Review article
Review article
Symmetry and Asymmetry, Crossing and Polarity: The Avian Synthesis
Enrico Marani, Tiboel Siegenbeekstraat 15, 2313 HA, Leiden the Netherlands
Received Date:April 16, 2025; Published Date:May 01, 2025
Abstract
“Two things commonly escape notice in considering the ingenuity of symmetrical patterning, a) being co-equal, each half of a nervous system is potentially an independent entity; and thus, b) the necessity arises for one or the other side to decide which of two possible behaviours, that of the left or of the right, the organism will follow [1]”. This article contains three parts. Part 1 relates crossings to symmetry by treating three hypothesis Cajal’s, stereometric and double twist explanations, while part two discusses planar polarity and asymmetry. Part 3 directs to symmetry and asymmetry in the avian “split” brain since no corpus callosum is present.
Keywords: Invariant asymmetry; placodes; avian song; planar polarity; hubs
Part 1: Crossings
Introduction
Wherefore should fibre systems in the brain cross to the other side or decussate at the same side in vertebrates? A series of hypotheses have been published on this topic by various scientists. Treated are three of them: The oldest is the explanation of the Nobel Prize winner and father of the neurosciences Ramon y Cajal [2]. The second is called the topological-stereometric explanation [3]. The third is the axial twist hypothesis [4,5]. (Two nearly identical axial twist explanations were independently published, see [6]). After Cajal’s explanation, using the visual system, several other clarifications have been published with emphasize on the optic system. The main six (1976 to 2005), briefly mentioned below, belong to the top of the iceberg of explanations of the crossings in the vertebrate nervous system. However, our attention goes to the noteworthy first three mentioned also for the sake of brevity.
The three-dimensional predictive approach of brain wiring or
topological-stereometric explanation [3] also establishes the rules
governing storage of brain connections. The start is with Cajal’s
laws of neuronal optimization:
a) Neuronal arrangements have to minimize space,
b) They have to maintain sufficient size and
c) They must minimize conduction time
These are simple rules to store neurons and connections
effectively within the brain, since the skull has restricted brain
space and the brain has to cope economically with the various
organ demands of the body. Thus, crossings have to fulfil applied
conditions of the general laws:
a) Crossings should be minimized,
b) Crossings must be tightly regulated and
c) Information is needed to plan the crossings during
development
These rules are the consequences of the general laws [7]. In principle, crossing does not induce asymmetry (see part 2), although asymmetry can be the cause for symmetric crossings (see axial twist hypothesis). Most crossings are symmetric.
Cajal’s Explanation of Crossing
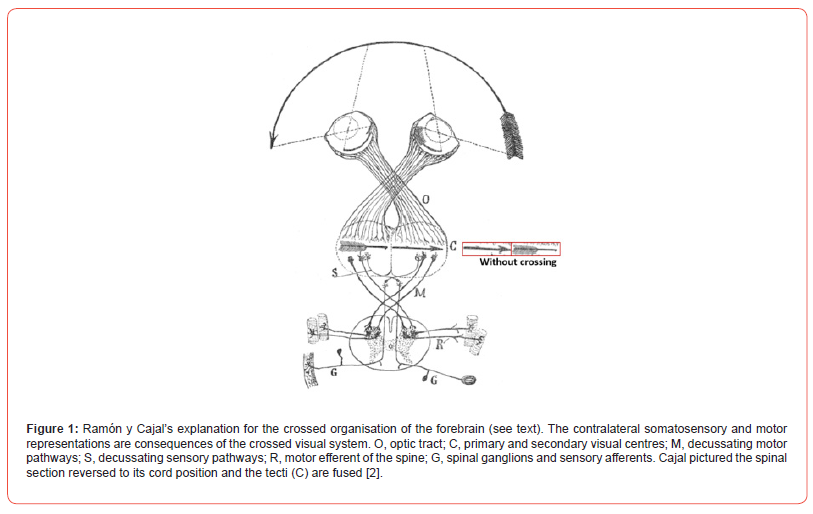
The eye will cast reverse images on the retina, the camera obscura effect present in boxes with a small opening, but also using a lens. In (Figure 1), the arrow should be depicted head to pin-tail, without crossing this visual information (see Figure 1 red block). The projection of the correct image can only be obtained by crossed information transport, and its uncrossed tectum (C), projecting to cortical areas, should be reached. The shortest way (O in Figure 1 thus minimizing space and conduction time) is the crossing of the axons from the eye to both tectal structures (C). In consequence, somatosensory and motor information also need to be crossed to reach areas belonging to and matching with it (M and S). This explanation is eloquent and graceful. Nevertheless, critics argue that the difficulty of real alignment is due to eye movements and the resulting movement of images.
The visual forebrain areas are poorly interconnected in various vertebrates. Cajal thought that all vertebrates used the corpus callosum for this purpose. In birds, the rather small anterior commissure (decussatio supraoptica, related to the principal optic and rotundus nucleus; [8-10]) takes over, since the corpus callosum is absent in birds. Nevertheless, avian commissures are present but restrictedly connect left and right cortical areas, e.g., the anterior and posterior commissure and supraoptic connections (part 3; [11]). Moreover, there is no direct connection between the avian visual cortex and the motor cortex. Directly related to Cajal’s explanation are several other propositions: image-forming eye hypothesis, the binocular vision hypothesis and, on other grounds, the bilateral symmetrical hypothesis, the avoidance behaviour hypothesis and the limb evolution hypothesis. All are put to the optic system test with more or less success ([12-14] for criticism and more references).
The Topological-Stereometric Explanation
On the condition that brain and body could be represented as being a two-dimensional flat structure crossings are absent (Figure 2a). However, brain and body both are three dimensional. Thus, folding the flat structures into a three-dimensional structure directly produces the crossing of connections (Figure 2b). A real crossing is determined by its mirror image (Figure 2c): “a mirror reflection about a symmetry plane generates an inseparable link if and only if the connections cross in that projection”. Even in the case of a two-dimensional structure connected with a threedimensional structure crossing occurs (Figure 2d). For the avian brain (Figure 2) this means: the cortical plates can be considered two dimensional (somewhat bent) structures, while the brainstem is three dimensional. Thus, connections between both cortices don’t need crossings, in fact the situation in Figure 2a. However, the connections from cortex into the brainstem lead to crossings as represented in Figure 2d. Crossing of systems can happen ipsilateral of the structures involved or contralateral.
