 Research Article
Research Article
Transmissibility of Primary Cases Not Completely Vaccinated and Effectiveness of Incomplete Vaccination Against Sars-Cov-2 in Secondary Cases in The Household. Study of 12 Families from February 1 to November 30, 2021 (Before Omicron), in A General Medicine Office in Toledo, Spain: It Is a Very Bad Idea Not to Complete Vaccination
Jose Luis Turabian*
Specialist in Family and Community Medicine, Health Center Santa Maria de Benquerencia, Regional Health Service of Castilla la Mancha (SESCAM), Spain
Jose Luis Turabian, Specialist in Family and Community Medicine, Health Center Santa Maria de Benquerencia, Regional Health Service of Castilla la Mancha (SESCAM), Toledo, Spain.
Received Date: February 12, 2022; Published Date: March 17, 2022
Abstract
Background: Experts radically changed, during 2021, their opinion on the efficacy of a single dose of vaccine, from being sufficient to being insufficient to reliably prevent infection, but its real impact on the protection of family members is not known.
Objective: Epidemiological evaluation of the transmissibility of SARS-CoV-2 from primary COVID-19 breakthrough infection in not fully vaccinated cases to another family member not fully vaccinated and effectiveness of incomplete vaccination schedule in members of home.
Methodology: An observational, longitudinal and prospective study of families with a primary COVID-19 breakthrough infection in not fully vaccinated people case and at least one secondary case of COVID-19 breakthrough infection in not fully vaccinated family member was conducted, from February 1, 2021, to November 30, 2021 (before omicron), in a general medicine office in Toledo, Spain.
Result: 12 families (with 38 people in total) with a primary case of COVID-19 breakthrough infection in not fully vaccinated people were included. Gross secondary attack rate of covid-19 breakthrough infections in not fully vaccinated people after index cases of covid-19 breakthrough infections in not fully vaccinated people within the family was 100%. Effectiveness of the incomplete vaccine against transmission among household among contacts was 1%.
Conclusion: In the context of general medicine in Toledo (Spain), during 2021 (before omicron), not to complete the vaccination was a very bad idea.
Keywords: COVID-19; SARS-CoV-2; Household contact; Secondary attack rate; Vaccination; Breakthrough infection
Introduction
The COVID-19 pandemic, a public health emergency of international concern, is caused by widespread infection with SARS-CoV-2 and the development of an infectious disease of the respiratory tract [1]. As of December 2020, a single dose of the COVID-19 vaccine was thought to be highly effective (although both doses were advised). So, as the Covid-19 vaccines reached more people, the question arose whether the second dose of the vaccines (from Pfizer, Moderna, Astra) could be delayed allowing more people to get vaccinated more quickly. Experts suggested that people should be calm, since even after a single dose of any of these vaccines, they have very high levels of protection [2]. Before the appearance of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant B.1.617.2 (delta), vaccination reduced the transmission of SARS-CoV-2 from vaccinated persons who became infected, potentially by reducing viral loads. Although vaccination still reduces the risk of infection, similar viral loads in vaccinated and unvaccinated people who are infected with the delta variant call into question the extent to which vaccination prevents transmission [3].
However, since March 2021, the coronavirus has continued to mutate. As of early July 2021, the delta variant had become the most dominant strain of SARS-CoV-2 circulating in the US. Moderna and Pfizer’s mRNA vaccines were not specifically designed to prevent the delta variant. While they still generally provide excellent protection after the full two doses [4], new research suggested that a single dose provides less immunity to coronavirus strains than existed in 2021 versus the original strain. Thus, they demonstrated that the emerging Delta variant partially, but remarkably, escaped neutralizing monoclonal antibodies and polyclonal antibodies elicited by previous infection with SARS-CoV-2 or by vaccination [5]. In addition, serum samples from vaccinated individuals neutralized the omicron variant to a much lesser extent than any other variant tested (alpha, beta, or delta) [6].
Consequently, experts modified their advice, suggesting that a single dose of the COVID-19 vaccine from Moderna or Pfizer is not enough to reliably prevent infection [7]. As of December 2021, while in some high-income countries the complete vaccination schedule had already been administered to almost 90% of their population, such as in Spain [8], in low-income countries only slightly more than 6% of the population had received a single vaccine dose [9]. So how effective is a single dose of each of the COVID-19 vaccines? Estimates are controversial and subject to criticism [10] and evidence on the long-term effectiveness of partial vaccination is limited [11]. On the other hand, the transmission of the SARS-CoV-2 virus occurs mainly between people through respiratory droplets expelled through the nose and mouth when coughing, sneezing or talking or when people touch their eyes, nose or mouth after contact with contaminated objects and surfaces [12]. In this context, households are the site of most SARS-CoV-2 transmission globally [13].
