 Case Report
Case Report
Sudden Onset of Paraplegia: A Rare Presentation of a Ruptured Abdominal Aortic Aneurysm
Loubna A Fares1, Layal A Olaywan2, Batoul M Mourda1, Hani M Shahin2 and Rima Chaddad*3
1Department of Emergency medicine, Faculty of Medical Sciences, Lebanese University, Beirut, Lebanon
2Department of Critical Care, Faculty of Medical Sciences, Lebanese University, Beirut, Lebanon
3Department of Cardiology, Faculty of Medical Sciences, Lebanese University, Beirut, Lebanon
Rima Chaddad, Department of Cardiology, Faculty of Medical Sciences, Lebanese University, Beirut, Lebanon.
Received Date:February 01, 2021; Published Date: March 04, 2021
Abstract
Abdominal aortic aneurysms represent an asymptomatic disease that is characterized by the localized pathological dilatation of the infrarenal aorta. Abdominal aortic aneurysms are generally diagnosed incidentally and treated surgically. However, underdiagnosis and lack of high-risk population surveillance contributes to high rates of complications and mortality due to the ultimate rupture of abdominal aortic aneurysms. When symptomatic, abdominal aortic aneurysm rupture usually presents with abdominal or back pain, abdominal pulsatile mass and hemodynamic instability. Neurologic presentations are usually rare and remain poorly characterized. In this report, we present a case of ruptured abdominal aortic aneurysm presenting as acute onset of paraplegia followed by syncope in a 74-year-old male. Typical abdominal aortic aneurysm symptoms were not observed, and surgical treatment was attempted following diagnosis. The patient passed away after exhibiting a sudden onset of hypotension followed by asystole. That being said, clinical and physical findings indicate that neurological complications, and not classic symptoms, could indicate abdominal aortic aneurysm rupture. Spinal cord ischemia due to inadequate blood supply is a possible cause of acute and sudden paraplegia. Adequate monitoring and preventative measures should thus be adopted in order to prevent shearing, occlusion and/or thrombosis of the artery of Adamkiewicz. Moreover, diminished femoral pulses should be considered as potentially reflective of aortic aneurysms or dissection in routine clinical practice.
Headings: Abdominal aortic aneurysms; Cardiovascular disease; Case report; Paraplegia
Introduction
An abdominal aortic aneurysm is defined as a localized pathological dilatation of the infrarenal aorta to at least 1.5 times that of the normal artery diameter [1]. In clinical practice, this roughly translates to arteries being diagnosed as aneurysmal should they present with a diameter of 30mm or more, albeit without ignoring gender and individual variations in original artery size [1]. More importantly, aneurysms present with a gradual and irreversible expansion of an artery. This is referred to as the ‘growth’ of an abdominal aortic aneurysm, which is continuous, and ultimately results in rupture if left untreated [2]. The proper surveillance and surgical management of patients presenting with abdominal aortic aneurysm thus emerges as a critical consideration. In fact, abdominal aortic aneurysm rupture remains intimately associated with extremely high mortality rates, both if managed surgically or if left untreated [3,4]. Abdominal aortic aneurysms exhibit continuous non-linear enlargement, which puts patients at a yearly increasing risk of morbidity and mortality. In fact, patients presenting with large aneurysms are more likely to suffer from rupture, particularly should they not be subject to constant surveillance [2]. In other words, if left untreated and unmonitored, abdominal aortic aneurysms could grow to lifethreatening sizes, ultimately rupturing and causing massive intraabdominal bleeding, and death. Older age remains among the main risk factors of abdominal aortic aneurysms, along with smoking, family history of abdominal aortic aneurysms and hypertension [2,3,5]. It thus seems that while distinct entities, abdominal aortic aneurysms and atherothrombotic disease present with common risk factors contributing to the manifestation of abdominal aortic aneurysms among older patients.
