 Research Article
Research Article
Temporal Trends of Esophagus Cancer Morbimortality in the State of Santa Catarina in the Period of 2009-2018
Lorenzo Becker Della Giustina1, Thiago Mamôru Sakae¹*, Luigi Becker Della Giustina¹ and Flávio Ricardo Liberali Magajewski¹
1Department of Medicine, University of Southern Santa Catarina, Tubarão, Brazil
Thiago Mamôru Sakae, Department of Medicine, University of Southern Santa Catarina, Tubarão, Brazil.
Received Date: February 01, 2021; Published Date: March 5, 2021
Abstract
Background: Esophageal cancer (EC) is one of the most common malignant tumors in the world and is associated with a late diagnosis and poor prognosis in most cases, with an average 5-year survival ranging from 15 to 20%. The main risk factors are smoking, alcoholism and gastroesophageal reflux disease. Worldwide, epidemiological disparities of the disease were observed according to geographic location.
Objective
1) To analyze the temporal trend of esophageal cancer morbidity and mortality in Santa Catarina in the period 2009-2018.
2) To define the sociodemographic profile of affected patients, according to sex, age group, ethnicity and macro-region of occurrence.
3) To analyze the temporal trend of risk rates for hospitalizations and deaths.
4) To determine the proportion of hospitalizations in ICU beds, the proportion of diagnostic, clinical and surgical procedures, and the average length of stay.
5) To detail the proportional distribution of cases of hospitalization for the neoplasm, according to its topographic location.
6) To evaluate clinical variables and the outcome of patients with esophageal cancer undergoing treatment in Santa Catarina.
7) To correlate the time series of hospital morbidity of hospitalizations for esophageal cancer with those of mortality for this group of neoplasms.
Methods: Observational study of ecological type with quantitative approach and time series analysis. It studied the population resident in Santa Catarina with 20 years of age or older who was hospitalized for esophageal cancer in the period 2009–2018 in hospitals in the state of Santa Catarina (SC), whose procedures were financed by SUS, and the population residing in Santa Catarina with 20 years of age or older who died having esophageal cancer as the basic cause, according to the death certificates issued in the period 2009– 2018 in the state.
Results: During the study period, 8,920 hospitalizations and 3,644 deaths from esophageal cancer were registered. The average hospitalization rate for males (30.48/100,000 inhab) was 4 times higher than for females (7.57). The highest average rates of hospitalizations occurred in the 60 to 79 age group (66.43) and in the macro-regions of the Grande Oeste (37.05), the Meio Oeste e Serra Catarinense (23.66). The average length of stay for hospitalizations was 6.82 days in the period studied. The average mortality rate was higher for the population aged 80 years or older (44.85). The majority of deaths were registered in the population with white skin color (n = 3,202; 87.87%), and the average lethality rate of non-whites (48.95%) was approximately 22.55% higher than the found in the population with white skin (39.94%).
Conclusion: The results showed that the sociodemographic profile of patients suffering from esophageal cancer in Santa Catarina in the period 2009-2018, according to available variables, was men, aged between 60 and 79 years, of white ethnicity, and residents of the Grande Oeste macroregion. There was a temporal trend of stability in the hospitalization rates, proportion of intensive care and clinical and surgical procedures. There was a temporal trend of reduction in mortality, lethality rates and average length of stay.
Headings: Esophageal cancer; Morbidity and mortality; Temporal trend
Introduction
Esophageal cancer (EC) is one of the most common malignant tumors and one of the main causes of cancer death worldwide [1]. The first description of esophageal cancer probably occurred in Egypt more than 5,000 years ago [2]. Due to its anatomical and histopathological particularities, EC is associated with a late diagnosis and in advanced stages, requiring extensive treatments, a decrease in quality of life and poor prognosis in most cases. The five-year survival of the EC varies from 15 to 20% [3-6]. The GLOBOCAN study, which estimated incidence and mortality from 36 types of cancer in 185 countries, pointed out that in 2018 there were 572,034 new diagnosed cases and 508,585 deaths from EC, which placed EC as the 7th most frequently diagnosed cancer, and the sixth leading cause of cancer death in the world, representing 5.3% of cancer deaths in the same year. Approximately 70% of EC cases occur in men [1]. In the world, some African countries have the highest incidences, around 24 cases per 100,000 inhabitants [7]. In South America, the regions with the highest incidence are Uruguay and Southern Brazil [8].
In Brazil, Rio Grande do Sul is the state with the highest incidence, and the gross rate in 2018, according to the Brazilian National Cancer Institute (INCA), it was 20.15 and 6.91 per 100,000 for males and for females, respectively, about 2 times that of the state of São Paulo. The INCA incidence estimates for EC in 2018 in the state of Santa Catarina were 15.08 and 3.44 per 100.000 inhabitants for males and females, respectively.
Squamous cell carcinoma (SCC) and adenocarcinoma (AC) represent approximately 90% of esophageal malignancies [9]. Smoking and alcohol consumption, two carcinogenic agents when in contact with the esophageal mucosa, especially if combined, are the main risk factors for esophageal SCC. In addition, mechanical injuries to the esophagus (for example: achalasia, radiotherapy and swallowing hot drinks or sodium hydroxide (caustic soda) increase the susceptibility of esophageal cells to carcinogenic agents [10]. According to the study by Chen et al, in a 2015 metaanalysis, an association between consumption of drinks and food at high temperatures and SCC type EC was significant in the Asian and South American population (OR: 2.06; 95% CI: 1, 62-2.61 and OR: 1.52; 95% CI: 1.25-1.85, respectively), but it was not significant in the European population (OR: 0.95; 95% CI: 0.68- 1.34) [11]. Dietary factors such as the consumption of fruits and vegetables are protective, while the consumption of red meat or processed meat may be associated with increased risk, but the association shown in some studies was weak [12,13]. Genetic factors are also involved in susceptibility to EC [14]. The control of smoking and drinking are the most effective primary prevention measures to date [15,16]. As for adenocarcinoma, obesity and gastroesophageal reflux disease were thought of as the most relevant risk factors for esophageal AC [17,18]. Helicobacter Pylori infection, and possibly the use of nonsteroidal anti-inflammatory drugs, are protective factors [19,20].