Few data exist on the efficacy or lack of efficacy of a single dose of COVID-19 vaccine, and on the transmissibility of SARS-CoV-2 among partially vaccinated persons. An evaluation of the efficacy of the vaccine in a real-world setting is needed. The lack of data is especially relevant in the family/household. In addition, long-term follow-up to assess the long-term efficacy of a single dose is needed to inform a policy of delaying the second dose [14].
In this scenario, this study aims at the epidemiological evaluation of the transmissibility of SARS-CoV-2 from index cases with COVID-19 progression of infection in people not fully vaccinated to another member of the family did not initially infected (assessing the rate of secondary attack) and the effectiveness of the incomplete and complete vaccination schedule at home.
Material and Method
An observational, longitudinal and prospective study of families with a primary COVID-19 breakthrough infection in not fully vaccinated people case and at least one secondary case of COVID-19 breakthrough infection in not fully vaccinated family member was conducted, from February 1, 2021 to November 30, 2021 (before omicron), in a general medicine office in Toledo, Spain, which has a list of 2,000 patients> 14 years of age (in Spain, the general practitioners [GPs] care for people > 14 years of age, except for exceptions requested by the child’s family and accepted by the GP). The GPs in Spain work within the National Health System, which is public in nature, and are the gateway for all patients to the system, and each person is assigned a GP [15].
Outcomes of Interest
1. Assess secondary transmission of SARS-CoV-2 from people vaccinated with an incomplete schedule to other family members also with incomplete vaccination. In this sense, the Secondary attack rate of COVID-19 in the family was determined, which was calculated dividing the number of infection events by the person follow-up time [16].
2. Calculate incomplete vaccine and fully vaccine effectiveness (VE) against infection among household contacts of primary COVID-19 breakthrough infection in not fully vaccinated people cases.
Diagnosis of COVID-19 breakthrough infections in vaccinated people
Because the vaccines require about two weeks reaching their maximum effectiveness, a person is not considered fully vaccinated until two weeks after they completed the recommended number of doses for the vaccine they received [17].
To consider a person as fully vaccinated was required [18]
1. That they have received 2 doses of vaccine separated by a minimum of 19 days if the first dose was BNT162b2 mRNA vaccine (Comirnaty, Pfizer / BioNTech), 21 days in the case of ChAdOx1 nCoV-19 vaccine (Vaxzevria, Oxford / AstraZeneca) or 25 days in the case of mRNA-1273 vaccine (Spikevax, formerly COVID-19 Vaccine Moderna), and that a minimum period of 7 days has elapsed since the last dose if the last dose was with BNT162b2 mRNA vaccine (Comirnaty), or 14 days if it was with ChAdOx1 nCoV-19 vaccine (Vaxzevria) or mRNA-1273 vaccine (Spikevax). People who received a dose of Janssen vaccine (Johnson & Johnson vaccine) more than 14 days ago were also considered fully vaccinated.
2. Or, that having passed the disease they have received a dose of any of the vaccines, after the minimum period equal to that established for the second doses.
3. In the heterologous regimen in which Vaxzevria (Oxford / AstraZeneca) is used in the first dose and mRNA vaccines in the second, it was considered fully vaccinated after 7 days if the second dose was with Comirnaty, or after 14 days if it was with the Moderna vaccine
To consider a person as COVID-19 partially immunized was required: Having received only 1 doses of vaccine [BNT162b2 mRNA vaccine (Comirnaty, Pfizer / BioNTech), or ChAdOx1 nCoV- 19 vaccine (Vaxzevria, Oxford / AstraZeneca) or mRNA-1273 vaccine (Spikevax, formerly COVID-19 Vaccine Moderna), and those 14 days had passed after the first dose; but before the second dose. People who received an only dose of Janssen vaccine (Johnson & Johnson vaccine) were considered fully vaccinated [19].
Diagnosis of COVID-19
Diagnosis was performed with reverse transcriptase polymerase chain reaction (PCR) or antigen testing. Rapid antigen tests began to be carried out for symptomatic patients with less than 5 days of evolution from december, 2021. The PCR tests were performed both in symptomatic patients and in asymptomatic contacts. The cases included confirmed cases and asymptomatic carriers. Information on COVID-19 patients and their contacts was obtained from the registry systems used by general medical services in the consultation.
A symptomatic confirmed case with active infection was considered to be any person with a clinical picture of sudden onset acute respiratory infection of any severity that occurs, among others, with fever, cough or feeling of shortness of breath. Other symptoms such as odynophagia, anosmia, ageusia, muscle pain, diarrhea, chest pain or headache, among others, were also considered symptoms of suspected SARS-CoV-2 infection according to clinical criteria; and a positive PCR or rapid antigen test positive [20].