That being said, abdominal aortic aneurysms are often asymptomatic and thus remain generally undiagnosed. As such, abdominal aortic aneurysms are generally diagnosed upon rupture and the presentation of associated clinical symptoms. The latter include the sudden onset of abdominal or back pain and hemorrhagic shock and indicate a ruptured abdominal aortic aneurysm [4]. Neurological complications have been previously associated with abdominal aortic aneurysm both as an atypical presentation of the condition [6], and as an uncommon outcome of after endovascular aneurysm repair and open repair surgery [7]. Similar reports have described cases of aortic dissection (or false aneurysm) presenting as sudden paraplegia, albeit rarely [8,9]. In this report, we describe the case of a ruptured abdominal aortic aneurysm presenting as a painless sudden onset of paraplegia in an elderly male patient. The clinical management of the patient is described, along with a discussion of potential etiology.
Case Report
A 74-year-old male patient was transported by ambulance to the Emergency Department due to the sudden onset of paraplegia followed by a decreased level of consciousness 1 hour prior to presentation. The patient had no documented history of hypertension, was not receiving any medication and had never smoked.
Upon presentation, the patient was hypotensive (60/40 mmHg) and tachycardic (170 BPM). Physical examination revealed that the patient was somnolent but arousable, with a Glasgow Coma Score of 10 (GCS=10). The patient exhibited normal vesicular breath sounds, regular, albeit tachycardic, cardiac rhythm, while the abdomen was soft with pulsatile abdominal mass. The patient had cold, pulseless and areflexic lower, with motor power 0/5 bilaterally. Volume resuscitation with normal saline (total of 2 liters) was given until blood pressure was raised to reach a systolic blood pressure (SBP) of 90mmHg and heart rate was decreased, at which point the patient regained his consciousness. Urgent thoracoabdominopelvic CT scan with intravenous iodinated contrast revealed an infrarenal aortic aneurysm. The latter was 11x7.4x6.5 cm with surrounding severe peri-aortic and retroperitoneal density fluid mainly on the right, a presentation compatible with abdominal aortic aneurysm rupture. The remainder of the aorta showed irregular atherosclerotic changes with multi-level intimal ulcers. Mild aneurysmal dilatation of the common iliac arteries was also noted with thrombi of the common iliac artery, larger on the left with no opacification of the left common femoral superficial and deep femoral arteries (Figures 1,2 &3).
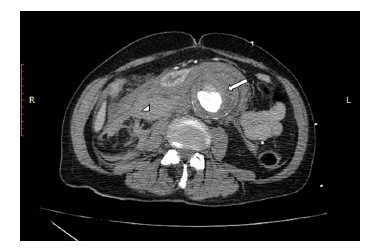
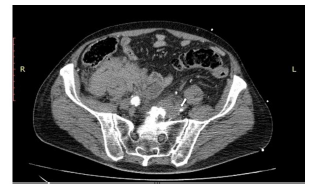
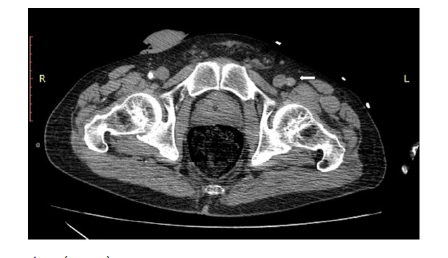
The patient was directly transferred to the operative room for emergent laparotomy for repair of the aortic aneurysm. Laparotomy incision was done under general anesthesia. Proximal dissection of the aorta revealed a considerable retroperitoneal hematoma. infra-renal cross-clamping of the aorta was undertaken with distal closure of both common iliac arteries. End to end anastomosis was completed between the aorta and Dacron graft and another end to side anastomosis was done between the graft and another Dacron graft. Despite declamping of the aorta, the patient presented with a sudden onset of hypotension followed by asystole. Cardiac resuscitation was unsuccessfully attempted for thirty minutes, and the patient ultimately passed away.