The economic impact of esophageal cancer for public and private health organs and institutions is high in most cases, mainly due to the anatomical, epidemiological and pathophysiological characteristics of the disease. Affected patients often need hospitalization, enteral or parenteral nutritional therapy, medications for symptomatic control, invasive surgical approaches with the need for trained multidisciplinary teams, radiotherapy and / or chemotherapeutic approach depending on the stage of tumor evolution, treatment of possible metastases and interventions in possible associated diseases in smoking and/or alcoholic patients [21]. In addition, patients in advanced stages may be dependent on admission to the intensive care unit (ICU) for long periods.
In addition to health care expenses, the economic impact is even greater if we consider that a significant part of the patients is part of the economically active population, resulting in increased indirect economic costs. The study by Tramotano et al. calculated the direct cost of treating patients with EC in the United States according to the stage of esophageal cancer of types SCC and AC, separately. The results were converted to 2018 US dollar amounts and pointed to an average total monthly cost in AC ranging from $ 70,280 in stage I to $ 55,328 in stage IV, and in SCC ranging from $ 84,538 in stage I to US $ 43,992 in stage IV [22]. Thus, esophageal cancer proves to be a serious disease, currently without public policies for population screening in Brazil, and with an important economic impact for the Brazilian Unified National Health System (SUS), showing that it is of public interest to analyze the temporal trend of morbidity and mortality due to esophageal cancer in the state of Santa Catarina, giving visibility to this problem and stimulating discussions in the scientific and health fields about new public health policies for the disease, optimization of expenses and integral treatment for affected patients. This study aimed to analyze the temporal trend of morbidity and mortality from esophageal cancer in Santa Catarina in the period 2009-2018 and, based on that, to define the sociodemographic profile of affected patients, the clinical evolution according to the macroregion of occurrence, in addition to determine the proportion of procedures performed, the proportional distribution of cases according to the topographic location and the average length of hospital stay.
Methods
Observational study of ecological type with quantitative approach and time series analysis. It studied the population resident in Santa Catarina with 20 years of age or older who was hospitalized for esophageal cancer (group C15 by the International Classification of Diseases-ICD 10) in the period 2009–2018 in hospitals in the state of Santa Catarina (SC), whose procedures were financed by SUS, and the population residing in Santa Catarina with 20 years of age or older who died having esophageal cancer as the basic cause (C15 by - ICD 10), according to the death certificates issued in the period 2009– 2018 in the state.
For the analysis of the time series of the rates, hospitalization for EC according to sex (female and male), age (20 to 39 years; 40 to 59 years; 60 to 79 years and 80 years or more), the macro-region of residence (Sul, Grande Florianópolis, Vale do Itajaí, Foz do Itajaí, Nordeste, Planalto Norte, Serra Catarinense, Meio Oeste e Grande Oeste), the need for hospitalization in intensive care unit beds (yes and no), diagnostic, clinical and surgical procedures (yes and no), length of stay (full days), ethnicity (white and non-white), deaths (frequency) and the population of Santa Catarina according to the same categories of gender, age groups, ethnicity and macro-region of residence were defined as dependent variables of the study. As an independent variable, the study period (2009 to 2018) was established.
Data collection was performed through access to public databases of the Unified Health System (SUS), being them: Hospital Information System (SIH) and Mortality Information System (SIM), both managed by DATASUS. The utility data for the study were obtained with the aid of the TABWIN software, which was installed on the researcher’s computer together with the definition files for reading the morbidity and mortality data. 120 files of reduced information of Hospitalization Authorizations (AIH) were used, and 10 files related to the annual death certificates (DO) corresponding to the state of SC. After being extracted and tabulated, the data were exported to the MS-Excel program, which was used to organize the data in spreadsheets, as well as to perform calculations, adjustments and to represent the results in the form of tables. For the calculation of rates and proportions, the ratio between the frequency of studied events (morbidities, mortality, type of procedure, etc.) by the population subject to the risk of occurrence of events was used, and the result was multiplied by the constant of 100,000 for rates, or 100 for proportions. The results were analyzed using the IBM - Statistical Package for the Social Sciences (SPSS). Version 18.0 program.
For temporal analysis, Spearman’s correlation coefficient (value 1 was considered a perfect correlation, values ≥ 0.800 and <1 a very strong correlation, values ≥ 0.645 and <0.800 a strong correlation, values ≥ 0.500 and <0.645 moderate correlation and <0.500 weak correlation), the coefficient of determination (R2), by simple linear regression (which percentually indicates the alignment of the studied rates in relation to the evolution of years in the period studied) and the p-Value from analysis of variance (ANOVA) were used. Values of p <0.05 were considered significant. As the present study is of the ecological type, based on secondary data in the public domain, without identification of the participants and used population aggregates as the unit of analysis, it was not necessary to assess the project by the Research Ethics Committee Involving Human Beings, in accordance with the terms of CNS Resolution 510/2016 (Article 1 Items II, III and V).
Results
During the period studied (2009-2018), a total of 4,371,365 hospitalizations were performed in the state of Santa Catarina, with 299,037 hospitalizations (6.84% of the total) corresponding to hospitalizations for neoplasms and, of these, 8,920 hospitalizations (0.20% of the total) corresponded to hospitalizations whose main cause was group C 15 - Malignant Esophageal Neoplasm, according to the ICD-10th Revision.
In the same period, using the Mortality Information System (SIM), 348,253 deaths were recorded in SC, 75,959 deaths from neoplasms (21.8% of the total), and of these, 3,644 deaths (1.0% of the total) by EC, representing 4.79% of deaths due to neoplasms that occurred in that period in the state. The average mortality rate for all types of cancer in the state in the period studied was 161.52 / 100,000 inhabitants, and the EC contributed with 7.75 deaths/100,000 inhabitants. Considering the hospitalization rate approximating the cancer incidence rate, the average mortality rate for cancer in the state in the period studied was 9.27%, while the average mortality rate for EC was 40.85%.
