Review article
Review article
An Afterwards Analysis of Neural Culture States Using Neurofilament Immunocytochemistry
Enrico Marani, Tiboel Siegenbeekstraat 15, 2313 HA, Leiden the Netherlands
Received Date:May 06, 2025; Published Date:May 19, 2025
Abstract
Various culturing and neurophysiological techniques are used to study neural or neuronal networks, often expressed by aggregates. The neural aggregates in chemically defined medium cultures tend to a stabilized amount of neurofilament positive cells and fibres after day 12 in vitro (12 DIV), with a minor difference of 1 DIV related to cell density plating. The total of positive cells and fibres is related to the diameter of the aggregates. The larger the aggregate diameter the higher the neurofilament positivity. This result opens the possibility to check afterwards the quality of cultures.
Keywords:Nervous system; mitochondria; multi electrode arrays; neurofilaments; neurodegenerative diseases
Definitions
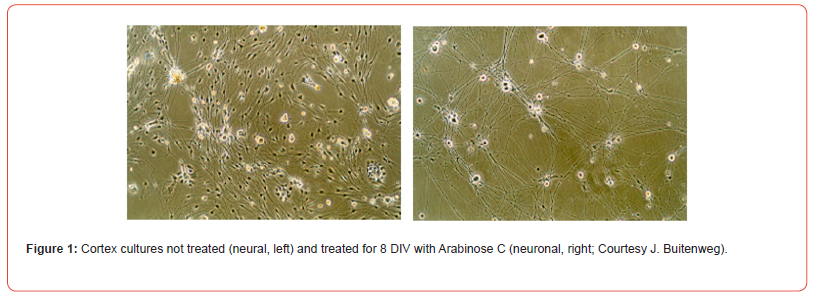
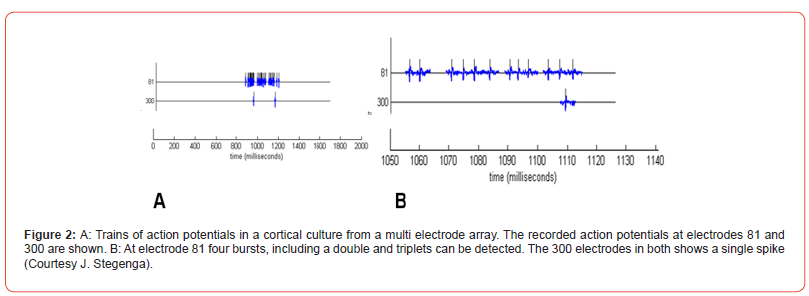
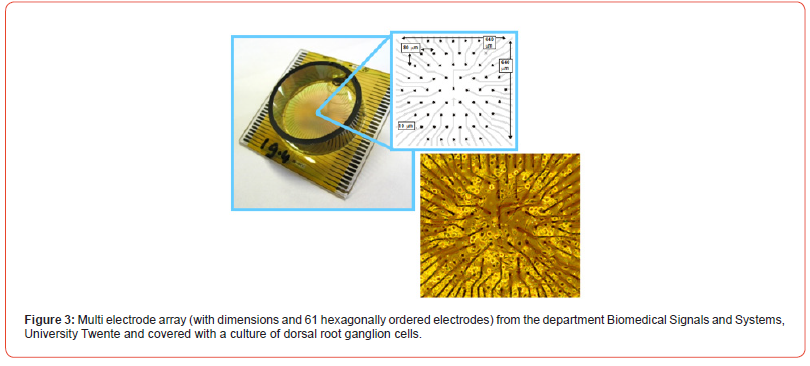
Neural networks differ from neuronal networks: neural networks will contain neurons but also other nervous system cell types. Neuronal networks exist of only neurons. Most networks are made by dissociation of (a part of) the nervous system, thus including other types, among which glia (Figure 1). Bursts: “A burst is a train of closely timed action potentials (spikes). It is convenient to treat doublets (two spikes) and triplets (three spikes) as short bursts. The functional significance of generating a burst of spikes is: i) bursts are a prerequisite to enhance the reliability of the communication between neurons, and ii) burst can be an effective mechanism for selective communication between neurons” [1]. First reliable bursts were noted at 7 days in vitro (DIV) [2] (Figure 2). Multi electrode arrays are 20-80 titanium nitride electrodes on silicon substrate with a Si3N4 insulating glass cover layer. Placed in a square grid with inter-electrode distance of 100μm and electrode diameter of 10μm. Electrodes can be made of Pt, Au, TiN, IrOx or conductive polymers [3]. A glass or plastic ring changed it into a culture chamber (see MEA ring options, multi-channel systems, 2021) (Figure 3).