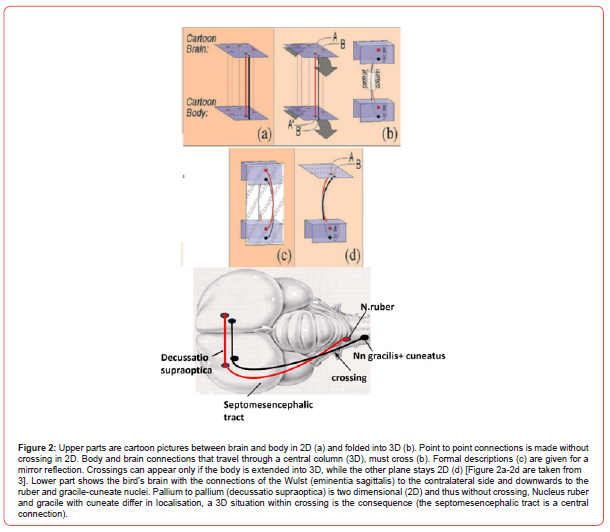
The topological-stereometric approach states:
a) Crossings are a mathematical necessity in 3D structures.
In simple connections these decussations can be within a tract
(see septomesencephalic tract in Figure 2). In complex systems
contralateral tract crossings are preferred.
b) Large numbers of neurons ask for a contralaterally wired
system to be stable.
c) Large systems need the decussation mechanism otherwise
an enormous number of genes are needed to organize identical
connections.
The Axial Double Twist Hypothesis
The double twist hypothesis is considered valid by the authors for all vertebrates and its rotation effects started with their common ancestors living presumably 450 million years ago. The double twist hypothesis is based on the compensation for embryological axial rotations present during morphogenesis. This should explain the decussations and decussation-absences in the central nervous system. Moreover, the consequence of the embryological axial rotations is the formation of an optic chiasm. All vertebrates do have an optic chiasm, although with different organisation types [15]. (In the course of the description of this rotation see Figure 3). All through its earliest development the embryo changes position and turns to the left. This is anti-clockwise to the position of the embryo. Thus, finally the embryo is located on its left side (see also part 2). The symmetry of the midsagittal vertico-dorsal plane has vanished (in Figures 3a&3b). This is unfavourable for organisms that need bilateral symmetric locomotor systems, e.g., fish. It does not matter for asymmetrical visceral organs (heart, digestive tract) in general. By compensatory mechanisms the bilateral symmetry can be restored.
This happens both clockwise and anticlockwise. The front of the embryo’s head goes on with the anticlockwise movement restoring symmetry in the head region. The body part restores symmetry by turning clockwise (Figures 3b&3c). The embryo undergoes a double twist: anticlockwise in the head region and clockwise in the rest of the body. The consequences are that the optic axons have to reach its brain parts in an inverted position. The eyes and forebrain are inverted, so the target area, the optic tectum, which is not inverted, can only be reached by the optic axon bundles if they cross to the other side (Figure 3d&3e). After crossing they reach the optic thalamus nucleus (lateral geniculate body, LNG, Figure 3d). This explanation for crossings is supported by results of the development of zebrafish and chickens [16]. Consequences are: Optic thalamus (LGN, anticlockwise turned) projects without crossing to the visual cortex (also anticlockwise turned), but the received thalamus information was already crossed.
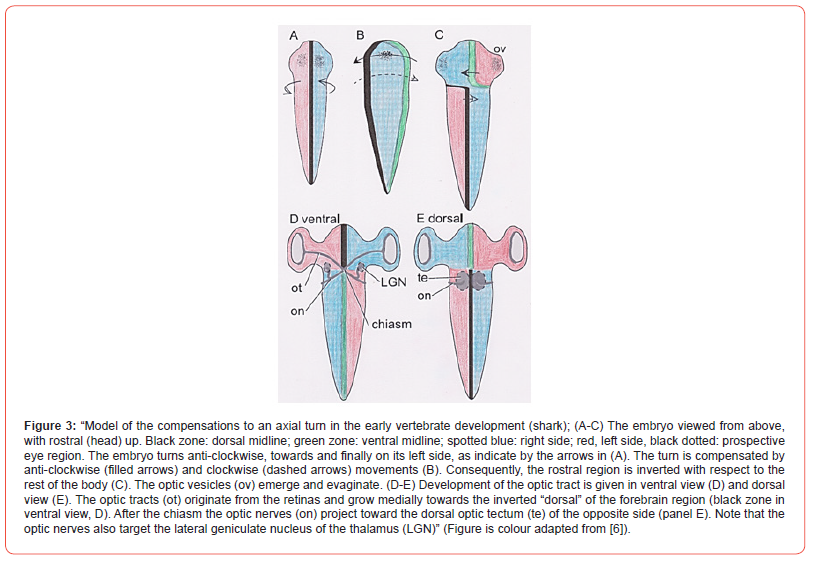
The visual cortex gets information from the contralateral eye. In sharks the visual input crosses twice: in the chiasm and later in the mesencephalon: in the chiasm due to the anticlockwise rotation and back due to the clockwise rotation in the mesencephalon, which is no forebrain structure. This is because the shark anterior cortex contains a large visual central nucleus. This nucleus represents the ipsilateral eye. This visual central nucleus gets its input from the optic tectum (te in Figure 3e) via the thalamus. The nerve fibres decussate again between tectum and the thalamus to give ipsilateral eye information. “Thus, in accordance with our hypothesis, the visual information crosses the midline twice: first in the optic chiasm [to bring contralateral eye information to tectum, remark ours] and again in the ventral mesencephalic tegmentum [to bring back ipsilateral eye information to the central nucleus, remark ours]”. By the way, one could consider the shark system as a violation of Cajal’s laws.
Synopsis 1
The kick-off of the discussion on crossings in the nervous system was given by Ramon y Cajal. Applying the simple laws of Cajal in a stereo-metric approach for the explanation of brain wiring reveals the necessity for crossing of the fibre systems in the brain. Small connections of 500- 1000 neurons can organize crossings within the tract, while large connections force contralateral decussations. Developmental rotations within the embryo support contralateral decussations as exemplified in the optic system. Therefore, without internal decussations of tracts and contralateral crossings no “stable” wired 3D brain can exist.