Secondary attack rate
Secondary attack rate was defined as the number of new cases divided by the number of people exposed to a primary case. The existence of second or third generation cases was not assessed. The cases for the determination of the attack rate included symptomatic cases and asymptomatic cases.
Household contacts
Household contacts were defined as people who shared a residence with the COVID-19 index case. We defined family members as those who had lived with primary cases in a house 4 days before and for more than 24 hours after the primary cases developed illness related to COVID-19. Presumed household transmission via an index case in households was cataloged using the definition of secondary transmission from 1 to 14 days [21]. The onset date of a confirmed case was defined as the date of the first appearance of self-reported clinical symptoms [22]. The onset date for an asymptomatic carrier was defined as the date a positive COVID-19 PCR test was obtained [22].
EV calculation against transmission among household contacts
We calculated the vaccine effectiveness against transmission via the secondary attack rate among close contacts of confirmed index cases:
Secondary attack rate in not fully vaccinated people / Secondary attack rate in not vaccinated people) × 100 [23, 24].
Collected variables
Primary case or secondary case, age, sex, symptoms, chronic diseases (defined as “any alteration or deviation from normal that has one or more of the following characteristics: is permanent, leaves residual impairment, is caused by a non-reversible pathological alteration, requires special training of the patient for rehabilitation, and / or can be expected to require a long period of control, observation or treatment” [25], classified according to the International Statistical Classification of Diseases and Health- Related Problems, CD-10 Version: 2019 [26], symptom duration in days, social-occupancy class (according to the Registrar General’s classification of occupations and social status code) [27, 28], if they were Health Care Workers, problems in the family context and low income household based on the genogram and in the experience of the GP for their continuity of care and knowledge of the family (genogram is a schematic model of the structure and processes of a family, which included the family structure, life cycle and family relational patterns. It was understood that “complex” genograms present families with psychosocial problems) [29- 32], number of family members, ethnic minority, symptomatic / asymptomatic COVID-19, time in days since last vaccine dose, severity of the disease (mild cases: clinical symptoms are mild and no manifestation of pneumonia can be found on images; moderate cases: with symptoms such as fever and respiratory tract symptoms, and the manifestation of pneumonia can be seen on the imaging tests; and severe cases: respiratory distress, respiratory rate ≥ 30 breaths / min; pulse oxygen saturation ≤ 93% with room air at rest; arterial partial pressure of oxygen / oxygen concentration ≤ 300 mmHg) [22] (To simplify comparison, moderate and severe cases were counted together), vaccine type: Comirnaty (Pfizer-BioNTech- BNT162b2 mRNA; Pfizer / BioNTech), Moderna-mRNA-1273 mRNA, Vaxzevria, and Janssen / Johnson & Johnson vaccine (Currently, the European Commission has licensed four vaccines: Comirnaty, Pfizer / BioNTech, licensed December 21, 2020; Moderna vaccine, licensed January 6; AstraZeneca vaccine, licensed 29 December and the Janssen / Johnson & Johnson vaccine, authorized on March 11. In Spain, these four vaccines are currently available, all of which have been approved by the European Medicines Agency [33].
Sample
All families in which a primary case of COVID-19 was diagnosed in a not fully vaccinated person, during the study period, and their all-medical information was available, were included.
Statistical analysis
The bivariate comparisons were performed using the Chi Square test (X2), X2 with Yates correction or Fisher Exact Test when necessary, (according to the number the expected cell totals) for percentages, and the Student test for the mean.
Result
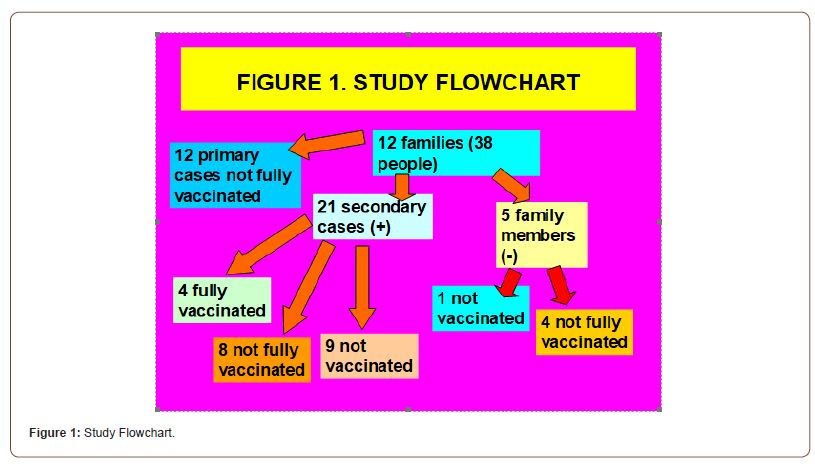
12 families (with 38 people in total) with a primary case of COVID-19 breakthrough infection in not fully vaccinated people were included. Family size was 3.1 +-1.4 (arithmetic mean +- standard deviation), with range: 2-7 people. Of these 12 primary cases, 8 were male and 4 females. There were 21 secondary cases (PCR or positive antigen test) (5 men and 16 women), and 5 negative family contacts. Secondary cases vs. primary cases were statistically significantly more women and had a longer mean time from last vaccination dose to COVID-19 diagnosis. Crude secondary attack rate was 21/26 (81%). Of the 21 secondary cases, 4 had the complete vaccination schedule, 8 had a partial schedule, and 9 were not vaccinated. Of the 5 negative family contacts, 1 was not vaccinated and 4 had a complete vaccination schedule (Figure 1) (Tables 1- 4).