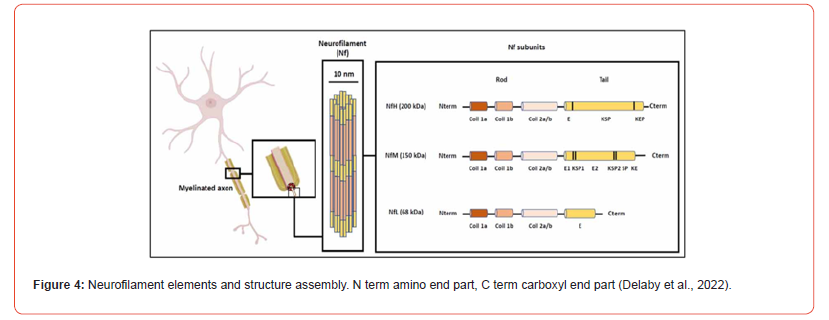
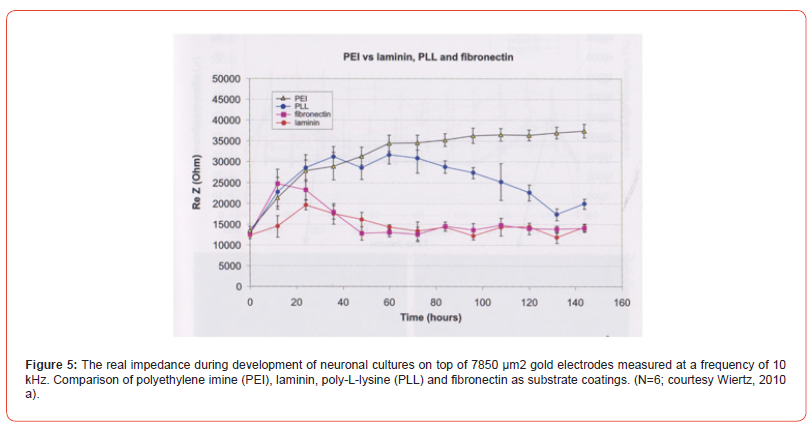
Neurofilament proteins are constructed from five neuronal intermediate filament proteins. Central base is a group of 310 amino acids with amino-and carboxyl ends. Heavy (200 kD), medium (150 kD) and light (70 kD) chain neurofilaments are discerned to which also belong alpha internexin and peripherin. The high and medium neurofilaments are characterized by a strong phosphorylation of the end groups. Neurofilaments, containing a diameter of 80-100 Å, supports neuronal growth of axons, axon and mitochondrial stability, and microtubule quantity. Assembly occurs intracellular and breakdown by the ubiquitin-proteasomal pathway [4]. Neurofilaments are related to various neurodegenerative diseases e.g.: Alzheimer’s disease, ALS and Huntington’s disease [5] (Figure 4). Impedance sensing. The study of the first 6DIV (144 hrs) has been done with impedance sensing on electrodes for several substrate coatings (Figure 5). PEI overcomes the possibility to group into aggregates and electrodes stay occupied. Fibronectine and laminin supports the aggregation or do not inhibit aggregate formation. PLL inhibits aggregate formation till 100-120 hrs. Coating substances do manipulate the aggregate formation within the first 6DIV.
Introduction
In general, various types of neurons together with their glia are brought in culture depending on the parts of the (central) nervous system used, for instance: cortex (various articles, say) [6], spinal cord ganglia [7,8], arcuate nucleus [9] hippocampus [10] and suprachiasmatic neurons [11]. Culture fluids are either MEM- (Minimum Essential Medium) based, generally supplemented with D-glucose, NaHCO3, glutamine and antibiotics (habitually penicillin and streptomycin) to which is added (fetal calf) serum or DMEM (Dulbecco’s Modified Eagle Medium) with analogue additions has been used. Different properties of culture media are out of the scope of this article. Serum-free media (e.g., now regularly produced by industries) [12,13] with or without added NGF (Nerve Growth Factor) are frequently used, surmounting serum impurities and its hormone changes with the advantage to hamper glia over-growth in neural cultures.
