Research Article
Research Article
Cadaveric Anatomical Study and Evaluation of The Coracoid Process as A Bony Landmark for Locating the Cephalic Vein
Jonathan L Sweeney1,3*, Albert Yurvati2, Armando Rosales1, Alfred V. Abraham5, Adam Beyer4 and Rustin Reeves1
1University of North Texas Health Science Center, 3500 Camp Bowie Blvd, Fort Worth, TX 76107, Center for Anatomical Sciences
2University of North Texas Health Science Center, 3500 Camp Bowie Blvd, Fort Worth, TX 76107, Chair and Professor of the Department of Surgery
3Texas College of Osteopathic Medicine: 3500 Camp Bowie Blvd, Fort Worth, TX 76107
4Virginia Commonwealth University, Richmond VA
5Baylor University Medical Center, Dallas TX
Jonathan Sweeney, University of North Texas Health Science Center, 3500 Camp Bowie Blvd, Fort Worth, TX 76107, Center for Anatomical Sciences, USA
Received Date: March 20, 2024; Published Date: March 27, 2024
Abstract
Objective: Clinicians require accurate anatomical information while gaining central venous access. The cephalic vein’s anatomical variations can make it challenging to locate. We asked if the coracoid process could be utilized as an accurate topographical landmark to locate the cephalic vein within 10 mm.
Methods: Bilateral shoulder dissections were conducted on 41 cadavers to determine the cephalic vein’s location in relation to the center of the coracoid process. Measurements were taken from the center of the coracoid process to the center of the cephalic vein. The cephalic veins were removed from the cadaver and the diameter obtained.
Results: Means of measurements relating to the coracoid process were: Straight line distance: 9.48 ± 4.45 mm, horizontal distance: 13.50 ± 6.45 mm, vertical distance: 11.03 ± 5.17 mm, and diameter: 1.59 ± 0.67 mm. A one sample student t-test on the straight-line distance, with the expected population mean set to 10 mm had a p-value = 0.0333906 and the test statistics was -1.860547. The mean diameter of the cephalic vein was 1.59 ± 0.67 mm. The mean depth of the cephalic vein was 13.53 ± 7.68 mm.
Conclusion: We showed that the cephalic vein is be located within 10 mm of the coracoid process with statistical significance. This study shows that if the clinician does not quickly locate the cephalic vein within 10 mm of the coracoid process, they can assume it is likely a variation or absent. This will aid clinicians in avoiding unnecessary time searching for the vein and instead rapidly transition to an alternative approach. With this new information, we hope to persuade more clinicians to make the cephalic vein cut down procedure their first and primary attempt for central venous access.
Keywords:Cephalic Vein Cut Down Procedure; Subclavian Puncture; Totally Implantable Venous Port Placement; Bony Anatomical Landmark; Coracoid Process; Vessel Diameter; Cadaveric Study; Clinical Decision Making
Abbreviations: CV: Cephalic vein; TIVAP: Totally implantable venous access port; SCP: Percutaneous subclavian puncture; CVCD: Cephalic Vein Cut Down; SD: Standard deviation; SWT: Shapiro-Wilk Test; CP: Coracoid process
Introduction
The cephalic vein (CV) is understood to travel the following path. The dorsal venous network of the hand gives rise to the dorsal metacarpal veins [1]. These veins give rise to the CV which traverses the anatomical snuffbox and runs cephalad on the lateral surface of the anterior forearm [1]. When the CV reaches the cubital fossa, it communicates with the basilic vein through the median cubital vein [1]. Next, the CV continues cephalad on the lateral portion of the humeral region of the upper limb before crossing medially through the deltopectoral groove [1]. The CV will then pierce the clavipectoral fascia and terminate into the axillary vein [1].
Totally implantable venous access port (TIVAP) is surgically placed when a patient requires continuous venous access. A TIVAP is beneficial for long-term chemotherapy, radiographical contrast administration, routine blood draws and, in some cases, parenteral nutrition [2]. Currently, there are two routine methods for placing a TIVAP: direct percutaneous puncture of a central vein and open insertion via cut-down method of a peripheral vein [2]. The most chosen techniques for the direct percutaneous puncture and the open insertion are the percutaneous subclavian puncture (SCP) method and the cephalic vein cut down (CVCD) procedure, respectively.
The SCP is performed by puncturing the subclavian vein via skin incision followed by a needle insertion just inferior to the clavicle. A second incision at the level of the second intercostal space is made and a small pocket is formed in the subcutaneous tissue to house the reservoir for the TIVAP. The reservoir and the intraluminal port are connected via a tube that is passed under the skin [2]. SCP has a 12% rate of serious complications, most notably, pneumothorax, but also includes arterial puncture, hemothorax, and brachial plexus injury [3]. Cardiovascular usage of the subclavian vein for pacemaker lead placement can cause “pinch off syndrome” or “subclavian crush syndrome” where the lead is pinned between the first rib and the clavicle [4].