Part 2: Asymmetry and Polarity: The Avian Song System
Introduction
Why is asymmetry present in the vertebrate body, as, for example, in birds? The exterior of humans is characterized by bilateral symmetry with respect to the midline. Nevertheless, asymmetry, also called lateralisation in the brain, in humans is well known for function and form. It goes from right-handedness to left sided speech localization in the human brain. The human heart (apex and aorta) and spleen are localized on the left and the liver is more placed on the right. Both form and function in the human body have internal asymmetry. Asymmetry is also present in the bird’s exterior e.g.: the wry bill (Anarhynchus frontalis) has a beak that is bent sideways and the crossbill (Loxia curvirostra) has an upper and lower bill tip that cross each other (Figure 4). Owls have asymmetrically placed ears. The asymmetry found in the avian exterior is generally in contrast to the mammalian appearance. The hyo-branchial system of the woodpecker’s tongue is related to the right nostril or the right eye [17]. In the bird’s connections of visual and song systems, lateralization of hemispheres is clearly present.

Moreover, kidneys and gizzard together induce an asymmetrical location and consequently right and left testes are different. In various bird species only, a left ovary is present, best known are the chicken and quail that have recently been studied at the single-cell level [18]. The bird’s right and left liver and lungs have different lobes. Bird’s right eye-left hemisphere function is often found dominant, for example in migration and in food collection in various birds. Food collection asymmetry is strongly variable in birds, left eye-right hemisphere is also often encountered, and it is frequently food-type dependent [19]. Functional food asymmetry is clearly present. This external and internal located asymmetry, already produced in the early embryo, is genetically determined and is affected by a roll to one side. King and Brown [20] even noted in their article on asymmetry: “The left side gets all the best genes”, and over 30 genes are detected to be involved in the avian asymmetry production [21]. Important contributions to the unravelling of asymmetry have been possible due to the chick embryo model [22].
Development of Invariant Asymmetry
Two types of asymmetry are discerned: invariant and fluctuating asymmetry [23]. Ontogenetic plasticity will refine the asymmetry. Best known example is the light effects on the visual system of chickens and pigeons [24]. Moreover, environmental and stochastic determination of asymmetry directions are also recognized [25]. A more common origin of animal asymmetry has been discussed by [26] in relation to MMP21, a (matrix) metallo-proteinase breaking the symmetry [27]. However, this MMP21 is absent in the genome of birds and reptiles and induces genetic research (e.g.: gonadal differentiation of chickens [28]).
Morphological invariant asymmetry is found earliest on day 23 of the human development (looping of the heart), on embryonic day 8.5 in the mouse and after 33-45 hrs of development in the chick. The initial cells of the embryonic disc are produced by its primitive node responsible for asymmetry [29], and its primitive streak, responsible for bilateral symmetry ([30]; Figure 6). These intra-embryonic cells make the mesoderm (precursors for bone and muscles) located in between ectoderm and endoderm of the embryonic disc.
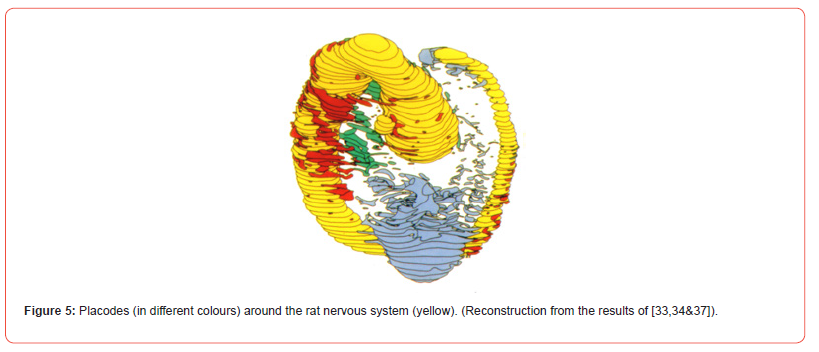
The disc will form the primitive embryo (Figure 6). Before the closure of the neural groove into a neural tube (see [31] for cascades), by the ascend of the bilateral symmetric neural walls, meso-ectoderm cells are already deposited into the embryo. These early neural crest cells, produce symmetrically mesodermal bone and muscles and their bilateral symmetric neuronal structures (spinal ganglia; Figure 6). The ectoderm cells also contribute and produce placodes. Placodes are specialized transient parts of ectoderm adding cells to the mesoderm compartment. Placodes also produce neuronal structures and contribute to the sense organs in the head, e.g., olfactory structures [32,33], cranial nerve parts and the lens of the eye (Figure 5) [34]. Placodes are mostly seen as symmetric structures, but do possess an internal asymmetry [35,36]. The first embryonic disc cells will multiply and grow in three directions called axes. The anterior-posterior axis, dorsal-ventral axis and the left-right axis. The left-right axis is strongly involved in the production of asymmetries. A series of protein cascades is needed that are genetically induced to produce asymmetry in the embryonic disc, the blastoderm.
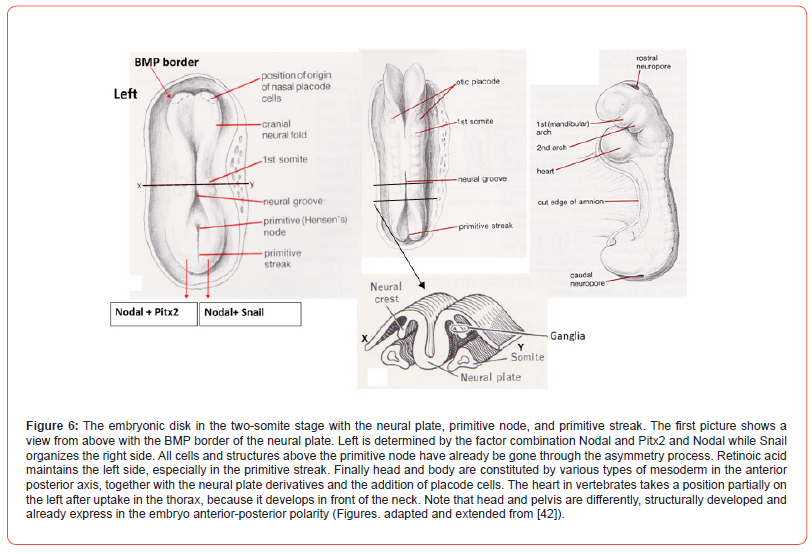
The avian blastoderm is floating and drifting on the egg yolk. Only some of the classic protein cascades involved are treated here at large. Within the blastoderm a neural part and an epidermal, surrounding part can be seen. The boundaries of the neural plate within the rest of the embryonic disc are determined by the Bone Morphogenetic Protein (BMP). Together with other substances such as Wnts (“wingless-related integration”), fibroblast growth factor and retinoic acid this border determines the change towards neuronal plate cells or towards epidermal cells [37,38]. While the anterior-posterior axis develops, followed by the dorsal-ventral axis and, with some delay, finally the left-right axis is added with its node and streak activity, operating consistent by three distinct asymmetric phases (denoted 1, 2, and 3 below). These three phases are based on polarity present within the organism (see part below, Figure 8) and asymmetries are genetically based. Fibroblast growth factors are a family of cell signalling proteins that are involved in embryonic development, specifically in cell proliferation and differentiation.