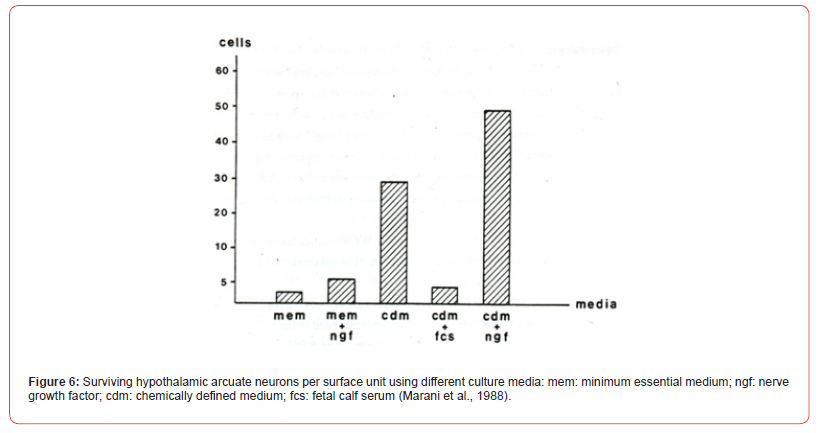
The Chemically Defined Media (CDM) without or with NGF let survive 5 to 10 times more neurons per surface unit respectively compared to MEM with or without NGF or chemically defined medium with added fetal calf serum (Figure 6). CDM importance for cultures of embryonic stem cells is widely accepted [14]. Production of neural precursors from cultured embryonic stem cells needs neural induction techniques [15]. Pure neuronal cultures are also made by mitotic suppression after 1DIV, mostly done by 1-b-arabinofuranosylcytosine (Ara C) for 3 to 4 days or longer (Figure 2) [16]. Purification of other cell types, especially of Schwann cells [17,18], is often a prerequisite for experimental research [19]. Dissociated dorsal root ganglion cells (DRG’s) can regroup and reconstitute a dorsal root ganglion in neural cultures [20,21]. Unambiguousness for the use of the types of culture media does not exist.
An underground or coating is repellent for neurons if it is hydrophobic. In hydrophilic circumstances neurons, but also other CNS cell types, will adhere to the surface. Coatings are natural ones like fibronectin, laminin or non-naturals like poly-D (Figure 5) and poly-L lysine, poly-L-ornithine, and they normally induce aggregates. Poly-Ethylene-Imine (PEI) is strongly hydrophilic provoking a nearly monolayer of neurons. Such a “random” distributed neural monolayer allows the creation of planar structures. Islands with hydrophilic (PEI) surfaces surrounded by hydrophobic (fluorocarbon) underground show that island-distances of over 100 mu cannot be crossed by neuronal neurites [22]. Monolayer neural cultures that self-organize into spontaneously bursting networks are in contrast with micro patterned cultures, by which the structural component as present in the central nervous system is mirrored. In general, the neuronal axons have the tendency to grow straight on, however the printed pattern on the bottom of the culture disk forces axons to follow these patterns.
Turning behavior can be statistically predicted. In structured neuronal networks neurons synapse with each other, neurotransmitters are produced and spontaneous electrical activity develops as in non-structured ones. Structured neuronal networks demonstrate enhanced recordable networks, enhanced cellular activity, increase in neuronal activity, greater survival of neurons, accelerated synaptogenesis, presumably by the five times higher presence of glia [23-26]. Detecting the state of neural networks during development by bursts has frequently been applied (Figure 2) [27]. The global state of networks has been done using time interfalls between bursts, duration and number of its action potentials, and its recruitment rate. Seal enhancement effects are unknown.
Single neurons show: the thinner the sealing gap between neuron and electrode the higher the potential that is measured. Sealing resistance is studied using impedance spectroscopy. Combination with intracellular recording and stimulation contributes to the further understanding of the neuron-electrode interface [28,29]. Impedance sensing in combination with microscopy has been used to study cell-cell and cell-substrate adhesion adding N-CAM protein and its antibody to the neural cultures [30-32]. Still the uncertainty of culture connectivity development and its final out-grown state interfere often with coating application, bursting studies and applied drug results, also due to using different methods and various brain areas, also impeding comparison of results. In short, a general measuring method of the state of the neural culture could be helpful. This article proposes a simple histochemical afterwards analysis method to overcome this doubt.
