 Research Article
Research Article
Quality Assessment of the Efficiency of the Warming Cabinet Method in Warming Fluids in Anaesthesia
Essam Abdelrazek1*, James Walker2, Johan Victor Rehnberg3, Sharon Docherty4, Ash Byrom5 and Akbar Vohra6
1Professor of Anaesthesia, Misr University for science & technology, Egypt
2Consultant Anaesthetist, Royal Bournemouth Hospital, UK
3Anaesthetic senior clinical fellow, University Hospital, Southampton, UK
4Senior Lecturer, Bournemouth University clinical research unit, UK
5Senior operation department practitioner, Royal Bournemouth hospital, UK
6Department of Cardiac Anaesthesia and Critical Care, Manchester University Foundation Trust, UK
Essam Abdelrazek, Professor of Anaesthesia, Misr University for Science & Technology, Egypt.
Received Date: November 19, 2019; Published Date: December 16, 2019
Abstract
In this observational study, we assessed the efficiency of the use of a 40° C warming cabinet method for heating intravenous (IV) fluids administered to patients during surgery. 24 bags of Ringers lactate were placed in a warming cabinet set at 40° C. Fluid temperatures at two points of the delivery system (bag and patient end) were measured every 3 minutes for 15 minutes after being removed from the warming cabinet.
Results: The bag temperature (34-31 °C) was significantly lower than 40°C but remained above room temperature throughout the study period. The patient–end temperatures were significantly lower than bag temperatures for all time-periods (p<0.01) and were similar to room temperatures within 3 minutes. We conclude that the quality of the warming cabinet technique used in operating theatres to warm fluids prior to IV infusion, is clinically inefficient and fails to deliver the IV fluids at a warm enough temperature and may therefore put patients at risk of unanticipated hypothermia. We recommend that the practice of the studied method of fluid warming needs to be revised and replaced by another method which could be more clinically effective and reliable in keeping the patient’s temperature within normal limits under anaesthesia.
Keywords: Hypothermia; Prevention; Hypothermia; Physiology effects
Introduction
Hypothermia is a well-known complication of general as well as regional anaesthesia [1,2]. It could have detrimental effects on the patient’s hemodynamic, respiratory, neurological, and metabolic conditions [3]. Post-operative shivering in recovery is usually very unpleasant and distressing experience for patients as well as the staff in the recovery room [4]. This can be a cause of delayed recovery from general anaesthesia. It may increase the patient’s feeling of pain in the immediate post-operative period, which could be difficult to control without improving their postoperative temperature [5]. Intraoperative administration of pre warmed intravenous (IV) fluids (37- 40° C) has been shown to be one of the useful methods used to counter the anaesthesia/surgery induced hypothermia effects in patients undergoing surgery [6]. Different methods have been suggested to warm IV fluids given to patients undergoing surgery, with variable degrees of efficiency [7-10]. One of these methods is the use of electrically operated warming cabinets. This is considered to be a simple, easy, available, and relatively cheap method of warming fluids given to patients in the operating theatre. Therefore, it has become popular globally. However, evidence of the efficiency of this technique remains unclear [7-9].
At the Royal Bournemouth Hospital (RBH) using warming cabinets to warm the intravenous fluids given to patients under anaesthesia is a standard technique. However, clinical observation has suggested that the warming cabinet is not fully reliable because the warmed fluids appear to lose heat quickly on traversing down the giving set. This study was designed to assess the efficiency of using the warming cabinet technique as a method of pre warming IV fluids and by inference, it’s appropriateness for maintenance of patient temperature during surgery.
Methods
Approval of an Ethics Committee was not necessary as there were no patients involved. Based on the results of an initial pilot study, a mean difference of 10° C (standard deviation±2.25) between the starting temperature on leaving the warming cabinet and two points A (the fluid bag) and B (the distal part of the giving set - i.e. patient end) was deemed statistically significant. A sample size of 24 fluid bags would be required based on significance of p<0.05 and 90% power. According to the standard practice at our institution, the warming cabinet (W157, LEEC Limited, Nottingham, U.K.) was used to warm the fluid bags. Twenty-four fluid bags of equal volume (1 L) were placed in the warming cabinet set at 40° C 12 hours prior to testing. Using the wall mounted room temperature sensor (Sauter Ltd. Company®, Basil, Switzerland), the room temperature was kept at 21ºC±0.23ºC. The fluid sample temperatures were measured using the C21 Comark thermometer (Comark instruments company, Norwich, U.K.). This is a batteryoperated digital thermometer designed to measure temperature of wide range between -50 to 150° C with reliable accuracy.