These fibroblast growth factors induce the initial neuronal symmetry break (1) together with other substances. It is important for the direction or side of the asymmetry: left side is initiated by fibroblast growth factor-8 with lefty-1 (left-right determination factor-1), which holds for all species studied [39], the right side is instigated by fibroblast growth factor-8 and activing (which also regulates morphogenesis of the prostate, lung, and kidney). To it inhibition and activations of other cascades of proteins are needed but not treated here. In this manner disc plate cells are produced in an “asymmetric” environment. The chick embryo shows nodal expression in the primitive or Hensen’s node that is a pit-shaped embryonic plate structure (Figure 6). The nodal pathway imposes the structural direction of the asymmetry (2), which is in most cases to the left, due to the transcription factor Pitx2. Right side structures are determined by the combination nodal and snail (Figure 6). In such cases, here Pitx2 versus snail, the asymmetry is exclusively headed on the left side of the embryonic plate [40].
The nodal signalling protein belongs to the transforming growth factor-β family of proteins. The nodal cascade needs stimulation of the Notch signalling pathway to be asymmetrically effective (3). The nodal-notch cascade finally organizes the asymmetry (see recent and classic overviews [41,42]). The zebrafish (Danio rerio) is the main experimental animal in the study of asymmetries. A series of brain asymmetries are known. One concerns the epithalamus, placed above the thalamus at the end of the brainstem. Its dorsal part, the habenula needs attention. The fish epithalamus is related to freeze behaviour: “the primary function of the zebrafish habenula could be to suppress motor activity under adverse conditions (something unpredictable and potentially dangerous that happens to fish). Indeed, the lateral habenula parts (thus one-sided) can inhibit dopaminergic neurons, which are key modulators of movement and motivation in this fish. This function of the habenula possibly dates back to very early evolutionary times. Presumably this pathway worked like “a circadian system of ancient vertebrates” [43].
Asymmetry is regarded as evolutionary old [44]. The asymmetry of the thalamo-fugal connections for the bird optic system has to be considered. Learning and action-reaction in birds is right eye-left hemisphere dominant. Bird memory seemingly needs an asymmetric organisation. “Visual imprinting in chicks and song-learning in songbirds are prominent model systems for the study of the neural mechanisms of memory. In both systems, neural asymmetry or lateralisation has been found to be involved in memory formation” [45]. While in mammals, except hominids, it remains difficult to investigate, both external and internal, asymmetries [46], a rather large series of studies in birds support asymmetries. A remarkable functional asymmetry concerns birdsong and the neural aspects of the syrinx needs explanation, especially the tracheosyringeal nerve branch of the hypoglossal nerve will be discussed.
The Syrinx
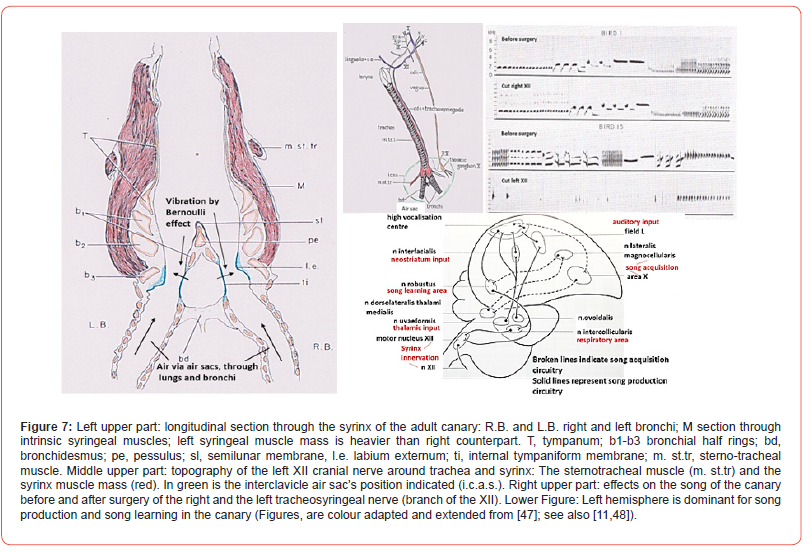
The air that circulates through the bird’s lungs, originates in the so-called air sacs and passes through air tubes into the bronchi. The Bernoulli effect states that increasing speed of the air passing through a tube will produce altered lower pressure within this air. Flexible walls will be drawn into the lumen of the tubes as also happens with the tympaniform membranes in the bird’s bronchi (Figure 7). Together with the external labia position and air-sac pressure regulation, membrane oscillations occur. These vibrations are determined in amplitude and frequency by intrinsic and extrinsic muscle groups. Each of the bronchi contains tympaniform membranes, has its own air supply by the left or right bronchus, and has its own muscles. Together with the bilateral innervation of the hypoglossal nerves, each bronchus could be considered a separate sound well.
Tracheosyringeal Nerve: Avian Functional and Dimorphic Asymmetries
First to notice is the larger muscle mass at the left side of the syrinx of the canary (Serinus canaria). Cutting the left or right branch of the hypoglossal nerve, the tracheosyringeal nerve, produce different results (Figure 7). Surgery of the right tracheosyringeal nerve does not change the song of the male canary as pictured by the two upper sound spectrograms. However, surgery of the left tracheosyringeal nerve destroys the main repertoire of the song (lower two spectrograms, Figure 7). The song is replaced by silent gaps, clicking sounds or distorted modulations. “Such birds sing vigorously, as judged by their posture and motion, yet look like actors in a silent cinema film” [47]. The conclusion of these surgical result of 49 male canaries is that the left tracheosyringeal nerve, branch of the left hypoglossus (Figure 7), is dominant for song control. Extra results show that this dominance is not related to auditory processing but is a syringeal bilateral innervated motor phenomenon [48].
