 Research Article
Research Article
Marine Debris in Green Sea Turtles along the Northern Coast of Taiwan
I-Jiunn Cheng*, Pin-Chun Chou, Ying-Tin Chan and Tsung-Hsien Li
Institute of Marine Biology, National Taiwan Ocean University, Keelung, Taiwan, ROC, 202-24
I-Jiunn Cheng, Institute of Marine Biology, National Taiwan Ocean University, Keelung, Taiwan.
Received Date: November 12, 2020; Published Date: December 09, 2020
Abstract
Sea turtles ingesting marine debris is a major problem worldwide. This is the first study ever done on the marine debris ingested by sea turtles in Taiwan, 191 dead green sea turtles from 2012 to 2019, mainly in northern coasts. We identified seven debris types and 15 colors. Plastic rope, soft and hard plastics, foam plastic and unidentified material were the most common ingested debris. White, transparent and mixture were either the common or the most common ingested color. Juveniles consumed more type of debris than the other age classes. Debris was found remain in the digestive tract for several weeks or longer prior to be found or excretion.
Keywords: Sea turtles; Marine debris; Plastic type; Plastic color; Digestive tract
Introduction
Marine debris is defined as any man-made solid waste product that is introduced into marine environment, and it has proven to have a widespread and negative impact on marine animals [1-4]. Most anthropogenic material ingested by living and dead individuals is made of plastics and pollutants [5]. The main sources of these debris are litter from ships, fishing and recreational boats, and garbage carried into the sea from land-based sources in industrialized and highly populated areas [1,2,6]. Coastal cities and towns are the main sources of plastic pollution, serving as point sources for the flow of plastic into the sea via urban and natural drainage systems [7]. Plastics are lightweight, strong, durable, and cheap, making them suitable materials for a wide range of products; they are ubiquitous on land, and make-up most of the marine litter worldwide [6,7]. Synthetic debris types include single-use plastics such as food packaging, fishing line and synthetic ‘soft bait’ lures [8]. Plastics are not biodegradable, but use in many shortlived disposable products, and have great dispersal capabilities, making them a severe global environmental threat to marine ecosystems [2,6,9]. Marine organisms may ingest plastic as a result of mistaken identity. As turtles are primarily visual feeders, they may misidentify items—such as shopping bags, and sheet plastic— as prey and actively select them for consumption [3,10-12]. The presence of plastic in their foraging areas increases the likelihood that they accidentally ingest these materials [12,13]. Marine debris can cause mortality in sea turtles directly and indirectly. Sea turtles generally die from debris ingestion when their digestive tract is obstructed, and ingested plastic materials reduce their digestive capability, often resulting in emaciation and severe starvation [13- 16]. Gastrointestinal perforation caused by swallowed hooks and hard plastic can cause chronic infection, peritonitis, gastrointestinal motility disorders, and eventual death. 52% of surviving sea turtles have likely ingested marine debris, and a reported 4% of necropsied sea turtles have died from it [15]. The sub-lethal effects of anthropogenic debris on sea turtles are presumably more common than the lethal ones, but are difficult to measure because animals can excrete marine debris through defecation, which likely becomes a major threat in the long-term. The alimentary track of sea turtles is particularly prone to plication and debris accumulation due to the inability of turtles to regurgitate items and their convoluted gastrointestinal tracts [17]. Ingesting debris can be worsening the physical conditions, diminished food stimulus, blockage of gastric enzyme secretion, lowered steroid hormone levels, delayed ovulation, reproductive failure and death following blockage of the intestinal tract [2-4, 18-20]. Debris ingestion can create a false sense of fullness and interfere with lipid metabolism, resulting in a build-up of intestinal gases and associated buoyancy disorders (floating syndrome), thus limiting the turtle’s ability to obtain food and avoid predation [4,21].Due to their hydrophobic properties, plastics are known to accumulate heavy metals and other toxins, such as PCBs, from the marine environment, which can also be released during digestion. These materials can alter hormonal processes, specifically those regulated by sex steroids [1,2,11,22,23]. Entanglement effects include abrasions, lesions, constriction, scoliosis, or the loss of limbs, as well as increased drag, which may reduce foraging efficiency and avoidance ability [7]. There are many studies conduct on plastic ingestion of different species, gender, growth stage, etc. [15,17]. However, it is important to compare the plastic ingestion between species, sexes, and growth stages of sea turtles in the same region, because different status and species sea turtles may come from different sites and may select ingest different type of debris. Then, one can determine if and how the plastics are ingested differently based on the different species or status. Then, the proper recommendations can be made.