Material and methods
Cell cultures were prepared by anaesthetizing pregnant WAGrats with aether and the foetuses (E19) were rinsed shortly with 70% ethanol. Under sterile conditions, the cortices were removed from the foetal brains and dissociated by mechanical disruption with a nearly sealed Pasteur pipette. Cell suspensions were centrifugated in chemically defined medium three times (5 min, 1000 rpm) to remove cell debris. One drop (0.07-0.08 ml) of high cell density (106 per cm2) or low cell density (105 cells per cm2) was gently plated on a glass disk coated with poly-D-lysine and placed in a Petri disc. Adherence of cells was allowed for one hour, after which 1-1.5 ml medium per dish was added. Chemical defined medium was used with or without 3.85* 10-4 mM nerve growth factor. Cultures were refreshed each two days and stored at 37o C in a 96-100% humidified and 5% CO2 containing atmosphere.
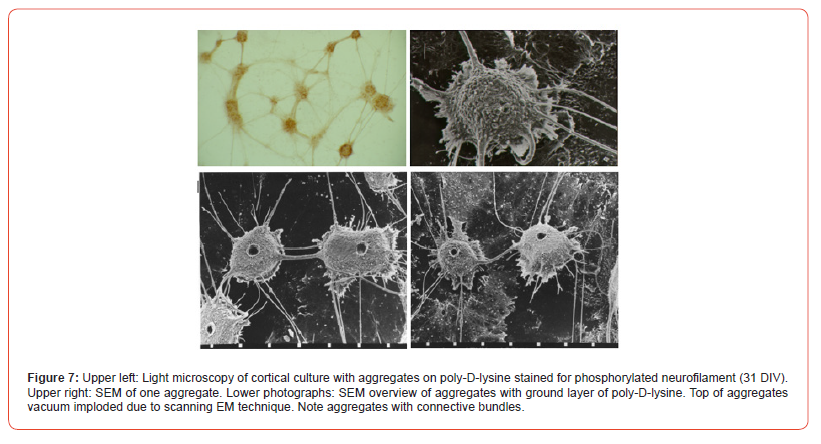
Immunocytochemistry (examples in Figure 7, cortex peroxidase and Figure 8, SCN fluorescence): cultures were rinsed twice with saline and fixated with acetone, 1 hour, 4o C, and overnight dried. Twice disks were rinsed with PBS and once with PBS 0.1% BSA, each for 5 min. Cultures were incubated with primary antibody NF90 (1:10.000). NF 90 has the ability to recognize also the 70kD subunit, which is the first to appear. Next day cultures were rinsed 3x PBS, 1x PBS0.1%BSA and incubated with HRP-conjugated rabbit-ant-mouse antiserum (1:400) for 1 hour, room temperature. Both antibodies were diluted in PBS 0.1% BSA and 1% normal goat serum. After the second incubation cultures were 3x rinsed in PBS and in 50mM Tris-maliate buffer pH 7.6 and incubated for 20 min with 0.04% 3.3-diaminobenzidine-4HCl and 0.02% H2O2 dissolved in the same buffer. For controls of non-specific reactions and endogenous peroxidase the same procedure was performed without first antibody.
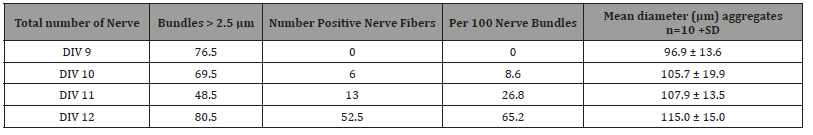
Cultures were counterstained with haematoxylin, differentiated in tap water, dehydrated through graded alcohols to xylene and covered with Depex while placed on object glasses. Quantification of neurofilament positive cell bodies and positive fibres was assessed in one culture per day for finally 12 and 31 DIV. Counts were performed in 10 reaggregates per culture with a low or high cell density. Cell bodies were counted positive if staining was as brown as in 12 DIV cultures from a previous normal series. Within one culture both cells and fibres were counted: reaggregates with low cell density (d ±100 μm N=10 per DIV) and with a high cell density (d ±150-200 μm, N=10 per DIV) were screened and the same aggregates were used to count positive nerve connections and the total of connections. Counting was done independently by two persons. A separate series was produced for 21 and 31 DIV for 150- 200μm/ high density aggregates.