Table 1: Description and Comparison of Incomplete Vaccinated Index Cases and Secondary Cases in The Family.
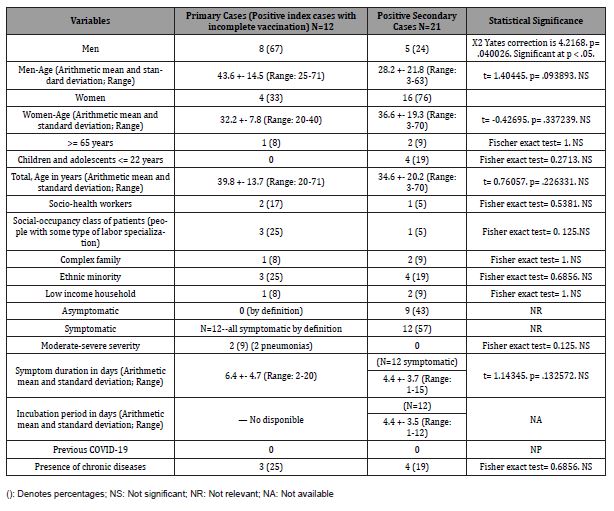
Table 2: Comparison of Symptoms in Incomplete Vaccinated Index Cases and Secondary Cases in The Family.
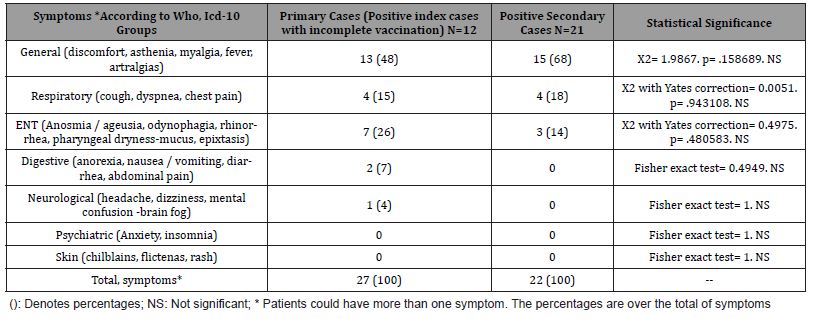
Table 3: Comparison of Chronic Diseases in Incomplete Vaccinated Index Cases and Secondary Cases in The Family.
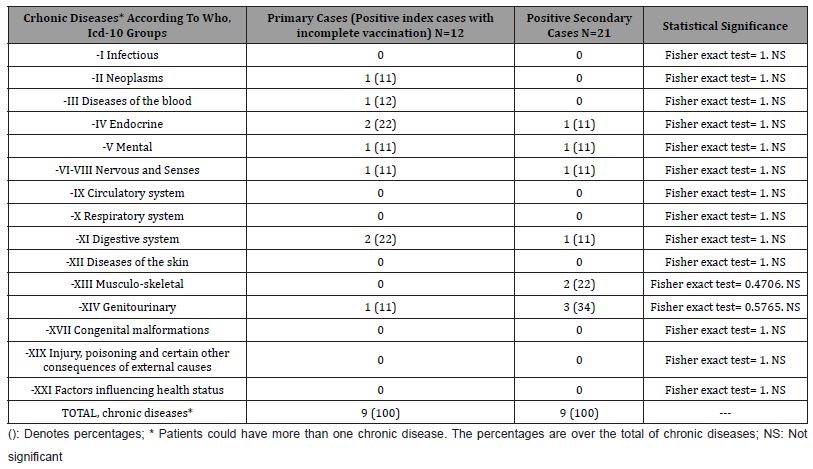
Table 4: Comparison of Vaccination Data in Incomplete Vaccinated Index Cases and Secondary Cases in The Family.