The Zebra finch (Taeniopygia guttata) shows the contrary. In this bird species the right hypoglossus nerve is dominant for song control with right side dominance for its hemisphere [49]. There is a song difference between male and female, the male sings and the female is mostly silent. Research showed that the female hypoglossal nucleus volume was 63% of the male, the female neuron somata within this nucleus were 86% of the male, the total number of female neurons was 90% of the male and the female syrinx musculature weight was 51% of the male [50]. Females masculinized by hormones do start singing. The part of the hyperstriatum that respond to sounds and normally induces song in the males, can stimulate the song in males, but not in the females. Although the same stimulation has been applied no response passes over the tracheosynringeal nerve of the female [51]. The question now is whether the female has the same neuronal connections [52].
Three Levels of Planar Polarity: Subcellular, Cellular and Tissue Polarity
Attention to asymmetry induces the awareness of polarity. The initial step is the breaking of the symmetry, followed by the generation of polarity in the axes. Polarity has a clear structural basis producing functional consequences in: the anterior-posterior (by animal poles), dorsal-ventral (by gravity) and the right-left (by asymmetry) axes. Polarity is considered: “The most fundamental characteristic of any pluricellular structure. The organism has at least two dissimilar poles, ends or surfaces” [53]. The term planar polarity [54] causes problems, since various definitions and approaches are current. In general, planar polarization is determined by one surface or one layer of cells in which cell to cell interaction organizes those two or more cells accept coordinated divergence, called cellular polarity [55], which is also important to establish 3D configuration of morphogenetic structures. For this interaction between cells special proteins are needed to mediate this act [56]. Planar cell polarity was first studied in the fruit fly Drosophila melanogaster [57] and later it has been applied to other experimental animals, especially in the cochlea of the mouse. The study of polarity in a two-dimensional plane and in the anterior– posterior patterning system has been useful due to the detection of the Frizzled/planar cell polarity.
Its directional signals are based on the same underlying mechanism in various organisms. A cellular interaction between receptor and its protein generates the polarity. In the Frizzled polarity system, its Frizzled transmembrane receptor act independently in the planar cell polarity system. This receptor is present in various cell types, including the otic-cochlear cells. Essential proteins needed are cell surface and cytoplasmic ones, names in brackets are the mammalian homologues: Frizzled (Fz, 1, 2, 3, 6), Strabismus (Vang 1, 2), Flamingo (Celsr 1, 2, 3) and the cytoplasmic Dishevelled (Dvl 1, 2, 3), Prickle (prickle-like 1, 2) and Diego (Diversin) proteins (Figure 8a-8c). These proteins after their production in the rough endoplasmic reticulum are released in the cytoplasm. Like a submarine that comes to the surface, these proteins reach the luminal (cochlea) or apical (Drosophila) surface of the cell involved. Microtubules are involved and required in this process to bring the Frizzled protein to the surface. Two groups are formed, a proximal and a distal one by activity of all six proteins (Figure 8c). Description of this gathering process has been omitted here [58].
The induced local polarity by these proteins is not restricted to that special cell, but will have effect on cell–cell interaction levels and/or on the tissue level (Figure 8) [59]. Another polarity cascade is the Fat/Dachsous pathway important in the placing of actin-rich hairs in animal fur (Figure 8d). Hair cells of the cochlea contain cilia that are organized in a V or W form on top of the luminal surface (Figure 8e). The height increases towards the point of the V and is called the staircase pattern. The V top is made by one large kinocilium (Figure 8 left). This structure “repeats” itself on all hair cell groupings. All the V bundles are directed away from the cochlear neural side that is ab-neural. (By the way, within the cochlea the Prickle protein act differently as explained in Figure 8a- 8c). In rat and mouse cochlea, the Dishevelled 1, 2, 3 accumulate abneural, while Strabismus (Vang 1, 2) and Frizzled (Fz 3, 6) group at the neural side and presumably organize the polarity within the hair cell rows [60-62]. The relation of kinocilium polarity and the influence of the other ciliary polarity are still under discussion. These interactions at the cellular level do have analogues at the tissue level.
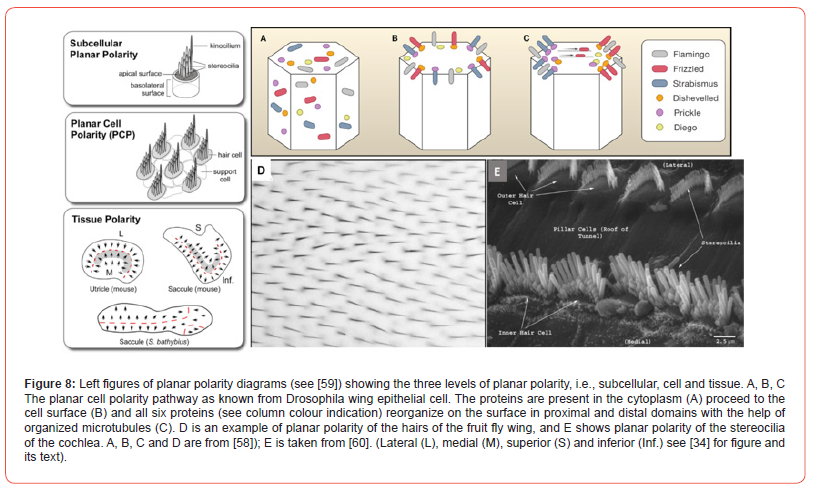
The vestibular maculae contain within the mature sensory epithelium opposite polarities. Within utricle and saccule maculae, the ciliary hair cell groups contain luminal opposite bundle orientations. The so-called Line of Polarity Reversal is the border between these opposite cilium distributions. While in the utricle, the polarity of the ciliary bundles is towards this line, in the saccule the ciliary bundles point away Figure 8 left diagrams, red line). Polarity is needed for the whole-body composition, e.g., during growth to maturation along the anterior–posterior axis. Normally, these various planar polarities for body development are combined. The antero-posterior body polarity with its dorso-ventral body polarity is converted into a bilateral symmetry of the mammalian body. Cell sociology already hypothesized the laws needed to understand this automation in animal development at cellular and tissue levels. Birds have been studied for planar cell polarity. It plays a role in skin feather implant, in the cochlear apparatus and in the lagena.
The lagena in pigeons showed that “hair cell planar polarities were oriented away from a central reversal line that ran nearly the length of the epithelium” [63], which is completely comparable to the cochlea results depicted in fig. 8 left.
