 Research Protocol
Research Protocol
Monitoring of Cadmium (II) Ions in Seawater from Suez Bay, Egypt
Khalid M El Moselhy1*, Eman M Saad2, Reda F Elshaarawy2 and Safaa A Mahmoud2
1Department of Marine Pollution, National Institute of Oceanography and Fisheries, Egypt
2Department of Chemistry, Suez University, Egypt
Khalid M El Moselhy, Department of Marine Pollution, National Institute of Oceanography and Fisheries, Egypt.
Received Date: December 30, 2019; Published Date: January 10, 2020
Abstract
Cd(II) ion is one of the highly toxic metal ions on the priority pollutant list, therefor this study is focused on the monitoring of Cd(II) ions in Suez Bay water, Egypt. The level of Cd (II) ions in Suez Bay water are ranging from 0.02 to 0.58 μg/L with insignificant difference in the local mean values; while it was significant (p<0.05) for the seasonal variations. Annual mean of the Cd ions in the present study are higher than the background level for open ocean and coastal water.
Keywords: Cadmium; Monitoring; water; Suez Bay
Introduction
Pollutants can introduce to the aquatic systems mainly through industrial and agriculture effluents. Metal ions are considered as one of the most famous water pollutants, due to their toxicity, nondegradability and accumulation in living organisms. Cadmium (II) ion is highly toxic pollutant found at the priority of toxicity list, as a carcinogenic toxic agent. Cd(II) ions are released into aquatic medium from electro-plating industries, batteries, phosphate fertilizers, mining, pigments, stabilizers and alloys [1]. The World Health Organization suggested that the acceptable concentration of cadmium in potable water should be 0.003 mg/L [2]. Thus, it is required to treat the concentration level of cadmium in wastewater before disposal into the environment. Accordingly, this study is conducted to monitor Cd(II) ions in seawater from the western side of northern part of the Gulf of Suez “Suez Bay”, Egypt.
Materials and Methods
The Gulf of Suez extends for about 250 km from Port Tawfiq Harbor to Shadwan Island. Six stations were chosen in the western side of Suez Bay for water sampling with regards to the sources of pollution in the current study area (Figure 1).

Station 1: Summer Palace Hotel beach (west of Port Tawfiq Harbor)
Station 2: El-Zeitiya Harbor
Station 3: El-Kabanon beach
Station 4: Electric Power Plant of Ataka
Station 5: Beach of NIOF and
Station 6: North of the Adabiya Harbour.
Subsurface water samples (20-50 cm depth) were collected seasonally from the different stations using water sampler, and kept in acid-washed polyethylene bottles, acidified and transferred in ice-box to lab for additional analysis. The total Cd(II) ion in water samples were measured by solvent extraction [3,4] using flame atomic absorption spectrophotometer (FAAS - Perkin Elmer model Aanalyst 100), in μg/L.
All chemicals and acids were of high analytical grade and deionized water was used to prepare all solutions. The containers and glassware were soaked in 10% nitric acid and later rinsed by distilled water. Method precision was confirmed by replicate measurements of Cd ions in the water samples, and the precision was found as 13.4 %.Cd(II) ions in water was analyzed by “ANOVA” to test differences between stations and seasons (significant values, p≤0.05 for all analysis). Statistical analysis was done using Statistica X software packages for windows, and Origin Pro 9.
Results and Discussion
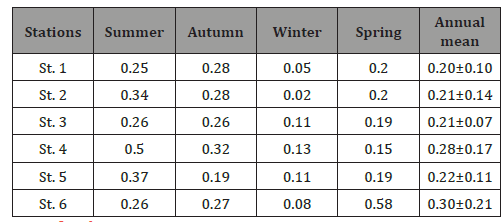
Seasonal and local distributions of Cd ion in samples of water (μg/L) collected from 6 different stations at Suez Bay were reported in (Table 1) and (Figure 2). It was ranged from 0.02 (St. 2) to 0.58 μg/L (St. 6) in winter and spring, respectively. St. 6 (north of Adabiya Harbor) is affected by the shipping, trading operations, untreated sewage from a nearby small community, and the effluent of industrial wastewater plants. ANOVA analysis reported insignificant difference in Cd ions among the studied stations (p > 0.05). Seasonally, summer recorded the maximum level of Cd ion while winter exhibited the minimum value, with moderately high concentration in autumn and spring seasons (Figure 3). The seasonal variation showed significant difference at p < 0.05; high Cd(II) ions concentration during hot seasons may be owing to increase of human activities and land-based contribution [5]. This feature could be attributed to the increase in metal accumulation rate due to higher temperature; high evaporation rate and increasing the toxicant amount flushed into the water, in particular sewage. Cd ions level in the current investigated area was in agreement with those recorded (ND-0.86 μg/L) by El Moselhy et al., El Moselhy and Hamed, El-Metwally, Zaghloul and Baleg [6- 10]. They were still lower than those recorded by Said [11] (0.12 – 1.35 μg/L). In addition, Cd ions level in Suez Bay in this study was higher than those of background concentrations in the open ocean (0.02 – 0.12 μg/L) and coastal water (0.01 – 0.17 μg/L) reported by Sadiq and Bryan & Langstone [12,13]. In contrast, the present Cd ions levels were lesser than the mandatory value for water quality, 5 μg/L [14], and threshold recommended by the Australian guidelines for marine waters, 2 μg/L [15].