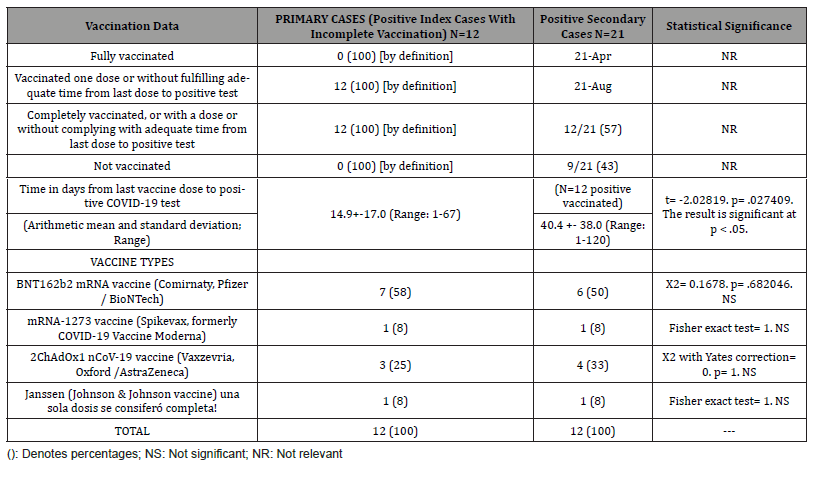
Table 5: Secondary Attack Rate Among Contacts in The Family of Confirmed Index Cases with Covid-19 Breakthrough Infections in Partially (Not Fully) Vaccinated People and Vaccine Effectiveness Against Infection.
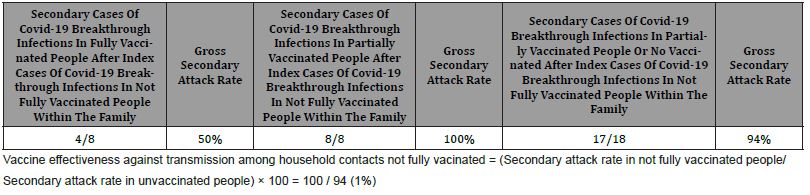
Gross secondary attack rate of COVID-19 breakthrough infections in fully vaccinated people after index cases of covid-19 breakthrough infections in not fully vaccinated people within the family was 50%. Gross secondary attack rate of COVID-19 breakthrough infections in not fully vaccinated people after index cases of COVID-19 breakthrough infections in not fully vaccinated people within the family was 100%. Gross secondary attack rate of covid-19 breakthrough infections in no vaccinated people after index cases of COVID-19 breakthrough infections in not fully vaccinated people within the family was 94%. VE against transmission among household contacts not fully vacinated was [(Secondary attack rate in not fully vaccinated people / Secondary attack rate in not vaccinated people) × 100] 100/ 94 = 1% (Table 5).
Discussion
Real-world evidence from vaccine rollout programs, through the end of 2021, has shown that full COVID-19 vaccinations are highly effective against severe illness, hospitalization, and death from SARS-CoV-2, including B.1.1.7 (alfa) and B.1.617.2 (delta), and reduce both asymptomatic infection and intra-household transmission by reducing the progressive spread of people who have become infected despite vaccination [3, 34].
How second booster doses of vaccines work
When the immune system first encounters a vaccine, it activates two important types of white blood cells. First of all there are the plasmatic B cells, which are mainly focused on the production of antibodies. Unfortunately, this type of cell has a short life, without the second injection, a rapid decline occurs. Then there are T cells that can stay in the body for decades until they hit their target, meaning immunity to vaccinations or infections can sometimes last a lifetime; but usually not many of these cells are produced until the second dose. The booster dose is a way of re-exposing the body to antigens that activate the immune system, to initiate the second part of the response. On the second exposure to the same vaccine, the B cells can divide rapidly and create a second spike in the amount of circulating antibodies. The second dose also kickstarts the B-cell maturation process, which involves selecting the immature ones with the best receptors to bind to a particular pathogen. Meanwhile, memory T cells also proliferate rapidly [10].
Duration in time of protection after the first dose
There are no clear data showing that protection after the first dose is maintained after 21 days. It is possible that the protection that people seem to have suddenly disappears after that point. Reliably calculating how long protection from a single dose might last is further complicated by the fact that all currently approved Covid-19 vaccines use entirely new technology [10]. In our study, secondary cases vs. primary cases had a statistically significant longer mean (arithmetic mean) time from last vaccination dose to COVID-19 diagnosis (40 vs. 14 days).