Several more models, still under discussion, explain the
breaking of the symmetry:
a) The nodal flow model; the cilia in the node move into one
direction, bringing fluid with its substances to the left side of
the embryo [64],
b) The two-cilia model; two populations of cilia are present
in the node, the central population produces the left flow,
while the immobile ones are the mechano-sensors that induce
intracellular influx of calcium at the flow side [65].
c) The Gap-junction model; in birds the asymmetry is
already present before the node’s existence. Due to the electric
coupling of these early cells by Gap-junctions asymmetry is
induced, since several substances (e.g., serotonin) can move
one sided to other cells [66-68].
Synopsis 2
The conclusion of part 2 is that total symmetry is not present at the subcellular-, cellular-, tissue- and body levels whether by planar polarity or by left-right asymmetry.
Part 3. Symmetry and Asymmetry of The Avian Brain: Its Synthesis
The Absence of The Avian Corpus Callosum: The Consequences
All placental mammals have the corpus callosum, which establishes the mutual functional hemispherical connections (Figure 9, left). The human corpus callosum contains ± 170 million fibres [69,70]. The diameter of the myelinated fibres counted can vary and beside myelinated fibres also unmyelinated ones are present, but hardly measured. Sperry [71] studied the total commissurotomy of the corpus callosum, known as “split brain”. He concluded: “We can now demonstrate with appropriate tests a whole of distinct impairments [72] that are most simply summarized by saying that the left and right hemispheres, following their disconnection, function independently in most conscious mental activities. Each hemisphere, that is, has its own private sensations, perceptions, thoughts and ideas all of which are cut off from the corresponding experiences in the opposite hemisphere. Each left and right hemisphere has its own private chain of memories and learning experiences that are inaccessible to recall by the other hemisphere. In many respects each disconnected hemisphere appears to have a separate “mind of its own”.
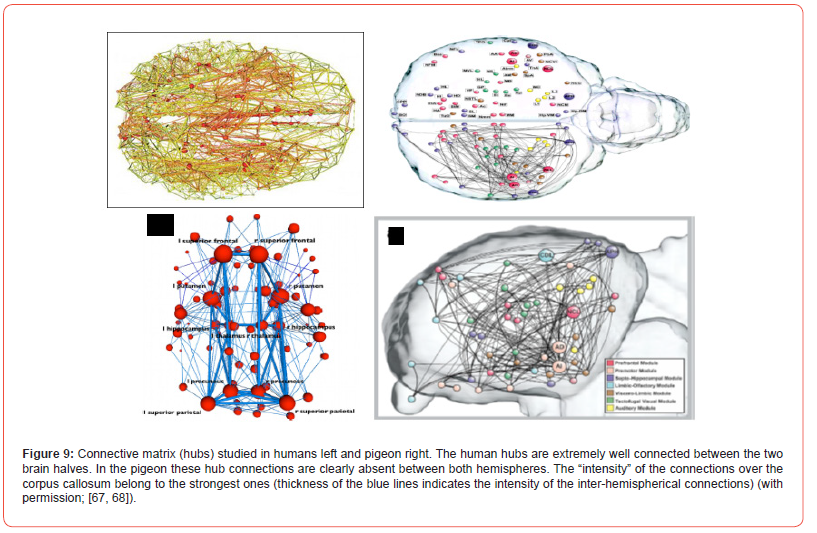
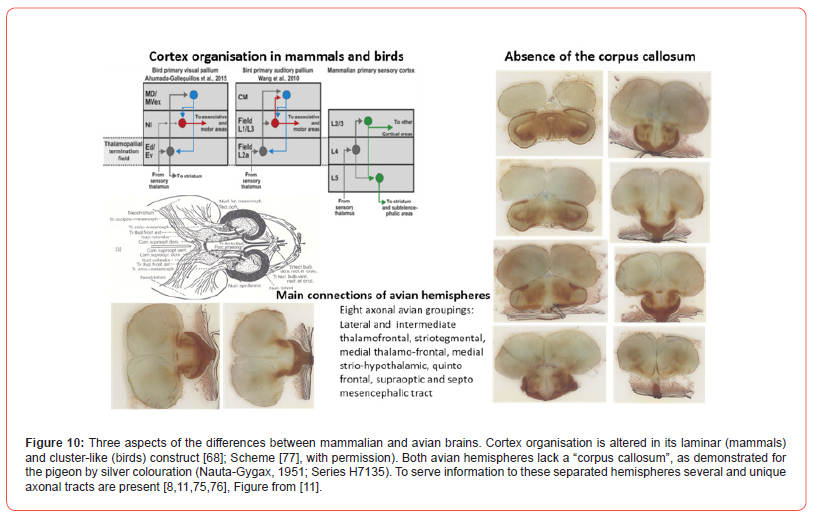
The avian brain halves are restrictedly interrelated by its commissura anterior, posterior and the commissura supraoptica, since the corpus callosum is absent in the avian brain. Interaction between both hemispheres is strongly reduced and is not comparable to the hemispherical communication of mammals as studied by hubs. The cortex structure of birds is also different. The avian pallium is based on nucleated clusters, while the mammalian cortex is known by its laminated neuronal organisation (Figure 10) [73]. The storage of neurons in the avian forebrain is different, resulting in more neurons/volume [74]. Information towards avian “split brain” hemispheres asks for symmetrical relay groupings, of which eight main connections are discerned: Lateral and intermediate thalamofrontal, striotegmental, medial thalamofrontal, medial strio-hypothalamic, quinto frontal, supraoptic and septo-mesencephalic tract [75,76] (Figure 10). The functional asymmetry as common in the human brain is also present in the avian brain, but in its own hemispherical separated way.
Lateralisation comprises several of the functional asymmetries in the avian brain, which clearly can differ in various bird species. Generally, the right eye-left hemisphere is dominant for mate recognition, category distinction, vocalisation, olfactory cues, while the left eye- right hemisphere knows dominance for spatial abilities, predator recognition, fear expression and aggression, and in various birds for food. The establishment of asymmetry in bird’s brains, we owe to Nottebohm [77,78]. In the canary the left hemisphere is exclusively the location for both song and song learning (Figure 7). Small and large lesions targeted to the nuclei involved in song production showed that in the canary the left hemispheric lesions disturbed song production heavily, while analogous lesions to the right hemisphere had clearly less effect on song production.
Synopsis 1-3
Part 1 on symmetry states: without internal tract decussations
and contralateral crossings no stable wired brain can exist.
Part 2 on asymmetry concludes: deviations of symmetry are
a necessary prerequisite to structure and to let the organism
function.