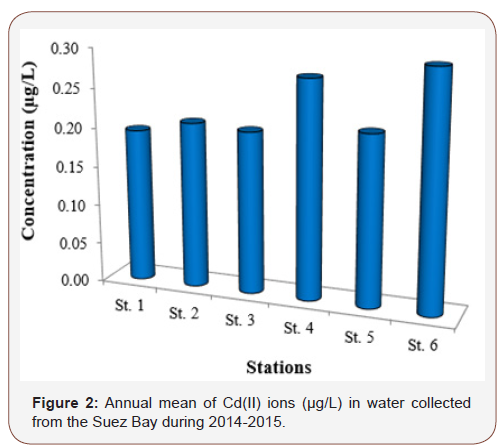
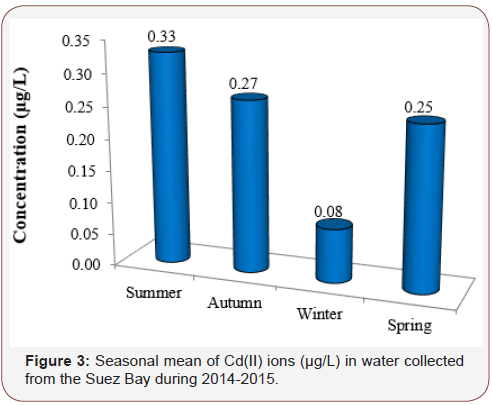
Table 1: Seasonal variations of cadmium (II) ions level (μg/L) in water collected from different stations of the Suez Bay during 2014-2015.
Conclusion
Suez Bay at the northern part of the Gulf of Suez is suffering from different pollution sources, especially oil and harbor activities. Accordingly, it expected to found high levels of pollutant such as heavy metals. The present study recorded concentrations of Cd ions in the water from the Suez Bay higher than previously reported for open ocean and coastal water, but still lower than values required for water quality. Therefore, cadmium and other metals ion must be continuously investigated in all compartment of the Suez Bay area to avoid any hazard impact of the marine life of this vital area.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Krika F, Azzouz N, Ncibi M C (2016) Adsorptive removal of cadmium from aqueous solution by cork biomass: Equilibrium, dynamic and thermodynamic studies. Arab J Chem 9: S1077-S1083.
- WHO (2008) Guidelines for Drinking Water Quality: Recommendations. World Health Organization, 3rd ed Geneva.
- Brewer PG, Spencer DW, Smith CL (1969) Determination of trace metals in seawater by atomic absorption spectrophotometry. ASTM STP 443: 70-77.
- APHA American Public Health Association (1999) Standard methods for the examination of water and wastewater (20th edition). Washington DC, USA.
- Abouhend A S, El Moselhy Kh M (2015) Spatial and seasonal variations of heavy metals in water and sediments at the northern Red Sea coast. Amer. J Water Resour 3 (3): 73-85.
- El Moselhy Kh M, Diab A A, Tolba M R, Mohamedein L I (1999) Levels of some heavy metals in coastal water, sediment and the limpet Patella sp. from the northern part of the Gulf of Suez (Suez Bay). Egypt J Aquat Biol& Fish 3 (2): 69-84.
- El Moselhy Kh M, Hamed M A (2006) Impact of land-based activities on hydrographic conditions and levels of heavy metals in water and sediments along the Mediterranean coast of Egypt. Egypt J Aquat Res 32 (2): 63-82.
- El Metwally M E M A (2014) Monitoring of heavy metals pollution in the Egyptian Red Sea coast and response of marine organisms. Ph. D Thesis, Zool Dept, Fac Sci, Mansoura Univ, 275.
- Zaghloul, Gh Y H (2015) Organic and Inorganic pollutants in Suez Canal. Ph. D Thesis, Fac Sci (Girls Br) Al Azhar Univ, pp 269.
- Baleg A O (2016) Ecological, biological and physiological studies on some fish species from Suez Canal and nearby areas. Ph. D Thesis, Fac Sc, Ain Shams Univ.
- Said R E M (2009) Ecological and biological studies on some marine annelids inhabiting the Red Sea, Egypt. M. Sc Thesis, Faculty of Science, Al-Azhar University, 169.
- Sadiq M (1992) Toxic metal chemistry in marine environments. Marcel Dekker, New Yourk.
- Bryan G W, Langston W J (1992) Bioavailability, accumulation and effects of heavy metals in sediments with special reference to United Kingdom estuaries: a review. Environ Pollut 76(2): 89-131.
- EPA (2001) Parameters of water quality: Interpretation and Standards. Published by the Environmental Protection Agency, Ireland 132.
- ANZECC Australian and New Zealand Environment and Conservation Council (1992) Australian water quality guidelines for fresh and marine waters.
-
Khalid M El Moselhy, Eman M Saad, Reda F Elshaarawy, Safaa A Mahmoud. Monitoring of Cadmium (II) Ions in Seawater from Suez Bay, Egypt. Ad Oceanogr & Marine Biol. 1(5): 2020. AOMB.MS.ID.000521.
-
Cadmium, Monitoring, water, Suez Bay, Seasonal variations, Electro plating industries, World Health Organization, Flame atomic absorption spectrophotometer, ANOVA, Statistica X software, Industrial wastewater, Coastal water, Marine water, Gulf of Suez, Harbor activities.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