The proportional distribution of cases of hospitalization for esophageal neoplasms in Santa Catarina, in the studied period, according to their topographic location, was as follows: invasive esophageal lesion, 6,528 cases (73.2%); cervical portion of the esophagus, 1,002 cases (11.2%); thoracic portion of the esophagus, 741 cases (8.3%); abdominal portion of the esophagus, 649 cases (7.3%). (Table 1) shows the rates of hospitalization for esophageal cancer according to the year, sex and age group that occurred in Santa Catarina in the period 2009-2018. During the studied period, there was no correlation of hospitalization rates by sex (Spearman = 0.370 for males, and 0.248 for females). The average hospitalization rate for males was 4.02 times higher than for females. There was a moderate correlation in the evolution of hospitalization rates for EC in the age group from 40 to 59 years old (Spearman = -0.552). There was a strong correlation in the evolution of hospitalization rates due to EC in the age group of 80 years or more (Spearman = 0.661). The average hospitalization rate by age group was higher between 60-79 years old. (Table 2) shows the rates of hospitalization for EC according to the year of occurrence and macroregion occurred in SC in the period 2009-2018.
Table 1: Hospitalization rates due to esophagus cancer (x100.000) by year of occurrence, sex and age group. Santa Catarina, 2009-2018.
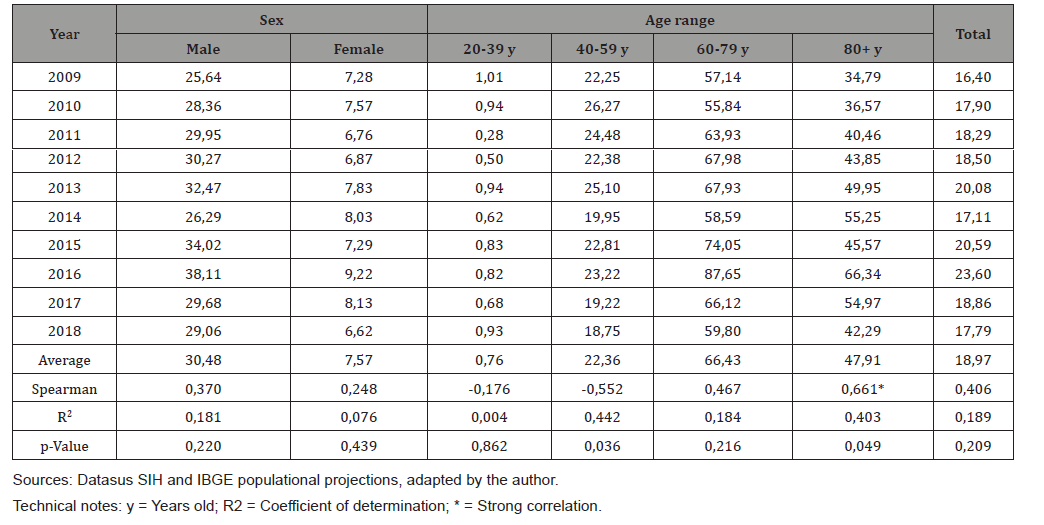
Table 2: Hospitalization rates due to esophagus cancer (x100.000) by year of occurrence and macroregion. Santa Catarina, 2009-2018.
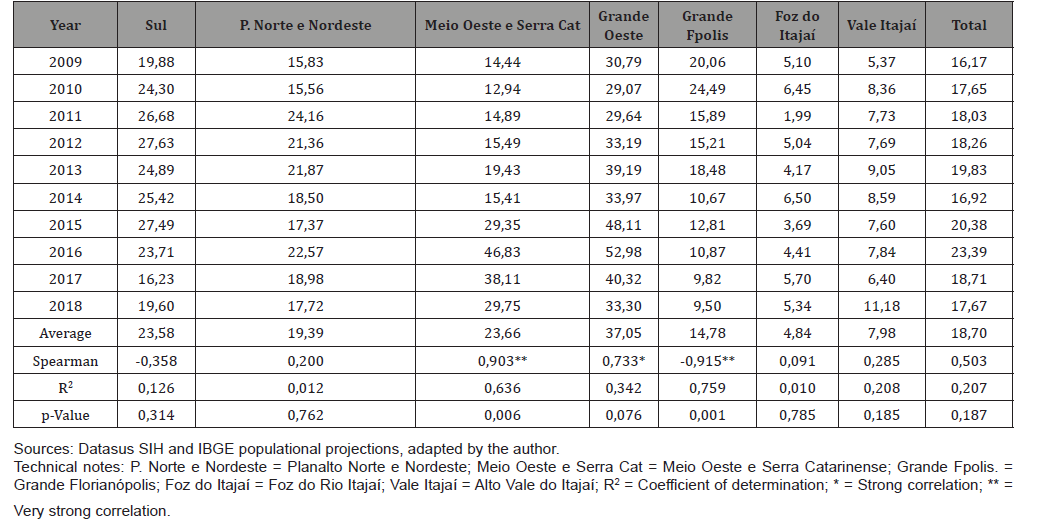
The analysis of hospitalization rates by macroregion indicated a very strong correlation with the period studied in the Meio Oeste and Serra Catarinense (Spearman = 0.903), and the coefficient of determination (R2) indicated that time explained the evolution of rates in 63.6% (R2 = 0.636). On the other hand, hospitalization rates indicated a very strong negative correlation with the evolution of time in the Grande Florianópolis macro-region (Spearman=-0.915) with statistical significance (p=0.001) and the determination coefficient (R2) indicated that time explained the evolution of rates by 75.9% (R2=0.759). The highest average hospitalization rate in the period was found in the Grande Oeste macro-region, which was 7.85 times higher than in Foz do Rio Itajaí and 1.56 times higher than the macro-region with the 2nd highest average rate, the Meio Oeste and Serra Catarinense.
Table 3 shows the proportions of hospitalizations in ICU beds (%), procedures (%) and average stay (in days) related to hospitalizations for EC according to the year of occurrence in SC in the period 2009-2018. Regarding diagnostic procedures, there was a strong negative correlation (Spearman = -0.682) and, according to the coefficient of determination, time explained the evolution of rates by 66.2% (R2 = 0.662). The average length of hospital stays tended to decrease in the period (Spearman = -0.915), with time explaining the evolution of rates by 86.3% (R2 = 0.863).
Table 4 shows the mortality rates due to EC (x100,000) according to the year of occurrence, sex and age group that occurred in Santa Catarina in the period 2009-2018. Mortality rates by sex showed a downward trend in the study period for males (Spearman=-0.758). The average mortality rate by sex was 4.14 times higher in males than in females. The mortality rate by age showed a downward trend in the study period with a strong correlation and statistical significance in the 40-59 age group (Spearman = -0.673 and p-value = 0.022), and a very strong correlation and statistical significance (Spearman = - 0.830 and p-Value = 0.002) in the 60-79 age group. The average mortality rate was higher for the population aged 80 years or older.