Part 3 on symmetry and asymmetry shows that absence of
avian symmetrical hemispherical crossing favours functional
asymmetry.
Conflict of Interest
The author declares that he has no competing interests.
- Doty RW (2003) Unity from duality. Acta Neurobiol Exp 63(3): 163-170.
- Ramón y Cajal S (1898) Estructura del kiasma optico y teoria general de los entrecruzamientos de las vias nerviosas. Rev Trim Micrograf 3: 15-645.
- Shinbrot T, Young W (2008) Why decussate? Topological constraints on 3D wiring. Anat Rec 291(10): 1278-1292.
- Lussanet de MHE, Osse JWM (2012) An ancestral twist explains the contralateral forebrain and the optic chiasm in vertebrates. Animal Biology 62: 193-216.
- Kinsbourne M (2013) Somatic twist: A model for the evolution of decussation. Neuropsychology 27(5): 511-515.
- Lussanet de MHE, Osse JWM (2015) Decussation as an axial twist: A comment on Kinsbourne (2013). Neuropsychology 29: 713-714.
- Llinas RR (2003) The contribution of Santiago Ramon y Cajal to functional neuroscience. Nat Rev Neurosci 4(1): 77-80.
- Nieuwenhuys R, Donkelaar HJ, Nicholson C (1998) The central nervous system of vertebrates. Springer 21: 1525-1636.
- Karten HJ, Hodos W, Nauta WJ, Revzin AM (1973) Neural connections of the “visual Wulst” of the avian telencephalon. Experimental studies in the pigeon (columba livia) and owl (Speotyto cunicularia). J Comp Neurol 150(3): 253-278.
- Karten HJ (2013) Neocortical Evolution: Neuronal Circuits Arise Independently of Lamination. Curr Biol 23(1): R12-R15.
- Marani E (2023) Lateralisation, hubs and cognition in the mammalian and avian brain. Acta Morphol Anthropol 30(3-4): 102-117.
- Banihani SM (2010) Crossing of neural pathways: Is it a response to the occurrence of separated parts for the body (limbs, eyes, etc) during evolution. Med Hypotheses 74(4): 741-745.
- Capazzoli NJ (1995) Why are vertebrate nervous systems crossed? Med Hypotheses 45(5): 471-475.
- Kashakilar SJ (1988) An explanation for the development of decussations in the central nervous system. Med Hypotheses 26(1): 1-8.
- Jeffrey G, Erskine J (2005) Variations in the architecture and development of the vertebrate optic chiasm. Prog Retin Eye Res 24(6): 721-753.
- Guillery RW, Mason CA, Taylor JS (1995) Developmental determinants at the mammalian optic chiasm. J Neurosci 15(7 Pt 1): 4727-4737.
- Bock WJ (2015) Evolutionary morphology of the woodpeckers (Picidae). Denisia 164: 37-54.
- Wang T, Leng D, Cai Z, Chen B, Li J, et al. (2024) Insights into left-right asymmetric development of chicken ovary at the single-cell level. J Genet Genomics 51(11): 1265-1277.
- Karnina K, Giljov A (2022) Lateralization in feeding is food type specific and impacts feeding success in wild type birds. Ecology and Evolution 12(2): e8598.
- King T, Brown NA (1999) Embryonic asymmetry: The left side gets all the best genes. Curr Biol 9(1): R18-R22.
- López-Gracia ML, Ros MA (2007) Left-right asymmetry in vertebrate development. Adv Anat Embryol Cell Biol 188: 1-121.
- Monsoro-Burq AH, Levin M (2018) Avian models and the study of invariant asymmetry: how the chicken and the egg taught us to tell right from left. Int J Dev Biol 62(1-2-3): 63-77.
- Balmford A, Jones IL, Thomas ARL (1993) On avian asymmetry: evidence of natural selection for symmetrical tails and wings in birds. Proc Royal Soc B 252(1335): 245-251.
- Manns M (2021) It is not just in genes. Symmetry 13(10): 1815.
- Palmer AR (2016) What determines direction of asymmetry: genes, environment or chance? Philos Trans R Soc B 371(1710): 20150417.
- Blum M, Ott T (2018) Animal left-right asymmetry. Curr Biol 28(7): R301-R304.
- Guimier A, Gabriel GC, Bajolle F, Tsang M, Liu H, et al. (2015) MMP21 is mutated in human heterotaxy and is required for normal left-right asymmetry in vertebrates. Nat Genet 47(11): 1260-1263.
- Zou X, Wang J, Qu H, Lv XH, Shu DM, et al. (2020) Comprehensive analysis of miRNAs, lncRNAs, and mRNAs reveals potential players of sexually dimorphic and left-right asymmetry in chicken gonad during gonadal differentiation. Poult Sci 99(5): 2696-2707.
- Gros J, Feistel K, Viebahn C, Blum M, Tabin CJ (2009) Cell movements at Hensen’s node establish Left/Right asymmetric gene expression in the chick. Science 324(5929): 941-944.
- Downs KM (2009) The enigmatic primitive streak: prevailing notions and challenges concerning the body axis of mammals. Bioessays 31(8): 892-902.
- Kacker S, Parsad V, Singh N, Hordiichuk D, Alvarez S, et al. (2024) Planar cell polarity signalling: Coordinated crosstalk for cell orientation. J Dev Biol 12(2): 12.
- Whitlock KE (2004) A new model for olfactory placode development. Brain Behav Evol 64(3): 126-140.
- Smits-van Prooije AE, Vermeij-Keers Chr, Marani E (1988) The development of the olfactory nerve from the nasal placode. Ultramicroscopy 26: 446-447.
- Marani E, Heida C (2018) Head and Neck. Morphology, models and function. Springer Cham Switzerland.
- Bancroft M, Bellairs R (1977) Placodes of chick embryo studied by SEM. Anat Embryol 151(1): 97-108.
- Serras F, Fraser F, Chuong CM (1993) A symmetric pattern of gap junctional communication in developing chicken skin. Development 119(1): 85-96.
- Smits-van Prooije AE, Vermeij-Keers Chr, Dubbeldam JA, Mentink MMT, Poelmann RE (1987) The formation of mesoderm and mesectoderm in prosomite rat embryos cultured in vitro, using WGA-Au as a marker. Anatomy and Embryology 176: 71-77.
- Schmidt C, McGonnell I, Allen S, Patel K (2008) The role of Wnt signaling in the development of somites and neural crest. Adv Anat Embryol Cell Biol 195: 1-64.
