JB Lee, HS Park, S Park, HJ Lee, KA Kwon, et al. (2019) Temsirolimus in Asian Metastatic/Recurrent Non-clear Cell Renal Carcinoma. Cancer Res Treat 51(4): 1578-1588.
- R Motzer, B Alekseev, SY Rha, C Porta, M Eto, et al. (2021) Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med 384(14): 1289-1300.
- SK Pal, B McGregor, C Suarez, CK Tsao, W Kelly, et al. (2021) Cabozantinib in Combination with Atezolizumab for Advanced Renal Cell Carcinoma: Results From the COSMIC-021 Study. J Clin Oncol 39(33): 3725-3736.
- AS Belldegrun, K Chamie, P Kloepfer, B Fall, P Bevan, et al. (2013) ARISER: A randomized double blind phase III study to evaluate adjuvant cG250 treatment versus placebo in patients with high-risk ccRCC-Results and implications for adjuvant clinical trials. Journal of Clinical Oncology 31(15): 4507-4507.
- TK Choueiri, T Powles, M Burotto, B Escudier, MT Bourlon, et al. (2021) Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 384(9): 829-841.
- V Grunwald, B Hadaschik (2019) Re: Preliminary Results for Avelumab Plus Axitinib as First-Line Therapy in Patients with Advanced Clear-cell Renal-cell Carcinoma (JAVELIN Renal 100): An Open-label, Dose-finding and Dose-expansion, Phase 1b Trial. Eur Urol 75(4): 697-698.
- B Escudier, A Pluzanska, P Koralewski, A Ravaud, S Bracarda, et al. (2007) Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 370(9605): 2103-2111.
- BI Rini, SK Pal, BJ Escudier, MB Atkins, TE Hutson, et al. (2020) Tivozanib versus sorafenib in patients with advanced renal cell carcinoma (TIVO-3): a phase 3, multicentre, randomised, controlled, open-label study. Lancet Oncol 21(1): 95-104.
- CN Sternberg, RE Hawkins, J Wagstaff, P Salman, J Mardiak, et al. (2013) A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: final overall survival results and safety update. Eur J Cancer 49(6): 1287-1296.
- CN Sternberg, ID Davis, J Mardiak, C Szczylik, E Lee, et al. (2010) Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 28(6): 1061-1068.
-
Neerja Trivedi and Devendra Kumar*. Physiological And Metabolic Alteration in Renal Cell Carcinoma: Importance for Therapeutic Outcome. Anat & Physiol Open Access J. 1(5): 2024. APOAJ.MS.ID.000523.
-
Frontal, Sinuses, Agenesis, Pneumatization, Prevalence, Cadavers, Embryology, Surgery, Symptoms, Sinusitis
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.