Table 3: Proportion of hospitalizations in ICU beds (%), procedures (%) and average length of stay (in days) related to esophageal cancer hospitalizations by year of occurrence. Santa Catarina, 2009-2018.
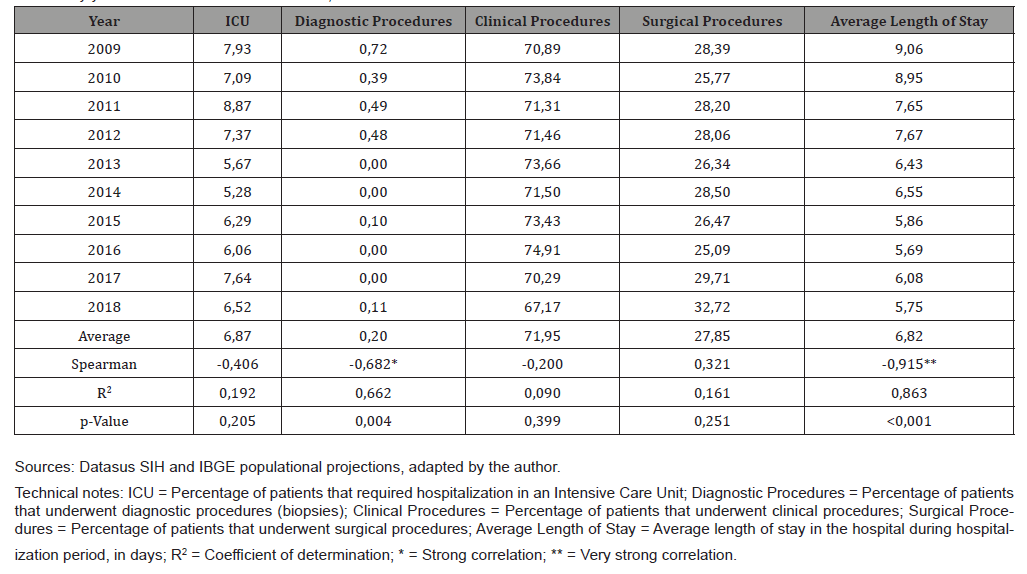
Table 4: Mortality rates due to esophagus cancer (x100.000) by year of occurrence, sex and age group. Santa Catarina, 2009-2018.
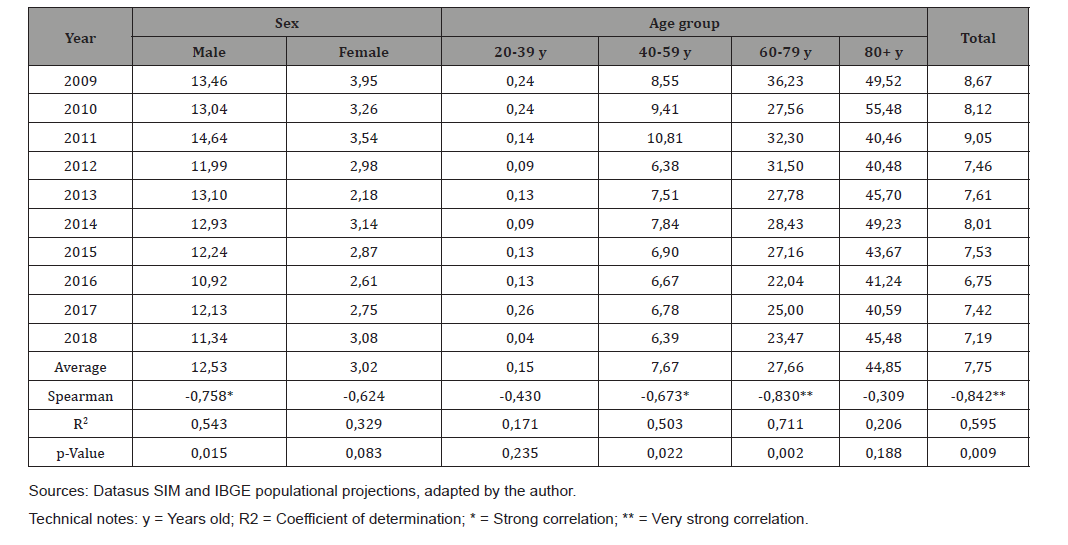
Table 5: Letality rates due to esophagus cancer (x100) by year of occurrence, sex and age group. Santa Catarina, 2009-2018.
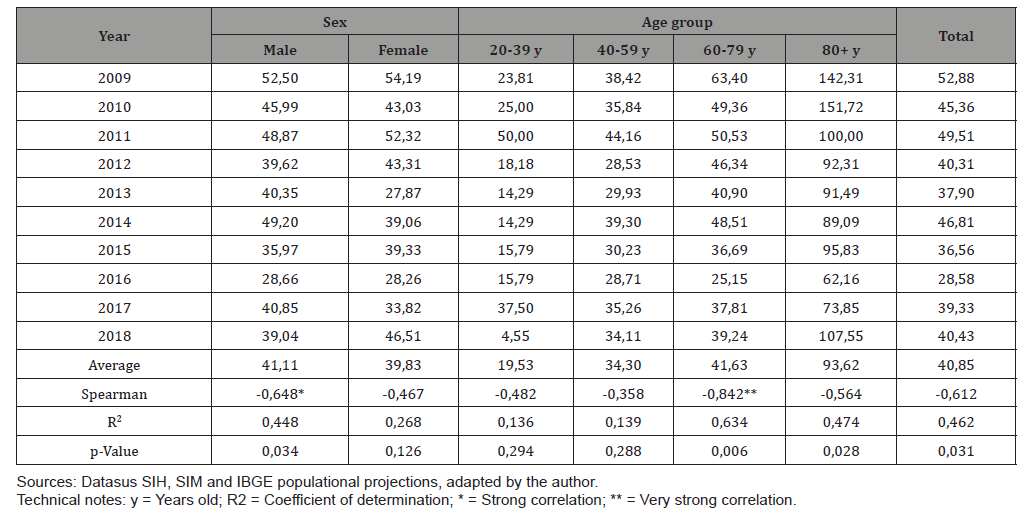
Mortality rates due to esophageal neoplasm in Santa Catarina, during the studied period, showed a tendency to decrease with a very strong correlation (Spearman = -0.842) with statistical significance (p-value = 0.009). (Table 5) shows the lethality rates by EC (x100) according to the year of occurrence, sex and age group that occurred in SC in the period 2009-2018. Male rates showed a downward trend with a strong correlation (Spearman = -0.648 and p-value = 0.034), in contrast to female rates, which showed an indefinite trend (Spearman = -0.467 and p-value = 0.126). The lethality rate by age showed a downward trend with a very strong correlation in the 60-79 age group (Spearman = -0.842 and p-Value = 0.006). The average lethality rate was higher for the population aged 80 years or older, and there was a downward trend with moderate correlation (Spearman = -0.564 and p-Value = 0.028). Considering the entire population of Santa Catarina, lethality rates showed a downward trend with moderate correlation (Spearman = -0.612). The lethality rate according to race/color showed a reduction trend in the period studied in white patients (Spearman = -0.685 and p-value = 0.024). Lethality rates in non-whites showed an undefined trend (Spearman = 0.515).