Once removed from the warming cabinet, the tested fluid bag was attached to a standard line giving set with total capacity of 20 ml fluid volume (Fresenius Kabi, Homburg, Germany). The fluid temperature was checked at two different points
I. Temperature point A (The bag end): Via a 14 G cannula inserted into the bag and connected to a three-way tap for easy and frequent aspiration of 20 ml fluid sample to enable measurement of bag-fluid temperature every three minutes for fifteen minutes.
II. Temperature point B (The patient end): Via a three-way tap placed at the distal end of the infusion giving set to enable easy and frequent aspiration of 20 ml fluid sample at the patient delivery point every three minutes for fifteen minutes.
All the fluid samples were collected using a 20 ml syringe and placed into a plastic cup for immediate temperature measurement performed by blinded investigators (JW and JVR).
Statistical analyses were performed using IBM SPSS Statistics 22.0. The mean value of the two recordings made at each point and time was used in the analysis. Unless otherwise stated, reported statistics are mean (SD). Comparisons were made using one sample t-tests and Repeated Measures ANOVA with post hoc comparisons where appropriate and the measurements were compared at each time point:
I. Temperature difference between Point A and Point B.
II. Temperatures at Point A versus the warming cabinet temperature
III. Temperatures at Point B versus the warming cabinet temperature.
IV. Temperature difference between Point A and room temperature.
V. Temperature difference between Point B and room temperature.
Results
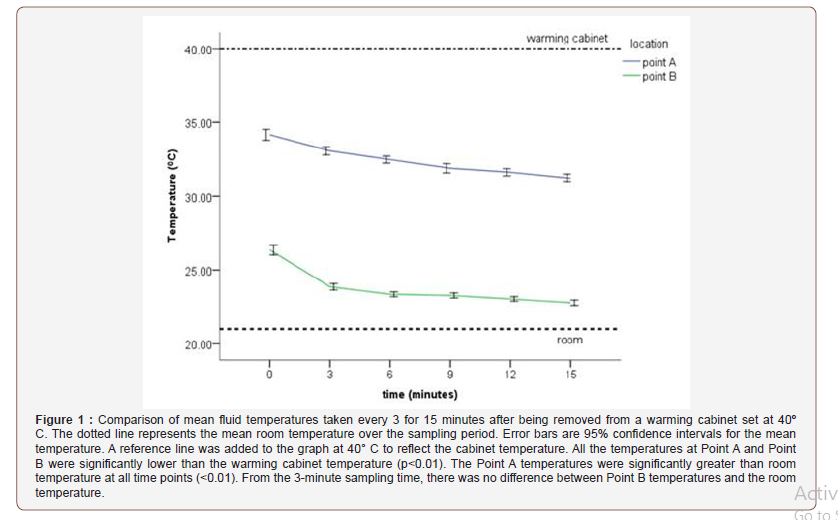
Throughout the study period, the cabinet temperature was maintained at 40° C and the ambient room temperature ranged from 20.7 to 21.5 ºC with a mean of 21±0.23ºC. All temperatures recorded at each sampling location for each sampling time are presented in Table 1 and Figure 1.
Table 1: Summary of mean (SD) temperature recordings (ºC) for each sampling time and location (n = 24) and the temperature difference between Point A and Point B. All times points were associated with a significantly lower temperature at Point B compared to Point A (p<0.01).
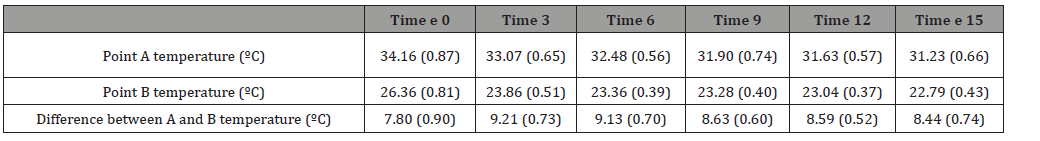
Differences in temperature between point A and point B (Table 1 and Figure 1)
There was an overall significant difference between the temperatures taken at Point A versus Point B (F1,46 = 4382.63, p < 0.001). Post hoc comparisons showed significant differences between Point A and B at each sampling time (p < .001). Temperatures were significantly lower at Point B for all time points with a minimum difference occurring at time 0 (7.8±0.9ºC); and a maximum difference at 3 minutes (9.21±0.73ºC). At the end of the study period, the difference between point A and Point B was 8.44±0.74ºC.