Material and Methods
Sample collection
Samples were mainly collected from Hualien County in eastern Taiwan to Taichung County in western Taiwan from 2012 to 2019 (Figure 1).
A total of 191 turtles were collected. Body length and body
weight were measured prior to necropsy. Body length was
expressed as curved carapace length (CCL), measured from the
tip to the notch of the dorsal carapace. Necropsy was carried out
on turtles that were either stranded dead or rescued but did not
survive during rehabilitation. The entire digestive tract—including
the esophagus, stomach, and intestines—was removed for analyses.
Gender was determined during necropsy by visually examining the
morphology of the gonad [24]. Age class was determined based on
body size; CCL<35 cm was considered hatchling, 35
Sample treatment
First, the gross morphology was observed to determine the body conditions and if the turtle was entangled by foreign materials. Necropsy was performed following the procedure of [24]. Samples were removed from analysis if their digestive tract was decayed beyond recognition, or was fractured during the sampling procedure. Intact digestive tracts were brought back to the laboratory and stored in a refrigerator for later analysis. In the laboratory, the digestive tract was cut open and the contents of the esophagus, stomach, and intestines were washed with tap water and filtered through a 0.05-cm mesh filter. Marine debris was collected from the filter and dried at room temperature. Debris from each digestive tract section was stored in plastic bags. The different parts of the digestive tract were separated following [2]. The esophagus stretches from the anterior end of the digestive tract to the gastroesophageal sphincter. The stomach is found from gastroesophageal sphincter to the constriction duodenal. The intestine is found from constriction duodenal to the end of the digestive tract. The digestive tract is further divided into five sections: sections 1 and 2 refer to the small intestine, and sections 3 to 5 refer to the large intestine.
Identifying marine debris
Marine debris was identified visually, with a stereomicroscope
if necessary. Debris was separated based on material type (soft
plastic, hard plastic, cotton thread, plastic rope, foam plastic,
textile, rubber, metal, or unidentified) and color (transparent,
semi-transparent, white, black, grey, silver, green, brown, orange,
yellow, purple, blue, green pink, red, or mixture). Soft plastic was
defined as a deformable non-thread plastic material, like straws