Discussion
The average rate of hospitalization for EC in SC between 2009 and 2018 was 18.97/100,000 inhabitants, with a trend of stability in the period studied. Similar findings were found in the study by Sarvepalli et. al, [23] that after analyzing 538,776 hospital discharges of patients with a primary diagnosis of EC in the United States, between 1998 and 2013, indicated an average hospitalization rate of 11.3/100,000 inhabitants, with a trend of stability in the period (growth of 0.03/100,000 inhabitants each year) [23]. This stabilization trend can be explained by the increase in treatment options and the increase in the application of palliative therapies in terminal patients, which, as indicated in the study by Plas et. al, have already been shown to reduce the number of hospitalizations of cancer patients [24].
The highest average rate of hospitalizations by age group was observed in the ages between 60 and 79 years (average rate of 66.43 hospitalizations / 100,000 inhabitants), 38.6% higher than the second highest average rate of hospitalizations, the age group of 80 years or more. This finding may be related to the aggressive characteristic of esophageal neoplasms, which are usually associated with low survival rates, which vary from 15 to 20% in 5 years [3-5]. The low survival rate, combined with the higher incidence of EC in the age group from 60 to 79 years, produces a reduction in the population with EC that can be hospitalized in the age group of 80 years or more [25,26]. In the 20 to 39 age group, the average gross distribution of hospitalizations was 0.76 hospitalizations/100,000 inhabitants, which confirms the findings of other worldwide studies that esophageal cancer is not often related to younger people [25-27], with hospitalizations in this age group representing approximately 2% of hospitalizations (n=169) and approximately 1% of deaths (n = 33).
People diagnosed with hereditary tylosis palmoplantaris, an autosomal dominant disease, are at an increased risk of developing squamous-type esophageal cancer at an early age due to the mutation in the RHBDF2 gene located on chromosome 17q25.1, with the risk of being affected by the neoplasia reaching 95% at 65 years of age, an association called Howel-Evans Syndrome [28,29]. In individuals with familial tendency for Barrett’s esophagus and esophageal adenocarcinoma, genetic mutations possibly associated with the incidence of EC in younger individuals have also been identified, such as the MRS1, CRTC1, BARX1 and FOXP1 genes, the latter being responsible for coding a protein involved in the formation of the esophagus [30,31]. It was observed that the hospitalization rates by sex showed great differences, and the average hospitalization rate for males was 4.02 times higher than that for females, a result that is in line with that of various rates of EC incidence by sex in South America, which according to a study by Bray et. al, it was 3.66 times higher for males in 2018 [1].
In addition to the differences in exposure to factors known to be associated with an increased risk of esophageal neoplasia such as tobacco, alcohol and Barrett’s esophagus, other hypotheses are considered possible explanations for the difference in incidence between the sexes in the epidemiology of EC, such as the protective potential of estrogen, the lower frequency of abdominal obesity in women and the lower incidence of gastroesophageal reflux in women [32]. The average length of stay (ALS) of hospitalizations for EC in Santa Catarina showed a strong downward trend in the period studied, once that in 2009 the average length of stay was 9.06 days, and in 2018, 5.75 days, a reduction of 36.53%. Other studies have also found a downward trend in the length of stay for EC. Sarvepalli et. al analyzed 538,776 hospital discharges of patients with a primary diagnosis of EC, in the United States, between 1998 and 2013, and indicated that there was a 19.1% decrease in length of stay in hospitalizations for EC during this 16-year series [23]. It is interesting to note that while the ALS of hospitalizations for EC in SC in 2009 was 9.06 days, in the Sarvepalli study the ALS found in 2009 was 7.3 days. In 2013, the last year of the study in that study, the ALS of hospitalizations for EC was 6.8 days while in Santa Catarina the ALS was 6.4 days. The main hypotheses to explain the magnitude of the reduction in the ALS of hospitalizations by the EC are the expansion of access to lines of care at earlier moments of the disease’s evolution, and the greater awareness of health professionals in relation to palliative and home care therapies in the last few decades, which may have enabled better attention to the health needs of patients, resulting in shorter hospitalizations [24].
Mortality rates due to EC showed a strong downward trend in the studied period. The average mortality rate was 4.14 times higher in males than in females, and this difference is directly related to the difference in the incidence of EC between the sexes [1,33], and also due to the characteristics of the male population such as using less health services for screening tests and consultations [34], which can delay the diagnosis and treatment of this group, with an increase in more reserved prognostics. Mortality rates by age showed a downward trend in the studied period for the age group ranging from 40 to 79 years old. In Santa Catarina there was a reduction in the total mortality rate due to EC of 17.07% between 2009 (8.67) and 2018 (7.19). Guerra et. Al [35], who evaluated the magnitude and variation of the cancer mortality burden in Brazil and Federation Units between 1990 and 2015, evidenced findings that are in line with the results of this study: 14.1% reduction in the total adjusted mortality rate by EC between 1990 and 2015 in the country, with a total adjusted mortality rate in the country of 10.5/100,000 inhabitants in 2015, as this rate followed the downward trend in the states of Rio de Janeiro, São Paulo, Santa Catarina and Rio Grande do Sul, and increased only in the states of Ceará and Paraíba. This reduction may have been a consequence, apart from the expansion of access to health services in the state and in the country, of the reduction in the prevalence of tobacco consumption, since Brazil has presented an important reduction in smoking in the last two decades [36], a drug strongly related to increased risk of EC, mainly of the squamous cell type, responsible for 70% of EC cases in the country, according to the study by Barrios et. Al [37].