Results
Reaggregates of dissociated fetal (E19) cortical rat neurons showed interconnections by bundles of protrusions (Figure 7). The bundles make synaptic contact within and outside the aggregates, notice the re-looping bundle (Figures 7&8). Within an aggregate interconnection are encountered and dendrites are present. Boutons en-passage and terminal ones are recognized by their varicosity-like appearance (Figure 8) [33]. Positive bundles can be followed into the centre of the few negative aggregates. Within connecting bundles thick and thin neurofilament positive fibres are noted. After the first 5-DIV in culture aggregates start being formed and the first accumulates are interconnected.
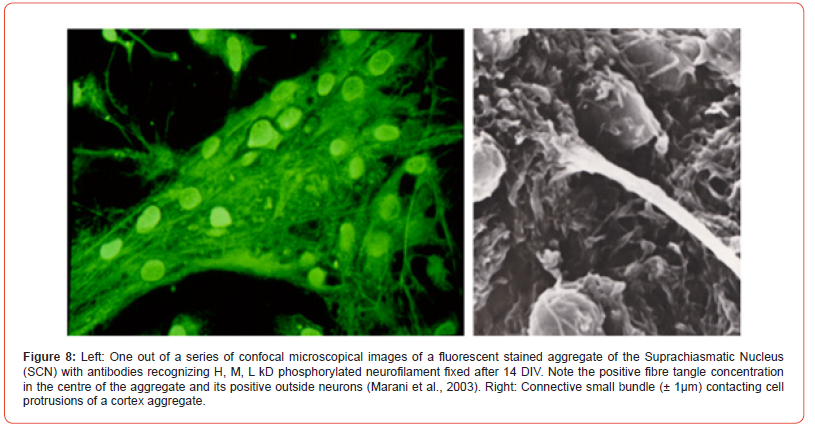
The extensive networks developed by joined axons and dendrites and they supported cell migration, especially near the centres of the cultures. Control procedure for immunoreactivity was negative at 9-DIV and non-specific precipitates were not found. Until 6-DIV aggregates stayed negative with a very light brownish diffuse overall staining. At day 7 in 150-200 μm aggregates and at 8-DIV in 100 μm aggregates the first NF positive perikarya were present in 100 μm ones in a single peripheral islet, containing the low cell density. From 9-DIV on NF positive fibres were observed. Gradually at older DIV’s positivity increased in the centres of welldeveloped aggregates (Tables 1 & 2).
Table 1: Overview of neurofilament positive cells in aggregates of 150-200 μm and 100 μm per DIV.
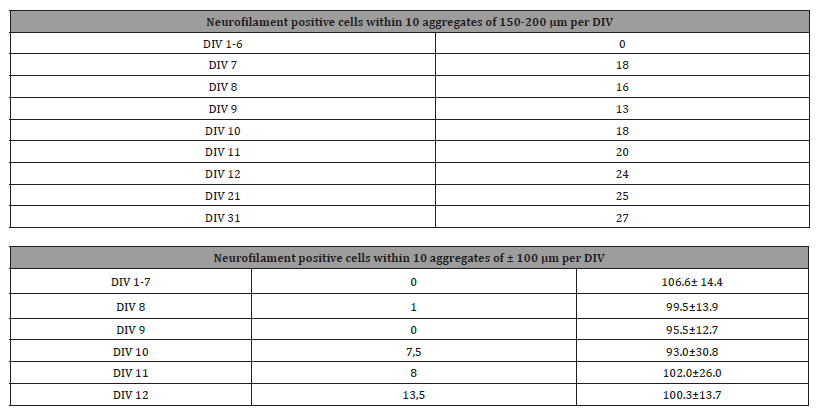
Table 2:Number of total bundles larger than 2.5 μm leaving aggregates and neurofilament positive nerve fibres per aggregate and per 100 nerve bundles, included bundles < 2.5 μm, till DIV 12 for aggregates of ± 100μm (diameter and SD).
Discussion
Neurofilaments are cytoskeletal proteins involved in: microtubule organisation, nerve conduction, neurotransmission and organelle dynamics. The neurofilament genetics presented NF importance in neurodegenerative diseases. NF-L is needed for assembly of the higher kD neurofilament proteins and is studied in relation to degenerative diseases. Developmental control in vivo of the NF-L has a yet an unknown biological meaning [34]. Neuronal cultures do play an important role in these neurofilament studies. Cell density in culture starts influencing the structural end situation: high densities, 106 per cm2, compared to low cell density, 105 cells per cm2, produces in general ± 40-50% more bundles and ± 50% more positive cells and a ± 30% higher number of positive fibres. After 12 DIV large aggregates (150-200μm) show a nearly equal number of positive fibres up to the measured 31 DIV.