Discussion
In the present report, we described the case of a patient who had presented with a sudden onset of paraplegia followed by syncope., which led to the diagnosis of an asymptomatic abdominal aortic aneurysm. When asymptomatic and intact, abdominal aortic aneurysms are often diagnosed incidentally when screening for or attempting to characterize another condition through radiography or sonography. It thus stands to reason that screening for abdominal aortic aneurysms, particularly among elderly men, was shown to reduce both its associated mortality and the overall incidence of ruptured aneurysms [10,11]. Abdominal or back pain, hypotension or shock, and abdominal pulsatile mass are widely recognized as characteristic abdominal aortic aneurysm symptoms. However, this triad of symptoms concomitantly present only in a quarter to half of all abdominal aortic aneurysm patients, which could lead to its misdiagnosis as other conditions (e.g. renal colic, diverticulitis, and gastrointestinal hemorrhage) [12].
Atypical AAA presentation have been described and include hemodynamically stable cases [13], acute cauda equina syndrome [14], and acute onset right testicular pain [15]. The incidence of neurological complications has not been specifically determined and these complications appear to be uncommon. That being said, reports have indicated that chronic abdominal aortic aneurysm rupture can present as lower-extremity or crural neuropathy [16,17]. Moreover, patients can exhibit other neuropathic conditions both at initial presentation and after abdominal aortic aneurysm repair. These conditions include ischemic sciatic neuropathy [18], testicular ischemia [19], and nerve root compression [20].
Clinical and surgical findings suggested that the paraplegia observed in the patient occurred as a result of diminished blood flow in the artery of Adamkiewicz. This was most likely caused by stasis and primary intraluminal thrombus formation in this artery, ultimately resulting in spinal cord ischemia and infarction. Acute paraplegia has been previously reported as a presentation of aneurysmal rupture [6,21]. However, acute paraplegia is more likely to occur as a result of aortic thrombosis or as a postoperative complication of abdominal aortic aneurysm repair [22,23]. When considering aneurysmal rupture-associated paraplegia, thrombotic emboli arising from aneurysmal mural thrombus and occluding an abnormally low Adamkiewicz artery were thought to be responsible for transient episodes of spinal cord ischemia in a patient presenting for acute onset of paraplegia [21]. Interestingly, the patient had records of previous completely reversible episodes of paraplegia and was later diagnosed with abdominal aneurysm rupture with an intraluminal thrombus. The etiology of acute and sudden paraplegia seems to be spinal cord ischemia, spinal cord infarction or trauma to the spinal cord. Neurological deficits were suggested to be the result of shearing, occlusion and/or thrombosis of the artery of Adamkiewicz [23].
The artery of Adamkiewicz, also known as arteria radicularis magna, is a radicular artery of particular importance. This vessel anastomoses with the anterior and posterior spinal arteries to supply the lower thoracic and lumbar spinal cord as well as the conus medullaris and can originate from an intercostal or lumbar artery [24]. When the artery of Adamkiewicz arises in the thoracic area, it supplies the thoracic spinal cord while lumbar collateral branches supply the distal spinal cord. When the artery of Adamkiewicz arises in the lumbar area, it supplies the distal spinal cord while the thoracic spinal cord receives its blood supply from the collaterals of intercostal arteries [24]. If blood flow is obstructed to the artery of Adamkiewicz, spinal cord ischemia may occur and progress to infarction if not recognized and will inevitably leads to acute paralysis of the lower extremities. As such, special care has been exercised in the surgical management of abdominal aortic aneurysms in order to prevent paraplegia associated with deficits in blood supply to the spinal cord by the Adamkiewicz artery and the segmental artery supporting it [25,26]. While spinal cord function was found to depend on blood supply by these arteries in approximately a third of abdominal aortic aneurysm patients, the Adamkiewicz artery is not solely responsible for the onset of spinal cord ischemia and paraplegia [27]. In most cases, abdominal aortic aneurysm-induced paralysis is permanent. There are reported cases of complete recovery among patients with lower extremity paralysis following endovascular treatment of abdominal aortic aneurysms [28-30], with cerebrospinal fluid drainage emerging as a common restorative method [28,30].