The average mortality rate in SC was significantly higher in the elderly population (older than 60 years), with a peak in the population aged 80 and over, confirming the finding of the study by Santos et. al, who also evidenced a progressive increase in mortality rates as the populations age increased in all geographic regions of Brazil between 1980 and 2014 [38]. This trend has also been observed in studies in European countries [39] and has been related worldwide to the cumulative effects of longitudinal exposure to potentially carcinogenic agents and events [14, 26, 40]. The absolute distribution of deaths from esophageal cancer according to sex and age group, in this study, followed the trend observed in other countries and also in Brazil, and was approximately four times higher for males (n = 2931; 80.4 %) than for females (n = 713; 19.6%), with peak incidence between 60 and 79 years (n = 1876; 51.5%). The age group of 80 years or more had the highest mortality rate (average rate of 44.85 deaths / 100,000 inhabitants). It is known that the risk of dying from esophageal cancer increases with age, for reasons already indicated in the previous paragraph [14,26,40].
Most deaths from EC were registered in the population with white skin color (n = 3,202; 87.87%), with the average lethality rate of non-whites (48.95%) being approximately 22.55% higher than than that found in the population with white skin (39.94%), showing epidemiological characteristics that vary according to the ethnicity of the patient affected by esophageal cancer, which have already been reported by other studies [41,42]. Cruz et al. [43], who analyzed the profile of patients with esophageal cancer diagnosed between 2001 and 2010 in Brazil, pointed out that 28% of patients with esophageal cancer referred to themselves as white, and 72% as non-white, a difference that can be explained by the profile of the population of the state of SC, which is mostly white, while in Brazil, most of the population identifies itself as non-white, according to the Brazilian Institute of Geography and Statistics (IBGE) [44]. Chen et. Al [45], after evaluating 49,766 cases of EC between 1998 and 2013 in the United States, pointed out that 77.1% of the patients studied were white (non-Hispanic), a group that had the highest survival rate, while black patients had the lowest survival rates, even when they had a diagnosis of squamous cell type neoplasm, associated with a more favorable prognosis. This study suggested that the lower survival rates found in ethnic groups could be associated with less opportunity for access to diagnosis and treatment, since a lower proportion of cancer surgeries for the treatment of EC was also observed among blacks.
The Human Development Index (HDI) is a measure calculated from three indicators of human development: longevity, education and income. The study by Bray et. al related the global transitions of cancer incidence and mortality rates with HDI variations since 2008, classifying countries by HDI as low (HDI <0.5), medium (0.5≤IDH <0.8), high (0.8≤IDH <0.9), and very high (HDI≥0.9). Bray pointed out that in 2008, in the regions of high and very high HDI, breast, lung, colon and prostate cancers accounted for approximately 50% of the total number of cancer cases, while in the regions of medium HDI, cancers of the esophagus, stomach and liver were also common and, together with the first four, represented 62% of the total cancer burden in areas of medium to very high HDI. In regions with a low HDI, cervical cancer was more common than breast and liver cancer [46].
In the studied period, the macroregions of Santa Catarina that presented the highest average rates of hospitalization for esophageal cancer per 100,000 inhabitants were those of the Grande Oeste (37.05), the Meio Oeste e Serra Catarinense (23.66) and the Sul (23.58), with a strong growth trend in the first two and a stability trend in the third. In 2010, these macro-regions had the three worst HDIs in the state (0.753, 0.743 and 0.755; respectively) [47]. The macro-region of Foz do Rio Itajaí had the lowest average rate of hospitalization for esophageal cancer per 100,000 inhabitants (4.84) and, also, the highest HDI (0.764). Grande Florianópolis, which had the third lowest average hospitalization rate per EC per 100,000 inhabitants (14.78) with a very large downward trend in the period studied, reached the second highest HDI (0.761) in Santa Catarina. The data presented suggest a very consistent negative correlation between the regional HDI and hospitalization rates by EC. These results were corroborated in the study by Ribeiro and Nardocci, which indicated the existence of a negative correlation between the socioeconomic level of the area of residence and the esophageal cancer incidence and mortality [48]. It is important to note that the regions with the worst socioeconomic indicators generally have similar characteristics, such as a lower degree of urbanization, economic activities that are more dependent on the primary sector, eating habits and lifestyles more related to local culture [49], which can influence the results that were the object of this study. In addition, several studies have shown that the habit of consuming chimarrão in large quantities, with water at high temperatures, increases the risk of developing esophageal cancer [11, 27, 50, 51] as well as the habit of consuming red meat, already associated with carcinogenesis for many years [52], thus, this raises the hypothesis that such habits have an influence on the results of this study, since the three macro regions of SC with the highest rates of hospitalization due to EC border with the state of Rio Grande do Sul and share these cultural habits. The stability of hospitalization rates for esophageal cancer in the state raises the hypothesis that the incidence of this neoplasm remains constant in SC. Temporary hospitalization trends described in the main macroregions analyzed, such as the upward trend in the Grande Oeste, Meio Oeste e Serra Catarinense, in contrast to the downward trend in Grande Florianópolis, may serve as a basis for further studies, more focused on investigating possible social, food, economic and exposure to toxic agents in these regions, that may clarify the causal relationships and the antagonistic trends observed.