Bag temperature (point A) compared to warming cabinet temperature
The starting fluid bag temperatures at point A were significantly lower (p < 0.001) by a mean of 5.84 ºC (0.87) from the starting temperature of 40ºC after being attached to the giving set. However, throughout the study period, the mean bag temperature remained above 30 ºC (Figure 1).
Distal end delivery point temperature (point B) compared to warming cabinet temperature
The starting distal end delivery point temperatures at point B were significantly lower (p<0.001) than the warming cabinet temperature (40 ºC) by a mean of 13.6 (0.81) ºC. Throughout the study, the mean temperature measured at point B continued to decrease. At the end of the study period, the mean difference between the two temperatures was 17.21 (0.43) ºC (Figure 1).
Differences between Point A temperature and room temperature
The bag temperature was significantly greater (p<0.01) throughout the study period. It was 13.0±0.87ºC greater at time 0 and this difference gradually decreased to 10.0±0.66ºC greater than room at the end of the study period (Figure 1).
Differences between Point B temperature and room temperature
The Point B temperature was 5.2±0.81ºC greater than room temperature at time 0 but this difference quickly decreased with no significant difference (1.6±0.43ºC) at the 3-minute sampling time (Figure 1).
Discussion
It is acknowledged that perioperative hypothermia is a common and serious complication of surgery under general anaesthesia, regional anaesthesia or even deep sedation [1,2]. Interference with the normal thermoregulation processes that occur at two different levels is proposed. The hypothalamus, which is the primary center for thermoregulatory control of maintaining the patient’s temperature within normal limits and at the spinal cord level where some integration and thermoregulation may also occur. Peripherally, hypothermia under anaesthesia may occur secondary to peripheral vasodilation leading to heat loss from the patient through radiation, conduction, convection as well as evaporation [11]. Failing to counteract this anesthetic induced complication will put the patient at risk of several detrimental effects including, prolonging the duration of action of anesthetic agents and muscle relaxants. This may subsequently delay the post anesthetic recovery and even cause post anesthetic thermal discomfort. Mild perioperative hypothermia significantly increases perioperative blood loss and augments allogenic transfusion requirements [11]. A reduction of the patient’s core temperature by only 1.9ºC triples the incidence of surgical wound infection following colon resection and increases the duration of hospitalization by 20% [12]. This may be caused by a combination of hypothermia-induced adverse effects on antibody and cell mediated immune defenses, and alteration in oxygen availability in the peripheral wound tissues. Mild lowering in the patient’s temperature may also triple the incidence of postoperative myocardial complications [1,2,3,12]. In view of this, even mild perioperative hypothermia should be avoided because it can cause significant perioperative complications and add to the patient’s care costs. Responsibility for patient normothermia, invariably falls on the anesthetist.
There are many recommendations and suggestions for maintenance and monitoring of temperature. Skin surface warming for 20 minutes immediately before anaesthesia (pre-warming) minimizes initial redistribution hypothermia [13]. It is suggested that the patient’s body temperature should be above 36° C before induction of anesthesia and should be measured continuously throughout surgery. Active warming should be applied when anaesthesia time is >60 minutes [14]. Effective methods of active warming are forced-air warming or conductive warming, provided that enough skin surface is available [13]. Increasing the operating room temperature and warming of irrigation fluids are adjunctive methods [15]. Several methods have been already used to warm the IV fluids given to patients under anaesthesia with variable degrees of efficiency [7-10]. Evaluating all the different methods of warming the IV fluids given to patient under anaesthesia is out of the scope of this study.
Warming fluids in an electrically operated cabinet is commonly used in many hospitals including our own. In this study, we found that the measured mean temperature of the fluid bags kept in a warming 40-degree cabinet were lower than the set temperature but remained above 30 for at least 15 minutes when used in a room temperature of 21±0.23ºC. When compared to the environmental temperature, the mean fluid-bag temperature was 12.96±0.87ºC higher at time 0 min. At the end of the 15-minute period of exposure outside the cabinet was 10.03 ±0.66ºC higher than the room temperature.