Conclusion
The present study reported the case of a 74-year old male patient diagnosed with an abdominal aortic aneurysm presenting as acute paraplegia. Consistently with existing evidence, abdominal aortic aneurysm rupture with lower extremity paralysis may not be accompanied by the classic triad symptoms of abdominal aortic aneurysm (e.g. abrupt chest, abdominal or back pains). Femoral pulses must therefore be examined in patients presenting with lower back pain and leg weakness and are suggested to be indicative of potential aneurysm or aortic dissection should they be abnormal.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Marie D Gerhard Herman, Heather L Gornik, Coletta Barrett, Neal R Barshes, Matthew A Corriere, et al. (2017) 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 135(12): e686-e725.
- Norman PE, Semmens JB, Lawrence Brown MMD, Holman CDAJ (1998) Long term relative survival after surgery for abdominal aortic aneurysm in Western Australia : Population based study. BMJ 1998 317(7162): 852-856.
- Sakalihasan N, Michel JB, Katsargyris A, Kuivaniemi H, Defraigne JO, et al. (2018) Abdominal aortic aneurysms. Nat Rev Dis Primers 4(1): 34.
- Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, et al. (2011) Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg 41 Suppl 1: S1-S58.
- Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, et al. (2010) Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg 52(3): 539-548.
- Chatlani PT, Van Dessel MG, Krishnan KR, McLoughlin GA (1989) Abdominal aortic aneurysm presenting as paraplegia: Case report. Paraplegia 27(2):146-147.
- Berg P, Kaufmann D, Van Marrewijk CJ, Buth J (2001) Spinal cord ischaemia after stent-graft treatment for infra-renal abdominal aortic aneurysms. Analysis of the Eurostar database. Eur J Vasc Endovasc Surg 22(4):342-347.
- Tsiouris A, Morgan JA, Paone G (2012) Type A aortic dissection presenting with isolated paraplegia. Heart Surg Forum Dec 15(6): E316-E317.
- Nandeesh BN, Mahadevan A, Santosh V, Yasha TC, Shankar SK (2007) Acute aortic dissection presenting as painful paraplegia. Clin Neurol Neurosurg 109(6): 531–534.
- Ashton HA, Gao L, Kim LG, Druce PS, Thompson SG, et al. (2007) Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br J Surg 94(6): 696-701.
- Wanhainen A, Hultgren R, Linné A, Holst J, Gottsäter A, et al. (2016) Outcome of the Swedish Nationwide abdominal aortic aneurysm screening program. Circulation 134(16):1141-1148.
- Eisenhoffer JL, Marston WA, Ahlquist RA, Johnson GA (1992) Misdiagnosis of ruptured abdominal aortic aneurysms. J Vasc Surg 16(1): a34344.
- Abdel Aziz A, Maskery N (2009) An unusual presentation of a ruptured abdominal aortic aneurysm. Crit Ultrasound J 1(1): 21-22.
- Engamba SA, Garaleviciene D, Baldry J (2017) Contained ruptured abdominal aortic aneurysm presenting as cauda equina syndrome. BMJ Case Rep 2017: bcr2016216602.
- Khan T, Mahomed A, Ghoorun B, Ali H (2018) Acute testicular pain secondary to a leaking abdominal aortic aneurysm (AAA). BMJ Case Rep 2018: bcr2018226577.
- Defraigne JO, Sakalihasan N, Lavigne JP, Damme H, Limet R (2001) Chronic rupture of abdominal aortic aneurysm manifesting as crural neuropathy. Ann Vasc Surg 15(3):405-411.