or plastic bags. Hard plastic was defined as a non-deformable nonthread
plastic material, like fragments of table ware or plastic cups.
Plastic rope was defined as thread-like plastic material, like fishing
line or fishing nets. Foam plastic was defined as foaming plastic,
including artificial sponges or crash cushions. Textile included
cloth. Rubber included parts of tile. Metal included fishing hooks.
Unidentified material was defined as any material that was decayed
beyond recognition. Color was determinate visually. In addition to
anthropogenic materials, natural debris were also collected and
identified, i.e. wood (branches, wood products). All materials were
counted and weighed (to 0.001 mg) and expressed as a quantity,
weight, and abundance (percentage of its weight to the total of all
debris found). To avoid repeating calculations, only particles larger
than 0.5 cm long were included. This is because most material
smaller than 0.5 cm long is a fragment of medium to large debris
in the digestive tract. In order to determine the frequency at which
each consumed debris item occurred, the frequency of occurrence
(FO) was calculated as FO (%) = (Ni/Nt) * 100, where Ni is the
abundance or weight of item i and Nt is the total abundance or
weight of all debris. FO was separated into three categories:
>50%: the most common, 50% < FO ≤ 10%: common, and <10%:
uncommon [25]. Debris that weighed too little to measure were
not included in the FO analysis. The index of relative importance
(IRI) was determined to measure the relative impact that each item
had on the turtle’s health. It was calculated as IRI of species = (%
occurrence * % weight)/100 [26]. We separated the IRI into three
categories based on von Brandis et al. [26]: 30< IRI ≤100: the most
important, 30 BCI = (turtle body mass/SCL3) * 1000 [27]. The body burden (BB) of each species was calculated to
determine the impact that each ingested debris had on the body
condition: BB = mg debris weight ingested/kg turtle weight [28]. The Shapiro-Wilk test was used to determine if the marine
debris showed a normal distribution. If not, the Kruskal-Wallis
test was used to compare the differences among material types
and colors of marine debris [29]. One-way Analysis of Variance
(ANOVA) was used to determine whether the samples had a normal
distribution. Turkey’s post-hoc test was used to determine the
differences among variables [29]. The Pearson correlation test was
used to determine if the body burden was correlated with the body