The CVCD is performed via surgical dissection down to the CV in the deltopectoral groove and insertion of a catheter and reservoir in the same incision. The CVCD has been shown to be a safe and feasible option for TIVAP placement, as well as having a lower complication rate when compared to the SCP [4]. This technique involves less trauma to the vessel and reduces damage to the surrounding structures [5]. The SCP has a risk estimation 1.09 times greater when compared to the CVCD [5]. Additionally, the CVCD technique did not observe the occurrence of pinch-off syndrome or subclavian crush syndrome [4].
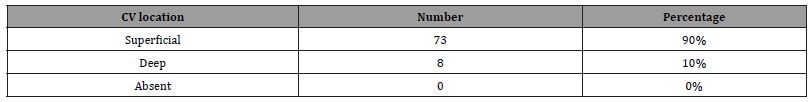
Despite the CVCD having less serious complications when compared to the SCP [6] and the CVCD being advised as the first choice for central venous access over the SCP [7] the SCP is still the standard first choice for central venous access amongst most clinicians. One challenge when conducting a CVCD is the CV documented anatomical variations. The CV is present 96% of the time and absent 4% of the time [7,1,9]. When present, the CV is found superficial to the fascia and adipose tissue of the deltopectoral groove 74% of the time and deep to the fascia, adipose and muscles of the pectoralis major and the deltoid muscles 26% of the time [7,1,9]. The CV also has a documented anatomical abnormality of a supraclavicular course occurring 2% of the time [7,1,9]. A common method employed for locating the CV is to find the deltopectoral groove topographically. The deltopectoral groove is the indentation created by the intersection of the deltoid muscle and pectoralis major muscle in the shoulder region [8]. The groove consists of a fat pad that separates the two muscles [8]. However, on initial dissection via CVCD, the CV will not be found if the anatomy follows a deep, absent, or supraclavicular physiology.
A study by Yeri, published in 2009 [10], recorded that the CV was located medial to the coracoid process (CP) at an average distance of 7.9 mm ± 6.0 mm with a range of 0-20 mm [10]. These measurements were taken from the closest edge of the CP to the closest edge of the CV. The study by Yeri presents the possibility of the CP as an accurate topographical landmark to locate the CV. However, when a clinician is preparing to conduct a CVCD on a patient, it may be difficult to palpate the medial edge of the CP with certainty. We proposed the distance from the center of the CP to the center of the CV be utilized to determine the usefulness of the CP as a topographical landmark. Straight line distance by itself can be helpful but gives an incomplete picture of the effectiveness of the CP as a topographical landmark. Horizontal, vertical distance, as well as angular offset of the CV from the straight-line segment between bilateral acromion processes give a full picture of the most common location of the CV and could enhance CVCD success rates. The usefulness of this additional information was a driving force behind the significance of this study. The first specific aim of this study is to determine if there is a consistent landmark that can be used by clinicians to locate the CV. The study hypothesis states the CV will be located ≤ 10 mm of the CP a statistically significant amount of the time.
According to current research, the diameter of the CV is a crucial data point in anatomical studies. When the CV is < 1 mm it will be unsuitable for pacemaker lead placement [11]. An additional study recorded that out of an n = 548, 324 patients had CV diameters of >1.5 mm and were found to be independently associated with the primary functional maturation of radio cephalic arteriovenous fistula maturation 86% of the time. Along with the radial artery diameter, CV diameter is the most significant factor associated with autogenous radio cephalic arteriovenous fistula maturation [9]. These statistics further justify the value of accurate diameters of the CV in anatomical studies. However, the method for gathering the diameter varies widely from study to study. Current techniques for measuring diameter include several methods ranging from computer pixel analyzing of photographs taken of specimens, ultrasound measurements in living patients, to measuring the whole CV with calipers when it is still in the cadaver. Despite taking precautions, every method has its shortfalls and may not be widely available. The second specific aim of this study was to determine a method of measuring vein diameter so that all studies will be unified and easily compared no matter what tools and resources are available.
Methods
According to Texas regulations on cadaver material, institutional review board approval is not required. This project utilized 41 cadaver donors from the University of North Texas Health Science Center’s Willed Body Program in Fort Worth, Texas. Bilateral dissections were conducted on the anterior shoulder region to locate the CV and measure its distance from the CP. Dissection area was made to span laterally midway through the deltoid muscle region to include supraclavicular variations of the CV should they be present. Initial dissection was conducted down to the fat pad of the deltopectoral groove. If the CV was located within this fat pad in the superficial designation, the dissection was halted, and measurements were taken. If the CV was not located, a further dissection was conducted through the muscle to determine if the CV was deep or absent. Once all measurements were taken, the dissection was photographed with a Canon EOS Rebel T6 (Oita, Japan). A tripod was utilized for consistent images. A probe was used when necessary to clearly demarcate the vein edges, and a blue pin was used to mark the center of the CP (Figure 1).