VE of partial immunization (14 days after the first dose but before the second dose)
The story of Colin Horseman, 85, one of the first people in the world to receive the initial dose of a Covid-19 vaccine (Pfizer- BioNTech) in the UK, who died of COVID-19 less than three weeks later, has been published. He received that first dose; he must have received the second dose two days before his death. In fact, most vaccines require booster doses to work: around 40% of people who have received a single dose of the MMR (measles, mumps and rubella) vaccine are not protected from all three viruses, compared to 4 % of those who have received the second; People in the first group are four times more likely to contract measles than those in the second, and there have been outbreaks in places where a high proportion of people have not completed the full MMR vaccination schedule.
The minimum acceptable level of efficacy of a given vaccine is 50% in preventing COVID-19 as indicated by the World Health Organization [35] and the US Food and Drug Administration (36). In this sense, the figures communicated in the different published data regarding the VE of a single dose of COVID-19 vaccine are confusing and variable according to the definition of effectiveness (number of people who tested positive for the virus, prevention of symptomatic disease, prevention of severe disease, hospitalization), population, type of vaccine and predominant variant of the virus, but in general they are between 36% and 80% [10, 14, 37,45]. We found a gross secondary attack rate of COVID-19 breakthrough infections in not fully vaccinated people after index cases of COVID-19 breakthrough infections in not fully vaccinated people within the family of 100%.
Prevention of transmission of SARS-CoV-2
EV was largely tested by looking at whether they prevented people from developing symptoms, not whether they prevented them from becoming infected with the virus. And we know that it is possible to have an asymptomatic infection. There is no evidence yet that one dose, or even two, of the existing vaccines will stop people from transmitting the virus to other people [3,10].
After a single dose, the SARS-CoV-2 vaccines Vaxzevria (AstraZeneca) and BNT162b2 mRNA vaccine (Comirnaty, Pfizer / BioNTech) impact differently on the peripheral immune compartment. Although both vaccines elicited the induction of spike-specific effector cells and spike binding antibodies, the different compilation of these immunological features suggests that the strategy for spike delivery impacts on how and to what extent immune priming against the main SARS-CoV-2 antigen proceeds [46]. There is no evidence that any of the current COVID-19 vaccines can completely prevent people from becoming infected, and this has implications for our prospects of achieving herd immunity.
If vaccines don’t completely stop transmission, it will increase the number of people we need to vaccinate to truly cross herd immunity thresholds and bring cases down to a level close to zero.
For example, one calculation suggests that for a vaccine that totally eliminates transmission, 60-72% of the population would need to have it to achieve full herd immunity. But if the vaccine was 80% effective, 75-90% of people would need it. However, most of scientists do not expect eliminate virus; for the time being, the goal is to reduce its transmission as much as possible [10]. In our study, VE against transmission among household contacts not fully vaccinated [(Secondary attack rate in not fully vaccinated people / Secondary attack rate in not fully vaccinated people) × 100] was 1%.
Could some vaccines drive the evolution of more virulent pathogens?
Conventional wisdom is that natural selection will eliminate highly lethal pathogens if host death greatly reduces transmission. Vaccines that keep hosts alive but still allow transmission could allow highly virulent strains to circulate in a population. When vaccines prevent transmission, as is the case with almost all vaccines used in humans, this type of evolution toward greater virulence is blocked. But when vaccines leak, allowing at least some transmission of pathogens, they could create the ecological conditions that allow hot strains to emerge and persist. Therefore, the use of leaky vaccines may facilitate the evolution of pathogen strains that put unvaccinated hosts at increased risk of severe disease [47]. In any case, what is certain is that the appearance of variants that avoid vaccines, will be more likely through viral propagation and replication [48].
Can the behavior change after receiving a single dose?
The fact of receiving a single dose can make people think that they will be sufficiently protected, and encourage risky behavior. It should be very clear with the message to be given, which should include two key concepts: You are not going to be protected; and there is no evidence that having received the vaccine prevents you from contracting the virus and transmitting it [10].
Limitations and strengths of the study
1. It must be taken into account that the changes in community transmission during the study period may also imply changes in one direction or another in cautious and personal protection behaviors, which could be an element of confusion in interpreting the results
2. The study may have missed asymptomatic cases that did not attend in GP consultation, as no surveillance or systematic screening was done.
3. There is potential for misclassification of household transmission if the secondary case infection was actually acquired outside the household or if the true household index case was not assessed.