Conclusion
The results showed that the sociodemographic profile of patients suffering from esophageal cancer in Santa Catarina in the period 2009-2018, according to available variables, was men, aged between 60 and 79 years, of white ethnicity, and residents of the Grande Oeste macroregion. There was a temporal trend of stability in hospitalization rates by sex and age group, except for the age groups from 40 to 59 years old, with a downward trend, and from 80 or more years old, with an upward trend. Temporal growth trends were found in the rates of hospitalization for EC in the macro-regions of Grande Oeste, Meio Oeste e Serra Catarinense, reduction trends in Grande Florianópolis and stability in other macroregions of the state. Temporal tendencies of stability were observed in the proportions of intensive care and in the execution of clinical and surgical procedures, while there was a temporal trend of reduction in the proportions of the execution of diagnostic procedures and in the average length of stay. Mortality rates were higher in men, in the age group of 80 or more years of age, and in SC they presented a temporal trend of reduction. Lethality rates were similar in both sexes, with a temporal trend of reduction only in the male population. The lethality rate was higher in the age group of 80 or more years old. There was a temporal trend towards a reduction in lethality rates in the state of Santa Catarina as a whole. While the time series of morbidity (hospitalization rates) due to esophageal cancer showed a general trend of stability in the period, the general rates of mortality, lethality and the average length of stay showed a downward trend, denoting the possible influence of advances in the quality of health services, which reduced the length of stay in hospitals while reducing mortality and lethality by the EC.
Acknowledgement
Magajewski FRL: drafting of the manuscript, interpretation of data and critical revision of the manuscript and design. Sakae TM: statistical analysis. Giustina LBD: manuscript translation and critical revision of the manuscript. All authors have given final approval of the submitted manuscript.
Conflict of Interest
No conflict of interest.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Rebecca L Siegel, Lindsey A Torre, et al. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6): 394-424.
- Eslick GD (2009) Esophageal Cancer: A Historical Perspective. Gastroenterol Clin North Am 38(1): 1-15.
- Njei B, McCarty TR, Birk JW (2016) Trends in esophageal cancer survival in United States adults from 1973 to 2009: A SEER database analysis. J Gastroenterol Hepatol 31(6): 1141–1146.
- Gavin AT, Francisci S, Foschi R, DW Donnelly, V Lemmens, et al. (2012) Oesophageal cancer survival in Europe: a EUROCARE-4 study. Cancer Epidemiol 36(6): 505–512.
- Zeng H, Zheng R, Guo Y, Siwei Zhang, Xiaonong Zou, et al. (2015) Cancer survival in China, 2003-2005: a population-based study. Int J Cancer 136(8): 1921–1930.
- Anandavadivelan P, Lagergren P (2016) Cachexia in patients with oesophageal cancer. Nat Rev Clin Oncol 13(3): 185-198.
- Yousefi M sadat, Sharifi Esfahani M, Pourgholam Amiji N (2018) Esophageal cancer in the world: incidence, mortality and risk factors. Biomed Res Ther 5(7): 2504-2517.
- Sallum RA (2016) Esophageal cancer. In: ZATERKA, Schlioma, EISIG, Jaime Natan. Gastroenterology Treaty: from Undergraduate to Graduate Studies. 2nd (Edn.), São Paulo: Atheneu, Chap. 45, pp. 508.
- Daly JM, Fry WA, Little AG, DP Winchester, RF McKee, et al. (2000) Esophageal cancer: results of an American College of Surgeons patient care evaluation study. J Am Coll Surg 190(5): 562–572.
- Prabhu A, Obi K, Rubenstein J (2014) The Synergistic Effects of Alcohol and Tobacco Consumption on the Risk of Esophageal Squamous Cell Carcinoma: A Meta-Analysis. Am J Gastroenterol 109(6): 822-827.
- Chen Y, Tong Y, Yang C, Yong Gan, Huilian Sun, et al. Consumption of hot beverages and foods and the risk of esophageal cancer: a meta-analysis of observational studies. BMC Cancer. 15(1): 449.
- Liu J, Wang J, Leng Y, Changxing Lv (2013) Intake of fruit and vegetables and risk of esophageal squamous cell carcinoma: A meta-analysis of observational studies. Int J Cancer 133(2): 473-485.
- Qu X, Ben Q, Jiang Y (2013) Consumption of red and processed meat and risk for esophageal squamous cell crcinoma based on a meta-analysis. Ann Epidemiol 23(12): 762-770.e1.
- Wu C, Wang Z, Song X, Xiao Shan Feng, Christian C Abnet, et al. (2014) Joint analysis of three genome-wide association studies of esophageal squamous cell carcinoma in Chinese populations. Nat Genet 46(9): 1001–1006.
- Bosetti C, Gallus S, Garavello W, Carlo La Vecchia (2006) Smoking cessation and the risk of oesophageal cancer: An overview of published studies. Oral Oncol 42(10): 957–964.
- Bosetti C, Franceschi S, Levi F, E Negri, R Talamini, et al. (2000) Smoking and drinking cessation and the risk of esophageal cancer. Br J Cancer 83(5): 689-691.
- Chow WH, Blot WJ, Vaughan TL, H A Risch, MD Gammon, et al. (1998) Body Mass Index and Risk of Adenocarcinomas of the Esophagus and Gastric Cardia. J Natl Cancer Inst 90(2): 150-155.
- Rubenstein JH, Taylor JB (2010) Meta-analysis: the association of oesophageal adenocarcinoma with symptoms of gastro-oesophageal reflux. Aliment Pharmacol Ther 32(10): 1222–1227.
- Xie FJ, Zhang YP, Zheng QQ, Hong-Chuan Jin, Fa Liang Wang, et al. (2013) Helicobacter pylori infection and esophageal cancer risk: an updated meta-analysis. World J Gastroenterol 19(36): 6098-6107.
- Falk GW, Buttar NS, Foster NR, Katie L Allen Ziegler, Catherine J DeMars, et al. (2012) A Combination of Esomeprazole and Aspirin Reduces Tissue Concentrations of Prostaglandin E2 in Patients with Barrett’s Esophagus. Gastroenterology 143(4): 917-926.e1.
- Napier KJ, Scheerer M, Misra S (2014) Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol 6(5): 112–120.
- Tramontano AC, Chen Y, Watson TR, Andrew Eckel, Chin Hur, et al. (2019) Esophageal cancer treatment costs by phase of care and treatment modality, 2000‐2013. Cancer Med 8(11): 5158–5172.
- Sarvepalli S, Garg SK, Sarvepalli SS, MP Parikh, V Wadhwa, et al. (2020) Inpatient burden of esophageal cancer and analysis of factors affecting in-hospital mortality and length of stay. Dis Esophagus 31(9): doy022.