A straight incision was made from the medial one-third of the clavicle to the axillary cleft at an approximate forty-five-degree angle. A second incision was made from the superior point of the first incision parallel to the clavicle until the edges of the lateral and inferior incisions were in line vertically. The resulting triangular fold of skin was reflected laterally and clamped down with forceps. Fat and fascia were removed in a stepwise fashion until the muscle fibers were visible, and the fat surrounding the CV was observed. The CV was then dissected further until visually confirmed (Figure 1)

The presence of the CV was noted, and its location was described as either superficial, deep to muscle, absent, or an anatomical variant. Superficial notation was selected when the CV was in the deltopectoral groove but was not covered superficially by the muscle belly of the pectoralis major and deltoid muscle. Superficial notation was also given if the vein was between the muscle bellies of the deltoid and the pectoralis major muscles. A notation of deep was given when the CV was covered superiorly by the muscle of either the deltoid or the pectoralis major muscle. If the CV was not initially located, a further dissection was conducted to confirm absence or anatomical variation.
The coracoid process was palpated, and the center was marked with a blue pin. A direct distance was measured in millimeters from the previously mentioned blue pin to the center of the CV. A horizontal and vertical distance was also measured in millimeters from the previously mentioned pin to the center of the CV (Figure 2).
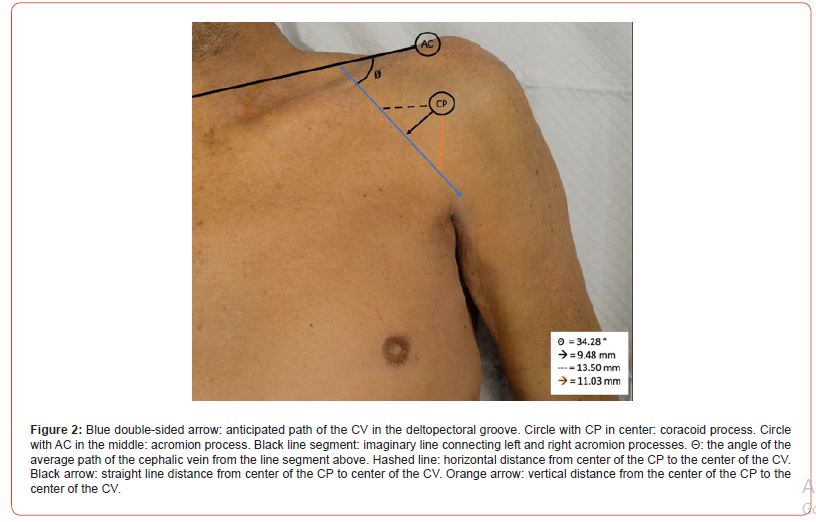
Depth was measured by taking a straight edge ruler and noting the depth of the cut from the outermost part of the dermis to the most superficial edge of the CV. A goniometer was positioned so the pendulum was parallel with the sternum and perpendicular with an imaginary line segment between bilateral acromion processes. The swinging pendulum of the goniometer was then aligned with the angle of the CV at the point closest to the CP. The resulting angle was measured.
Once the vein was extracted a straight-line incision was made across the entire length of the vein. Next, the CV was laid flat and opened and placed between two glass slides. The diameter of the inner lumen was measured with Mejia scopes, and the resulting circumference was obtained mathematically.
Results
A total of 41 cadavers were dissected. There were 23 males and 18 females. In one female cadaver, dissection could not be completed on the left side due to advanced tumor growth in the axillary region. This left side was not counted as absent but rather was excluded from the study due to the inability to complete a thorough dissection. This made for a total of n = 81 shoulder dissections conducted. All measurements are summarized in (Table 1) and (Figure 2). The location of the CV, as well as absence rates, and superficial versus deep are summarized in (Table 2). In previous research there was an absence rate of 4%. However, in our study we found the CV in all 81 specimens [12-21].
Table 1: Mean and Standard Deviation of study measurements. * Indicates statistical significance.
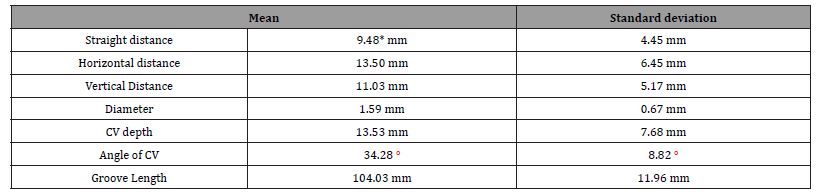
Table 2: Location of the CV found after dissection. Total number of each found and percentage of total.