Conclusion
In the context of general medicine in Toledo (Spain), gross secondary attack rate of COVID-19 breakthrough infections in not fully vaccinated people after index cases of COVID-19 breakthrough infections in not fully vaccinated people within the family was 100%. Por otra parte, VE against transmission among not fully vaccinated household contacts [(Secondary attack rate in not fully vaccinated people / Secondary attack rate in not vaccinated people) × 100] was 1%. Consequently it is a very bad idea not to complete the vaccination.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Mostaghimi D, Valdez CN, Larson HT, Kalinich CC, Iwasaki A (2021) Prevention of host-to-host transmission by SARS-CoV-2 vaccines. Lancet Infect Dis 22(2): e52-e58.
- Petri W (2021) How effective is the first shot of the Pfizer or Moderna vaccine? The Conversation.
- Eyre DW, Taylor D, Purver M (2022) Effect of Covid-19 Vaccination on Transmission of Alpha and Delta Variants. N Engl J Med 386: 744-756.
- National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases (2021) COVID-19 Vaccines are Effective.
- Delphine Planas, David Veyer, Artem Baidaliuk, Isabelle Staropoli, Florence Guivel-Benhassine, et al. (2021) Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 596(7871): 276-280.
- Rössler A, Riepler L, Bante D, von Laer D, Kimpel J (2022) SARS-CoV-2 Omicron Variant Neutralization in Serum from Vaccinated and Convalescent Persons. N Eng J Med 386(7): 698-700.
- Petri W (2021) How effective is the first shot of the Pfizer or Moderna vaccine? The Conversation; Updated July 14.
- COVID-19 vaccination strategy in Spain (2022) COVID19 vaccination executive report. Ministerio de Sanidad.
- Mohamed S, Dummett M (2021) New variants of COVID-19 exist in part because Big Pharma puts profit before life. Amnistía Internacional España 21 de diciembre.
- Gorvett Z (2021) How effective is a single vaccine dose against COVID-19? BBC 15th January.
- (2021) European Centre for Disease Prevention and Control Partial COVID-19 vaccination, vaccination following SARS-CoV-2 infection and heterologous vaccination schedule summary of evidence.
- UK Health Security Agency (2021) COVID-19 vaccination programme Information for healthcare practitioners Republished Version 3.11.
- Anika Singanayagam, Seran Hakki, Jake Dunning, Kieran J Madon, Michael A Crone, et al. (2022) Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: a prospective, longitudinal, cohort study. Lancet Infect Dis 22(2): 183-195.
- Amit S, Regev-Yochay G, Afek A, Kreiss Y, Leshem E (2021) Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet 397(10277): 875-877.
- Turabian JL (1995) Notebooks of Family and Community Medicine. An introduction to the principles of Family Medicine. Madrid Díaz de Santos.
- Nandí-Lozano E, Espinosa LE, Viñas-Flores L, Avila-Figueroa C (2002) Acute respiratory infection in children who go to a child development center. Salud Publica Mex 44(3): 201-206.
- South Carolina Department of Health and Environmental Control (2021) Breakthrough cases Tracking disease infection after vaccination.
- Ministerio de Sanidad (2021) COVID-19 early detection, surveillance and control strategy. Actualizado a 23 de julio de 2021. Ministerio de Sanidad Españ
- Estrategia devacunación COVID-19 (2021) What vaccines will we have available in Spain? Actualizació
- Instituto de Salud Carlos III (2020) Strategy for early detection, surveillance and control of COVID-19 Ministerio de Sanidad. Españ
- Lauren A Paul, Nick Daneman, Kevin L Schwartz, Michelle Science, Kevin A Brown, et al. (2021) Association of Age and Pediatric Household Transmission of SARS-CoV-2 Infection. JAMA Pediatr 175(11): 1151-1158.
- Suling Mao, Ting Huang, Heng Yuan, Min Li, Xiaomei Huang, et al. (2020) Epidemiological analysis of 67 local COVID-19 clusters in Sichuan Province, China. BMC Public Health 20(1): 1525
- Bianca E P Snijders, Alies van Lier, Jan van de Kassteele, Ewout B Fanoy, Wilhelmina L M Ruijs, et al. (2012) Mumps vaccine effectiveness in primary schools and households, the Netherlands, 2008. Vaccine 30(19): 2999-3002.
- Brechje de Gier, Stijn Andeweg, Rosa Joosten, Ronald Ter Schegget, Naomi Smorenburg, et al. (2021) Vaccine effectiveness against SARS-CoV-2 transmission and infections among household and other close contacts of confirmed cases, the Netherlands. Euro Surveill 26(31): 2100640.
- Strauss AL (1984) Chronic illness and the quality-of-life St Louis the CV Mosby Company.
- WHO (2019) International Statistical Classification of Diseases and Health-Related Problems ICD-10 Version.
- Royal Collage of General Practitioners (1986) The Classification and Analysis of General Practice Data Occasional Paper 26.