- Van der Plas AGM, Vissers KC, Francke AL, Gé A Donker, Wim JJ Jansen, et al. (2015) Involvement of a Case Manager in Palliative Care Reduces Hospitalisations at the End of Life in Cancer Patients; A Mortality Follow-Back Study in Primary Care. PLoS One 10(7): e0133197.
- (2020) SEER Cancer Statistics Review, 1975-2017.
- Gupta B, Kumar N (2017) Worldwide incidence, mortality and time trends for cancer of the oesophagus: Eur J Cancer Prev 26(2): 107–118.
- Wheeler JB, Reed CE (2012) Epidemiology of Esophageal Cancer. Surg Clin North Am 92(5): 1077–1087.
- Howel Evans W, McConnell RB, Clarke CA, (1958) Carcinoma of the oesophagus with keratosis palmaris et plantaris (tylosis): a study of two families. Q J Med 27(107): 413-429.
- Blaydon DC, Etheridge SL, Risk JM, Hans Christian Hennies, Laura J Gay, et al. (2012) RHBDF2 Mutations Are Associated with Tylosis, a Familial Esophageal Cancer Syndrome. Am J Hum Genet 90(2): 340-346.
- Orloff M, Peterson C, He X, Shireen Ganapathi, Brandie Heald, et al. (2011) Germline Mutations in MSR1, ASCC1, and CTHRC1 in Patients With Barrett Esophagus and Esophageal Adenocarcinoma. JAMA 306(4): 410-419.
- Dai JY, de Dieu Tapsoba J, Buas MF, Lynn E Onstad, David M Levine, et al. (2015) A newly identified susceptibility locus near FOXP1 modifies the association of gastroesophageal reflux with Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev 24(11): 1739-1747.
- Rashid F, Khan RN, Iftikhar SY (2010) Probing the link between estrogen receptors and esophageal cancer. World J Surg Oncol 8: 9.
- (2019) José Alencar Gomes da Silva National Cancer Institute. Estimate 2020: Incidence of Cancer in Brazil. Rio de Janeiro: INCA.
- Gomes R, Nascimento EF do, Araújo FC de (2007) Why do men use health services less than women? Explanations by men with low versus higher education. Cad Saude Publica 23(3): 565-574.
- Guerra MR, Bustamante Teixeira MT, Corrêa CSL, Daisy Maria Xavier de Abreu, Maria Paula Curado, et al. (2017) Magnitude and variation of the cancer mortality burden in Brazil and Federation Units, 1990 and 2015. Rev bras epidemiol 20: 102–115.
- Wünsch Filho V, Mirra AP, López RVM (2010) Smoking and cancer in Brazil: evidence and perspectives. Brazilian Journal of Epidemiology 13(2): 175-187.
- Barrios E, Sierra MS, Musetti C, David Forman (2016) The burden of esophageal cancer in Central and South America. Cancer Epidemiol 44: S53-S61.
- Dos Santos J, Meira KC, Simões TC, Raphael Mendonça Guimarães, Mauricio Wiering Pinto Telles, et al. (2018) Inequalities in esophageal cancer mortality in Brazil: Temporal trends and projections. PLoS One 13(3): e0193135.
- Castro C, Bosetti C, Malvezzi M, P Bertuccio, F Levi, et al. (2014) Patterns and trends in esophageal cancer mortality and incidence in Europe (1980–2011) and predictions to 2015. Ann Oncol 25(1): 283-290.
- Holford TR (1991) Understanding the effects of age, period, and cohort on incidence and mortality rates. Annu Rev Public Health 12: 425–457.
- Baquet CR, Commiskey P, Mack K, Stephen Meltzer, Shiraz I Mishra (2005) Esophageal cancer epidemiology in blacks and whites: racial and gender disparities in incidence, mortality, survival rates and histology. J Natl Med Assoc 97(11): 1471–1478.
- Xie SH, Rabbani S, Petrick JL, Michael B Cook, Jesper Lagergren (2017) Racial and Ethnic Disparities in the Incidence of Esophageal Cancer in the United States, 1992–2013. Am J Epidemiol 186(12): 1341–1351.
- Cruz AIBM, Pinto LFR, Thuler LCS (2018) Profile of Esophageal Cancer Patients Diagnosed between 2001 and 2010 in Brazil. Brazilian Journal of Cancerology 64(4): 471–477.
- (2020) Continuous National Household Sample Survey - Continuous PNAD | IBGE.
- Chen Z, Ren Y, Du XL, Jiao Yang, Yanwei Shen, et al. (2017) Incidence and survival differences in esophageal cancer among ethnic groups in the United States. Oncotarget 8(29): 47037–47051.
- Bray F, Jemal A, Grey N, Jacques Ferlay, David Forman (2012) Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. Lancet Oncol 13(8): 790-801.
- IDHM Municípios 2010 PNUD Brasil.
- Ribeiro A de A, Nardocci AC (2013) Socioeconomic inequalities in cancer incidence and mortality: review of ecological studies, 1998-2008. Health and Society 22(3): 878-891.
- Vargas DB, Vargas TAV, Theis IM (2008) The recent evolution of the regional productive systems of Santa Catarina. Dynamis Magazine 13(1): 92-101.
- Andrici J, Eslick GD (2013) Maté consumption and the risk of esophageal squamous cell carcinoma: a meta-analysis: Dis Esophagus 26(8): 807-816.
- Lubin JH, De Stefani E, Abnet CC, Gisele Acosta, Paolo Boffetta, et al. (2014) Maté drinking and esophageal squamous cell carcinoma in South America: pooled results from two large multi-center case-control studies. Cancer Epidemiol Biomarkers Prev 23(1):107–116.
- (2018) International Agency for Research on Cancer, Working Group on the Evaluation of the Carcinogenic Risks to Humans. Red meat and processed meat this publication represents the views and expert opinions of an IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, which met in Lyon, 6-13.
-
Lorenzo Becker Della Giustina, Thiago Mamôru Sakae, Luigi Becker Della Giustina, Flávio Ricardo Liberali Magajewski. Temporal Trends of Esophagus Cancer Morbimortality in the State of Santa Catarina in the Period of 2009-2018. 1(3): 2021. APHE.MS.ID.000512.
-
Esophageal cancer, Morbidity and mortality, Temporal trend, Cancer deaths, Tumors, Population, Mortality rate, Diagnosis.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