This may suggest that the over the study period the environment did not have a significant effect on the temperature in the fluid bag, whilst they sat outside the warming cabinet. Therefore, the bag continues to feel warm to the touch. Most anesthetists would therefore assume that this is useful because the bag temperature remains warm and gives the impression that we are infusing warm fluid to the patient. However, the process of fluid running down the giving set was associated with a marked reduction in this temperature. Clinically, we could say that these temperatures were almost the same as the environment after 3 minutes of running the bag on the drip stand.
The effect of the IV giving line on the temperature of the fluid as it ran down the tubing was significant. In particular, we found that the mean temperature at the distal end of the giving set (point B) dropped to only 1.79 ±0.20° C above room temperature after 15 minutes. This would suggest that the process of warming fluids in a cabinet would probably be of minimal benefit in maintaining the patient’s temperature within normal surgery duration. However, contradictory results have been reported in other studies. In 76 adults undergoing short duration surgery, Andrzejwski and colleagues [16] found that pre-warmed fluid administered under pressure within 30 min of its removal from a warming cabinet where it was kept at 41° C for at least 8 hours, was as efficient at preventing perioperative hypothermia as that delivered through an in-line warming system.
Woolnough and colleagues. assessed the effect of warming IV fluids in 75 women undergoing combined spinal epidural anaesthesia for Caesarian Sections (CS) and they concluded that pre-warmed fluids stored in warming cabinet set at 45° C and given under pressure when needed, is as efficient and cheaper than a hot line fluid warmer but there was no significant difference in the incidence of chills between CS patients receiving warm IV fluids and those receiving room temperature fluids [17]. Yokoyama and colleagues found that administration of pre-warmed IV 1L colloid followed by pre-warmed 1L crystalloid maintained core temperature during CS under spinal anaesthesia and induced higher Apgar scores and umbilical arterial pH. To maintain the temperature of the given pre-warmed colloid and crystalloid, the fluid was given using an IV warmer coil kept in a water bath warmer [18].
Goya and colleagues also studied the effect of warming IV fluids in the maintenance of core body temperature during CS under spinal anaesthesia in 64 patients randomized between two groups- one group received room temperature fluid (22° C) and the other warm I.V. fluid (39° C). They found that the core temperature reduction was less among the mothers who received warm fluids compared to the other group (p <0.01). However, there was no significant difference in the incidence of chills in the two groups [19]. McCorroll and colleagues also failed to show that warming IV fluids prevented hypothermia among women undergoing elective CS [20].
However, in our study, measuring the temperature of the tested fluid bags has found that the fluid has lost the gained heat within 3 minutes only. After 15 min from the beginning of the test the temperature of the fluid at the patient end was almost equal to room temperature. This agrees with John and colleagues’ assessment in their review article assessing the performance and clinical application of different perioperative warming devices, where they suggested that the main set back with the use of pre-warmed fluids is the potential cooling effect, that lower flow rates through long thin tubing has, on the delivery fluid temperature. Their suitability in pediatric cases has been questioned [21]. In addition, we note that the studies supporting the efficiency of the warming cabinet technique in pre-warming fluids during surgery, differed to our technique by giving the fluids quickly under pressure within short time.
Conclusion
Warming cabinet technique usually used in theatre to warm fluids before giving them intravenously to patients as a part of the anesthetic management is still a controversial technique. Our study suggests that the warming cabinet method is inefficient as it does not deliver the intravenous fluids to the patients as warm as we think is required. Moreover, the use of the warming cabinet is probably allowing a false sense of security for the anesthetists and potentially subjecting the patient to the risk of unnoticed hypothermia especially if the patient’s temperature is not monitored during surgery. Therefore, we recommend that the practice of using the studied method of fluid warming needs to be revised and may need to be replaced by another method which could be more clinically effective and reliable in keeping the patient’s temperature within normal limits. Further studies would be useful.
Acknowledgement
We thank Professor Farouk Messahel for reviewing the article. We also thank Mr S Mcfarlane and the operating room personnel for their help during the study.
Conflict of Interest
No conflict of interest.
References
- Merry AF, Mitchell SJ (2018) Complications of Anaesthesia. Anaesthesia 73: 7-11.
- Marcos D, Daniel EB (2010) Thermoregulation: physiology and clinical considerations during sedation and general anaesthesia. Anesth Prog 57(1): 25 -33.
- Tortorici Michael A, Kochanek, Poloyac SM (2007) Effects of hypothermia on drug disposition, metabolism, and response: A focus of hypothermia mediated alterations on the cytochrome P450 enzyme system. Crit Care Med 35(9): 2196-2204.