- Donaldson RJ, Donaldson LJ (1983) Essential Community Medicine Lancaster MTP Press.
- Turabian JL (2017) Family Genogram in General Medicine a Soft Technology that can be Strong an Update. Res Med Eng Sci 3(1).
- Russell LT (2020) Capturing Family Complexity in Family Nursing Research and Practice. J Fam Nurs 26(4): 287-293.
- Watts C, Shrader E (1998) How to do (or not to do) The genogram: a new research tool to document patterns of decision-making, conflict, and vulnerability within households. Health Policy Plan 13(4): 459-64.
- H McIlvain, B Crabtree, J Medder, K C Stange, W L Miller (1998) Using practice genograms to understand and describe practice configurations. Fam Med 30(7): 490-6.
- Consejería de Sanidad Castilla La Mancha (2021) Vaccination campaign against COVID-19.
- Shuo Feng, Daniel J Phillips, Thomas White, Homesh Sayal, Parvinder K Aley, et al. (2021) Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med 27(11): 2032-2040.
- World Health Organization (2021) WHO target product profiles for COVID-19 vaccines Updated.
- Food and Drug Administration (2020) Development and licensure of vaccines to prevent COVID-19 guidance for industry.
- Mark G Thompson, Jefferey L Burgess, Allison L Naleway, Harmony L Tyner, Sarang K Yoon, et al. (2021) Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers - Eight U.S. Locations. MMWR Morb Mortal Wkly Rep 70(13): 495-500.
- Jamie Lopez Bernal, Nick Andrews, Charlotte Gower, Eileen Gallagher, Ruth Simmons, et al. (2021) Effectiveness of Covid-19 vaccines against the B.1.617.2 (delta) variant. N Engl J Med 385(7): 585-594.
- Ontario Agency for Health Protection and Promotion (Public Health Ontario) (2021) COVID-19 real-world vaccine effectiveness -what we know so far Toronto ON Queen’s Printer for Ontario.
- ZOE COVID Study (2021) Cases rising rapidly among those with incomplete vaccinations.
- Gabriel Chodick, Lilac Tene, Tal Patalon, Sivan Gazit, Amir Ben Tov, et al. (2021) Assessment of Effectiveness of 1 Dose of BNT162b2 Vaccine for SARS-CoV-2 Infection 13 to 24 Days After Immunization. JAMA Netw Open 4(6): e2115985.
- Fernando P Polack, Stephen J Thomas, Nicholas Kitchin, Judith Absalon, Alejandra Gurtman, et al. (2020) Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383: 2603-2615.
- Public Health England (2020) Annex A Report to JCVI on estimated efficacy of a single dose of Pfizer BioNTech (BNT162b2 mRNA) vaccine and of a single dose of ChAdOx1 vaccine (AZD1222).
- Dan-Yu Lin, Yu Gu, Bradford Wheeler, Hayley Young, Shannon Holloway, et al. (2022) Effectiveness of Covid-19 Vaccines over a 9-Month Period in North Carolina. N Engl J Med p. 2117128.
- Agencias (2021) A dose of the vaccine only protects against variants in people who have overcome Covid-19 In those without previous Covid-19, the immune response was less strong after a first dose, potentially leaving them at risk of contracting the variants. Consalud Es.
- Michael Müller, Johann Volzke, Behnam Subin, Silke Müller, Martina Sombetzki, et al. (2022) Single-dose SARS-CoV-2 vaccinations with either BNT162b2 or AZD1222 induce disparate Th1 responses and IgA production. BMC Med 20(1): 29.
- Andrew F Read, Susan J Baigent, Claire Powers, Lydia B Kgosana, Luke Blackwell, et al. (2015) Imperfect Vaccination Can Enhance the Transmission of Highly Virulent Pathogens. PLoS Biol 13(7): 13(7): e1002198.
- Burioni R, Topol EJ (2021) Has SARS-CoV-2 reached peak fitness? Nat Med 27(8): 1323-1324.
-
Jose Luis Turabian. Transmissibility of Primary Cases Not Completely Vaccinated and Effectiveness of Incomplete Vaccination Against Sars-Cov-2 in Secondary Cases in The Household. Study of 12 Families from February 1 to November 30, 2021 (Before Omicron), in A General Medicine Office in Toledo, Spain: It Is a Very Bad Idea Not to Complete Vaccination. Annal of Pub Health & Epidemiol. 1(5): 2022. APHE.MS.ID.000523.
-
COVID-19, SARS-CoV-2, Household contact, Secondary attack rate, Vaccination, Breakthrough Infection, Vaccines, Respiratory Syndrome, Omicron, Symptomatic Cases, Transmission, Symptoms, Personal Protection, Humans
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