Smaller aggregates (100 μm) show ± 50 % less positive cells at day 12 DIV and are considered fully outgrown at 12 DIV [35]. Expression of neurofilament in cells is earlier than the expression in fibre bundles. A delay of 1 DIV is noted. Dissociated neurons need a recovery period in the chemically defined medium of around 7 DIV. This delay phase compares to the delay of neurofilament expression in the rat neural tube. As in the neural tube the expression of neurofilament is first in cells (motoneurons) and later in the spinal cord tracts [36,37]. Heterogeneity in vivo for neurofilament is a property of the developmental and mature rat spinal cord and is refound in the aggregates containing positive and negative elements for neurofilaments. The increase of synapses coincides with neurofilament increase in positive areas.
Checks and Balances
Checking the quality of the cultures afterwards supports
reliable results:
• Control cultures are produced at 12 DIV in the same medium,
coating, type of neural/neuronal cells and kept under the same
culturing conditions.
• Perform neurofilament immunocytochemistry.
• Count positive neural/neuronal cells at several aggregates by
choice small (100μm) or large (150-200μm) and fibres >2,5
μm.
• Use measured normal results in comparison to the experimental
results by its neurofilament immunocytochemistry after
experiments.
• Strong differences are or due to the experiments or to critical
culture situations.
- Izhikevich EM, Desai NS, Walcott EC, Hoppensteadt FC (2003) Bursts as a unit of neural information: selective communication via resonance. Trends Neurosci 26(3): 161-167.
- Stegenga J, Le Feber J, Marani E, Rutten WLC (2008) Analysis of cultured neuronal networks using intraburst firing characteristics. IEEE Trans Biomed Eng 55(4): 1382-1390.
- Blau A (2013) Cell adhesion promotion strategies for signal transduction enhancement in microelectrode array in vitro electrophysiology: An introductory overview and critical discussion. Curr Opin Coll & Interf Science 18(5): 481-492.
- Gafson AR, Barthélemy NR, Bomont P, Carare RO, Durham HD, et al. (2020) Neurofilaments: neurobiological foundations for biomarker applications. Brain 143(7): 1975-1998.
- Delaby C, Bousiges O, Bouvier D, Fillée C, Fourier A, et al. (2022) Neurofilaments contribution in clinic: state of art. Front Aging Neurosci 14: 1034684.
- Jimboo Y, Tateno T, Robinson HPC (1999) Simultaneous induction of pathway specific potentiation and depression in networks of cortical neurons. Biophys J 76(2): 670-678.
- Gross GW, Williams AN, Lucas JH (1982) Recording of spontaneous activity with photoetched microelectrode surfaces from spinal cord neurons in culture. J Neurosci Methods 5(1-2): 13-22.
- Mouveroux JMP, Lakke EAJF, Marani E, Stelzle M (2001) Profuse and selective growth in vitro of rat spinal axons on a micropatterned poly-ethylene imine grid. 23rd Ann Int Conf IEE Eng Med Biol Soc NJ 855-1331.
- Marani E, Corino M, van den Berg RJ, Rietveld WJ, Deenen M, et al. (1988) Ionic conductances in cultured pre-infundibular cells of the hypothalamic arcuate region. Neuroendocrinology 48(4): 445-452.
- Shen M, Piser TM, Seybold VS, Thayer SH (1996) Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J Neurosci 16(14): 4322-4334.
- Walsh IB, van den Berg RJ, Marani E, Rietveld WJ (1992) Spontaneous and stimulated firing in cultured rat suprachiasmatic neurons. Brain Res 588(1): 120-131.
- Romijn HJ, Van Huizen F, Wolters PS (1984) Towards an improved serum free, chemically defined medium for long term culturing of cerebral cortex tissue. Neurosci Biobehav Rev 8(3): 301-344.
- Van Bergen J, Lakke EAJF (1996) Chemically defined culture media: Rational recipes or witches’ brew? Biomed Rev 6: 111-119.
- Proetzel G, Wiles MV (2002) The use of a chemically defined media for the analyses of early development in ES cells and mouse embryos. Methods Mol Biol 185: 17-26.
- Turksen K (2002) Embryonic stem cells: Methods and protocols. Humana Press, New Jersey.
- Schwieger J, Esser KH, Lenarz T, Scheper V (2016) Establishment of a long-term spiral ganglion neuron culture with reduced glial cell number: Effects of AraC on cell composition and neurons. J Neurosci Methods 268: 106-116.