- Tsubota H, Nakamura T (2012) Chronic contained rupture of an abdominal aortic aneurysm manifesting as lower extremity neuropathy. J Vasc Surg 55(2): 548.
- Kibria SG, Gough MJ (1999) Ischaemic sciatic neuropathy: A complication of endovascular repair of abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 17(3): 266-267.
- Hall MJ, Duprat GI, Poulin TL (2010) Testicular ischemia following endovascular infrarenal abdominal aortic aneurysm repair: A rare complication. J Vasc Interv Radiol 1603-1604.
- Dahl T, Lange C, Ødegård A, Bergh K, Osen SS, et al. (2005) Ruptured abdominal aortic aneurysm secondary to tuberculous spondylitis. Int Angiol 24(1): 98-101.
- Whitwell GS, Vowden P (2002) An unusual presentation of a ruptured abdominal aortic aneurysm. Eur J Vasc Endovasc Surg 23(5): 465-466.
- West JR, Keller CS, Lombardi J (2019) A patient with lower extremity weakness after recent endovascular repair of an abdominal aortic aneurysm. Am J Emerg Med 38(4): 851.e1-851.e3.
- Tochii M, Takagi Y, Hoshino R, Yamashita M, Sato M, et al. (2009) Paraplegia following the emergency surgical repair of a nonruptured symptomatic abdominal aortic aneurysm: Report of a case. Surg Today 39(7): 603–605.
- Lindeire S, Hauser JM (2020) Anatomy, Back, Artery of Adamkiewicz. StatPearls [Internet].
- Nijenhuis RJ, Jacobs MJ, Schurink GW, Kessels AGH, van Engelshoven JMA, et al. (2007) Magnetic resonance angiography and neuromonitoring to assess spinal cord blood supply in thoracic and thoracoabdominal aortic aneurysm surgery. J Vasc Surg 45(1): 71–78.
- Arora L, Hosn MA (2019) Spinal cord perfusion protection for thoraco-abdominal aortic aneurysm surgery. Curr Opin Anaesthesiol 32(1):72–79.
- Griepp RB, Ergin MA, Galla JD, Lansman S, Khan N, et al. (1996) Looking for the artery of Adamkiewicz: A quest to minimize paraplegia after operations for aneurysms of the descending thoracic and thoracoabdominal aorta. J Thorac Cardiovasc Surg 112(5): 1202–1215.
- Azevedo Mendes M, Szecel D, Hans GA, Sakalihasan N (2016) Delayed Paraplegia After Endovascular Treatment of a Thoracoabdominal Aortic Aneurysm Successfully Managed Using Cerebrospinal Fluid Drainage. J Cardiothorac Vasc Anesth 30(5): 1358-1360.
- Morisaki K, Matsumoto T, Matsubara Y, Inoue K, Aoyagi Y, et al. (2016) A Rare Complication of Spinal Cord Ischemia Following Endovascular Aneurysm Repair of an Infrarenal Abdominal Aortic Aneurysm with Arteriosclerosis Obliterans : Report of a Case. Ann Vasc Dis 9(3): 255-257.
- Bajwa A, Davis M, Moawad M, Taylor PR (2008) Paraplegia Following Elective Endovascular Repair of Abdominal Aortic Aneurysm: Reversal with Cerebrospinal Fluid Drainage. Eur J Vasc Endovasc Surg 35(1): 46-48.
-
Loubna A Fares, Layal A Olaywan, Batoul M Mourda, Hani M Shahin, Rima Chaddad, Rida salman. Sudden Onset of Paraplegia: A Rare Presentation of a Ruptured Abdominal Aortic Aneurysm. Annal of Pub Health & Epidemiol .1(3): 2021. APHE.MS.ID.000511.
-
Abdominal aortic aneurysms, Cardiovascular disease, Case report, Paraplegia, Patients, Surgery, Diagnosis, Hypotension, Neurological complications.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