- Vaughan MS, Vaughan RW, Cork RC (1981) Postoperative hypothermia in Adults: Relationship of Age, Anesthesia, and Shivering to Rewarming. Anesth Analg 60(10): 746-751.
- Ott DE, Reich H, Love B, McCorvey R, Toledo A, et al. (1998) Reduction of Laparoscopic-induced hypothermia postoperative pain and recovery room length of stay by preconditioning gas with the Insuflow®Device: A prospective controlled multi-center study. JSLS 2 (4): 321-329.
- Smith CE, Gerdes E, Sweda S, Myles C, Punjabi A, et al. (1998) Warming intravenous fluids reduces perioperative hypothermia in women undergoing ambulatory gynecological surgery. Anesth Analg 87(1): 37-41.
- Henker R, Bernardo LM, O Connor K, Sereika S (1995) Evaluation of four methods of warming intravenous fluids. J Emerg Nurs 21(5): 385-390.
- Ohtsuka N, Yamakage M, Chen X, Kamada Y, Namiki A (2002) Evaluation of four techniques of warming intravenous fluids. J Anesth 16(2): 145 -149.
- Schultz JA, Sims C, Bissonnette B (1998) Methods for warming intravenous fluids in small volumes. Can J Anaesth 45(11): 1110 -1115.
- Anshus JS, Endhal GL, Mottley JL (1985) Microwave heating of intravenous fluids. Am J Emerg Med 3(4): 316-319.
- Polderman KH (2009) Mechanism of action, physiological effects, and complications of hypothermia. Crit Care Med 37: S186-S202.
- Reynolds L, Beekmann J, Kurz A (2008) Perioperative complications of hypothermia. Best Practice and Research Clinical Anesthesiology 22(4): 645-657.
- Janicki PK, Higgins MS, Janssen J, Johnson RF, Beattie C (2001) Comparison of two different temperature maintenance strategies during open abdominal surgery: Upper body forced air warming versus whole body water garment. Anesthesiology 95(4): 868-874.
- Alexander Torossian (2008) Thermal management during anaesthesia and thermoregulation standards for the prevention of inadvertent perioperative hypothermia. Best Pract Res Clin Anaesthesiol 22(4): 659-668.
- Kurz A (2008) Physiology of Thermoregulation. Best Pract Res Clin Anaesthesiol 22(4): 627-644.
- Andrzejowski JC, Turnbull D, Nandakumar A, Gowthaman S, Eapen G (2010) A randomized single blinded study of the administration of prewarmed fluid vs active fluid warming on the incidence of peri-operative hypothermia in short surgical procedures. Anaesthesia 65(9): 942-945.
- Woolnough M, Allam J, Hemingway C, Cox M, Yentis SM (2009) Intraoperative fluid warming in elective Caesarean section: a blinded randomized controlled trial. Int J Obstet Anesth 18(4): 346 -351.
- Yokoyama K, Suzuki M, Shimada Y, Matsushima T, Bito H, et al. (2009) Effect of administration of pre-warmed intravenous fluids on the frequency of hypothermia following spinal anesthesia for Caesarian delivery. J Clin Anesth 21(4): 242-248.
- Goyas P, Kundera S, Sharma S (2011) Efficiency of intravenous fluid warming for maintenance of core temperature during lower segment Caesarian sections under spinal anaesthesia. Journal of Obstetric Anesthesia Critical Care 1: 73-77.
- McCorroll SM, Cartwright P, Weeks SKs (1986) Warming intravenous fluids and the incidence of shivering during Caesarian sections under epidural anaesthesia. Canadian Anaesthesia Society Journal 33: S72-S73.
- John M, Ford J, Harper M (2014) Peri operative warning devices: performance and clinical application: review article. Anaesthesia 69(6): 623-638.
-
Essam Abdelrazek, James Walker, Johan Victor Rehnberg, Sharon Docherty, Ash Byrom, Akbar Vohra. Quality Assessment of the Efficiency of the Warming Cabinet Method in Warming Fluids in Anaesthesia. Annal Pub Health & Epidemiol. 1(1): 2019. APHE.MS.ID.000502.
-
Hypothermia, Prevention, Physiology effects, Surgery, Anesthesia, Patients, Intravenous fluids, Neurological, and Metabolic conditions, Evidence.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