- Vleggeert-Lankamp CLAM, Pego AP, Lakke EAJF, Deenen M, Marani E, et al. (2004) Adhesion and proliferation of human Schwann cells on adhesive coatings. Biomaterials 25(14): 2741-2751.
- Vroemen M, Weidner N (2003) Purification of Schwann cells by selection of p75 low affinity nerve growth factor receptor expressing cells from adult peripheral nerve. J Neurosci Methods 124(2): 135-143.
- Marani E, Lakke EAJF, Eds Mai JK, Paxinos G (2012) Peripheral nervous system topics. in: The human nervous system. PP: 82-140.
- Van Dorp R, Jalink K, Oudega M, Marani E, Ypey DL, et al. (1990) Morphological and functional properties of rat dorsal root ganglion cells cultured in a chemically defined medium. Eur J.Morphol 28(2-4): 430-444.
- Marani E, Deenen M, Maassen JA (1992) The expression of CD15 in dissociated cultured rat dorsal root ganglion cells. Histochem J 24(11): 833-841.
- Ruardij T, Goedbloed MH, Rutten WLC (2000) Adhesion and patterning of cortical neurons on polyethyleneimine-and fluorocarbon-coated surfaces. IEEE Trans Biomed Eng 47(12): 1593-1599.
- Chang JC, Brewer GJ, Wheeler BC (2001) Modulation of neural network activity by patterning. Biosens Bioelectron 16(7-8): 527-533.
- Chang JC, Brewer GJ, Wheeler BC (2006) Neuronal network structuring induces greater neuronal activity through enhanced astroglial development. J Neural Eng 3(3): 217-226.
- Corey JM, Wheeler BC, Brewer GJ (1991) Compliance of hippocampal neurons to patterned substrate networks. J Neurosci Res 30(2): 300-307.
- Mouveroux JMP (2003) Towards controlled patterning of axonal outgrowth in vitro, across microelectrode arrays. Thesis, Leiden University.
- Van Pelt J, Corner MA, Wolters PS, Rutten WL, Ramakers GJ (2004) Longterm stability and developmental changes in spontaneous network burst firing patterns in dissociated rat cerebral cortex cell cultures on multielectrode arrays. Neurosci Lett 361(1-3): 86-89.
- Buitenweg JR, Rutten WLC, Marani E (2002) Extracellular stimulation window explained by a geometry-based model of the neuron–electrode contact. IEEE Trans Biomed Eng 49(12): 1591-1599.
- Buitenweg JR, Rutten WLC, Marani E (2003) Geometry-based finite-element modeling of the electrical contact between a cultured neuron and a microelectrode. IEEE Trans Biomed Eng 50(4): 501-509.
- Wiertz RWF (2010a) Regulation of in vitro cell-cell and cell-substrate adhesion. Thesis, University Twente.
- Wiertz RWF, Marani E, Rutten WLC (2010b) Inhibition of neuronal cell-cell adhesion measured by the microscopic aggregation assay and impedance sensing. J Neural Eng 7(5): 056003.
- Wiertz RWF, Marani E, Rutten WLC (2011) Neural cell-cell and cell-substrate adhesion through N-cadherin, N-CAM and L1. J Neural Eng 8(4): 046004.
- Marani E, Rutten WLC, Deenen M (2003) Neuronal aggregates in cell culture. Trakia J Sci 1: 23-25.
- Van Asperen JV, Kotaich F, Caillol D, Bomont P (2024) Neurofilaments: Novel findings and future challenges. Curr Opin Cell Biol 87: 102326.
- Fedoroff S, Richardson A (2013) Protocols for neural cell culture. Springer Sci Media, LLC, NY.
- Oudega M (1990) Development of the rat spinal cord. Thesis, University Leiden.
- Oudega M, Lakke EA, Marani E, Thomeer RT (1993) Development of the rat spinal cord: immune- and enzyme histochemical approaches. Adv Anat Embryol Cell Biol 129: 1-166.
-
Enrico Marani*. An Afterwards Analysis of Neural Culture States Using Neurofilament Immunocytochemistry. Anat & Physiol Open Access J. 2(1): 2025. APOAJ.MS.ID.000528.
-
Nervous system; mitochondria; multi electrode arrays; neurofilaments; neurodegenerative diseases; iris publishers; iris publisher’s group
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.