A mean and standard deviation (SD) were obtained for all data points. Straight line distance was the only data point where a student t-test was conducted. The data were analyzed using a Shapiro-Wilk Test (SWT) to check for normality. The SWT showed a rightward/positive skew and a p-value of 0.0597345. This result was under suspicion, so the data were normalized using a square root data transformation. The SWT on the transformed data was p-value of 0.9876 with a normal distribution and without any skew. A Turkey Fence test was done and showed no outliers in the data set. Next, the data were analyzed using a one sample, left-tailed, student t-test against an expected population mean. The population mean was set to 10 mm so that the H0 would predict the data would be ≥ 10 mm while the Ha would predict the data was < 10 mm. The α was set to 0.05. The resulting p-value was 0.0333906 and the test statistics was -1.860547. The H0 was rejected showing that the CV was located within 10 mm of the CP with statistical significance. Since clinicians will likely have more than 10 mm of open incision while gaining access to the CV, the t-test was repeated with an expected population mean of 15 mm. The resulting p-value was 3.66613e-16 and the H0 was rejected. When α was set to 0.0001, the results remained significant. Diameter of the CV was obtained by taking the circumference of an open vein and dividing by π. The formula used was d=C/π.
Discussion
This study showed that the CP can be utilized as an accurate topographical landmark to locate the CV within 10 mm. Furthermore, this study showed that when the parameters are widened to 15 mm, the statistical significance is increased to ɑ = 0.0001. Clinicians can have confidence while utilizing the CP to locate the CV that this method will show the CV to be found a statistically significant portion of the time. Furthermore, if clinicians utilizing this method do not locate the CV quickly, they now have statistical data that shows the CV is likely not present, either by absence or anatomical variation. Based on the data, clinicians can quickly transfer to an alternative method for central venous access. The added confidence to make this quick transition is one of the main resources this study gives to clinicians.
This study’s method of diameter testing was successful and simple. This method can be completed with few resources which makes it ideal for labs working on a smaller budget. It is recommended that a circumference-based diameter testing be a standard vein measurement in cadaver-based anatomy studies. This study had some limitations. Due to utilizing cadavers from a health science center that were intended for dissection within multiple school’s anatomy courses, we were limited to these cadavers with no choice for using fresh or additional specimens. This also meant that our dissections were very limited to the axillary region since we could not disturb other potential areas needed for dissection in the anatomy courses. In future studies, we recommend dissecting the entire CV from the hand to the axillary region. These added data would have helped to track the CV better and aid our overall understanding of the entirety of the CV. It was for this limitation that the scope of the study was focused on the CV in relation to the CP in the axillary region.
The placement of TIVAPs for central venous access is a procedure that is done routinely in hospitals and clinics across the world. The two main procedures are the CVCD and the SCP. The CVCD has been shown to have less complications [6] and is recommended to be the first choice [7]. However, the SCP is still the first choice for most clinicians. We hypothesized that difficulty locating the CV could be a reason that clinicians prefer the SCP over the CVCD. This difficulty locating the CV has shown to increase average operating times of the CVCD which can play a factor in clinician efficiency and anesthesia times can affect patient complication rate. This study set out to give clinicians statistical data to aid them in locating the CV via the CP. We hope that clinicians will make the CVCD their first choice utilizing the CP to locate the CV quickly, which we have shown statistically to be accurate. If a clinician utilizes this method and is not able to quickly locate the CV, they now have statistical evidence that the CV is likely not there and can rapidly transition to the SCP procedure utilizing the original incision from the attempted CVCD for the port pocket. With this technique, even if the CVCD is unsuccessful, it will not waste significant time or increase the number of incisions, but if it is successful it will help to reduce complication rates.
Conferences
This research was presented Virtually by Jonathan Sweeney M.S. at The Annual Meeting of the American Association of Clinical Anatomists on June 15-19th, 2020.
This research was presented Virtually by Jonathan Sweeney M.S. at The Herb Robbins Research Symposium at Dallas Baptist University on October 27, 2020.
Acknowledgement
We would like to thank the Willed Body Program at UNT Health Science Center.
Conflict of Interest:
The authors have no conflicting interests.
References
-
Jonathan L Sweeney*, Albert Yurvati, Armando Rosales, Alfred V. Abraham, Adam Beyer and Rustin Reeves. Cadaveric Anatomical Study and Evaluation of The Coracoid Process as A Bony Landmark for Locating the Cephalic Vein. Anat & Physiol Open Access J. 1(4): 2024. APOAJ.MS.ID.000520.
-
Cephalic Vein Cut Down Procedure, Subclavian Puncture, Totally Implantable Venous Port Placement, Bony Anatomical Landmark, Coracoid Process, Vessel Diameter, Cadaveric Study, Clinical Decision Making
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.