- Palmer AR (2004) Symmetry breaking and the evolution of development. Science 306(5697): 828-833.
- Gilbert SF, Barresi MJF (2000) Early development in birds (Chap 11). Developmental Biology. Sinauer associates inc, Oxford University Press.
- Tabin CJ (2006) The key to left-right asymmetry. Cell 127(1): 27-32.
- MacKinnon P, Morris J (1990) Oxford textbook of functional anatomy. Head and neck.
- Ocklenburg S, Gűntűrkűn O (2018) The lateralized brain. The neuroscience and evolution of hemispheric asymmetries. Academic Press.
- Blum M, Feistel K, Thurnberger T, Schweickert A (2014) The evolution and conservation of left-right patterning mechanisms. Development 141(8): 1603-1613.
- Moorman S, Nicol AU (2015) Memory-related brain lateralisation in birds and humans. Neurosci Biobehav Rev 50: 86-102.
- Springer SP, Deutsch G (1989) Left brain, right brain. Freeman Co.
- Nottebohm F (1977) Asymmetries in neural control of vocalization in the canary. In: Lateralization in the nervous system PP 23-44.
- Tsukahara N, Kamata N, Nagasawa M, Sugita S (2009) Bilateral innervation of syringeal muscles by the hypoglossal nucleus in the jungle crow (Corvus macrorhyngos). J Anat 215(2): 141-149.
- Williams H, Crane LA, Hale TK, Esposito MA, Nottebohm F (1992) Right-side dominance for song control in the zebra finch. J Neurobiol 23(8): 1006-1020.
- Wade J, Buhlman L, Swender D (2002) Post-hatching hormonal modulation of a sexually dimorphic neuromuscular system controlling song in zebra finches. Brain Res 929(2): 191-201.
- Williams H (1985) Sexual dimorphism of auditory activity in the zebra finch song system. Behav Neural Biol 44(3): 470-484.
- Shaughnessy DW, Hyson RL, Bertram R, Wu W, Johnson F (2019) Female zebra finches do not sing yet share neural pathways necessary for singing in males. J Comp Neurol 527(4): 843-855.
- Chandebois R, Faber J (1983) Automation in animal development. A new theory derived from the concept of cell sociology. Monogr Dev Biol 16: 1-202.
- Eaton S, Jülicher F (2011) Cell flow and tissue polarity patterns. Curr Opin Genet Dev 21(6): 747-752.
- Butler MT, Wallingford JB (2017) Planar cell polarity in development and disease. Nat Rev Mol Cell Biol 18(6): 375-388.
- Goodrich LV, Strutt D (2011) Principles of planar polarity in animal development. Development 138(10): 1877-1892.
- Strutt DI (2001) Asymmetric localization of Frizzled and the establishment of cell polarity in the Drosophila wing. Mol Cell 7(2): 367-375.
- Zallen JA (2007) Planar polarity and tissue morphogenesis. Cell 129(6): 1051-1063.
- Deans MR (2013) A balance of form and function: planar polarity and the development of the vestibular maculae. Semin Cell Dev Biol 24(5): 490-498.
- Raphael Y, Altschuler RA (2003) Structure and innervation of the cochlea. Brain Res Bull 60(5-6): 397-422.
- Axelrod JD (2001) Unipolar membrane association of Dishevelled mediates Frizzled planar cell polarity signalling. Genes Dev 15(10): 1182-1187.
- Ezan J, Montcouquiol M (2013) Revisiting planar cell polarity in the inner ear. Semin Cell Dev Biol 24(5): 499-506.
- Zakir M, Wu LQ, Dickman JD (2012) Morphology and innervation of the vestibular lagena in pigeons. Neurosci 209: 97-107.
- Nonaka S, Shiratori H, Saijoh Y, Hamada H (2002) Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature 418(6893): 96-99.
- McGrath J, Brueckner M (2003) Cilia are the heart of vertebrate left-right asymmetry. Curr Opin Genet Dev. 2003; 13(4): 385-392.
- Levin M, Mercola M (1998) Gap junctions are involved in the early generation of left-right asymmetry. Dev Biol 203(1): 90-105.
- Heuvel van den MP, Sporns O (2013) Network hubs in the human brain. Trends Cogn Sci 17(12): 683-696.
- Shanahan M, Bingman VP, Shimizu T, Wild M, Güntürkün O (2013) Large-scale network organization in the avian forebrain: a connectivity matrix and theoretical analysis. Front Comput Neurosci 7: 89.
- Aboitiz F, Scheibel AB, Fisher RS, Zaidal E (1992) Fiber composition of the human corpus callosum. Brain Res 598(1-2): 143-153.
- Tomasch J (1954) Size, distribution and number of fibres in the human corpus callosum. Anat Rec 119(1): 119-135.
- Sperry RW (1974) Lateral specialization in surgically separated hemispheres. Neurosci, third study program, PP: 1-20.
- Sperry RW (1969) A modified concept of consciousness. Psychol Rev 76(6): 532-536.
- Jarvis ED, Güntürkün O, Bruce L, Csillag A, Karten H, et al. (2005) Avian brains and a new understanding of vertebrate brain evolution. Nat Rev Neurosci 6(2): 151-159.
- Olkowicza S, Kocoureka M, Lucan RK, Porteš M, Fitch WT, et al. (2016) Birds have primate-like numbers of neurons in the forebrain. PNAS Nati Acad Sci USA 113(26): 7255-7260.
- Ariëns Kappers CU, Hubert GC, Crosby EC (1936) The comparative anatomy of the nervous system of vertebrates, including man. MacMillan Co, NY.
- Pearson R (1972) The avian brain. Academic Press, London, NY.
- Güntürkün O, von Eugen K, Packheiser J, Pusch R (2021) Avian pallial circuits and cognition: A comparison to mammals. Curr Opin Neurobio 71: 29-36.
- Nottebohm F, Stokes TM, Leonard CM (1976) Central control of song in the canary, Serinus canarius. J Comp Neurol 165(4): 457-486.
-
Enrico Marani*. Symmetry and Asymmetry, Crossing and Polarity: The Avian Synthesis. Anat & Physiol Open Access J. 2(1): 2025. APOAJ.MS.ID.000527.
-
Cajal’s; stereometric; double twist crossing hypotheses; invariant asymmetry; placodes; avian song; planar polarity; hubs; iris publishers; iris publisher’s group
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.