size (straight carapace length; SCL), and if the body condition (BCI)
was correlated with the abundance of ingested debris. Eighty-six percentage of the stranded turtles ingested marine
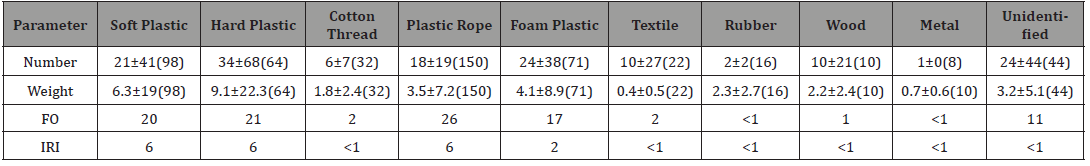
debris. Analysis showed that the hard and soft plastics, plastic rope,
foam plastic and unidentified material were the common while
unimportant ingested debris (Table 1). Table 1:Number and weight-specific debris ingested, FO (%) and IRI of different debris type ingested by green turtles. Unit: mg in weight. Statistical analysis showed that the turtles ingested more soft,
hard plastics and plastic rope than the metal, cotton thread, and
rubber. They also ingested more foam plastic than rubber, metal,
cotton thread, and textile. Turtles ingested more unidentified
material than rubber or metal (p < 0.001 in all cases). In term of
debris weight, green turtles ingested more hard plastic than textile
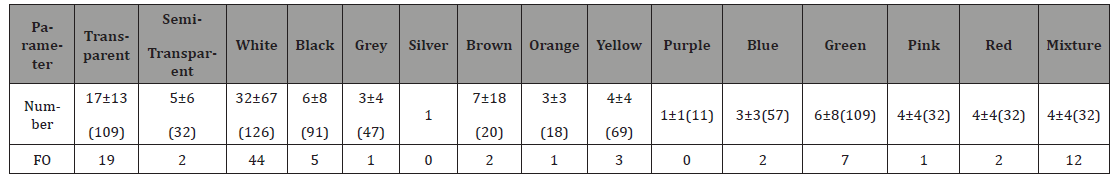
or wood (p < 0.001). For the color debris, white was the common
and most important ingested color, transparent was the common
and important ingested color, and mixture was the important
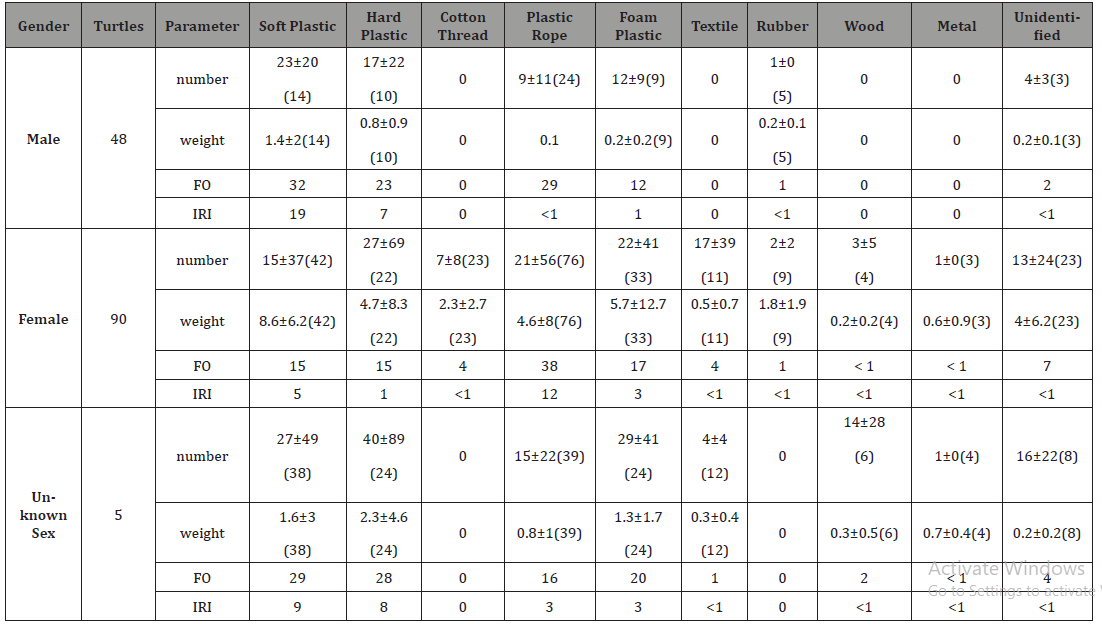
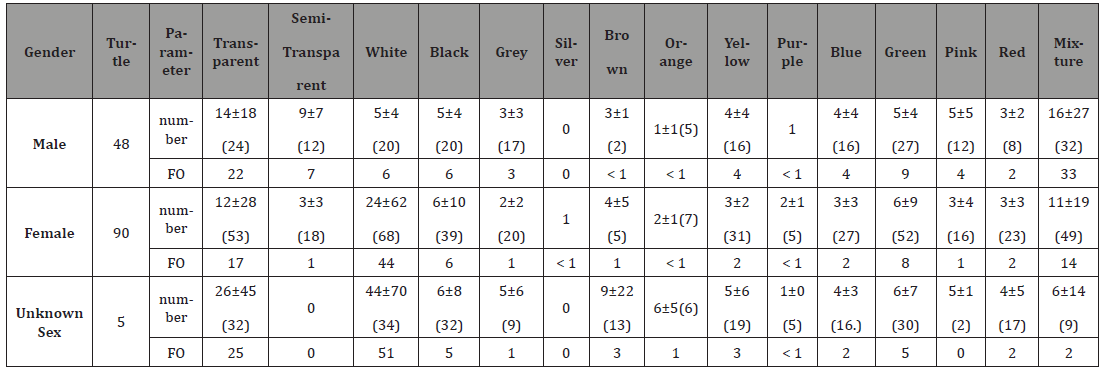
ingested color (Table 2). Table 2:Number-specific and FO (%) of ingested different color debris by green turtles. Forty-eight males, 90 females and 53 unknown sex were
recorded. The study found that for all genders, soft plastic and
plastic rope were the common and either important or unimportant
ingested debris. Hard plastic and foam plastic were the common
while unimportant ingested debris of male and unknown sex
turtles (Table 3). Statistical analysis showed that male turtles ingested less
rubber and cotton thread than the other debris (p< 0.001). In term
of debris weight, male turtle ingested more hard and soft plastics
than the cotton thread. They also ingested more hard plastic than
plastic rope (p< 0.001). Unknown sex turtles ingested more foam
plastic than metal (p= 0.001). For all genders, transparent was the
common ingested color. Mixture was the common ingested color of
male and unknown sex turtles, and white was the common ingested
color of the female and unknown sex turtles (Table 4). Statistical analysis showed that the turtles ingested more white
debris than purple and red, and ingested more transparent than
purple debris (p < 0.001). Comparison among different genders
showed that male turtle ingested unidentified debris than the
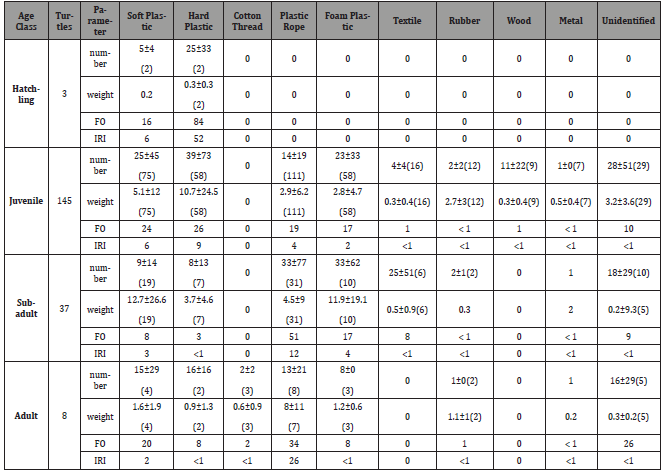
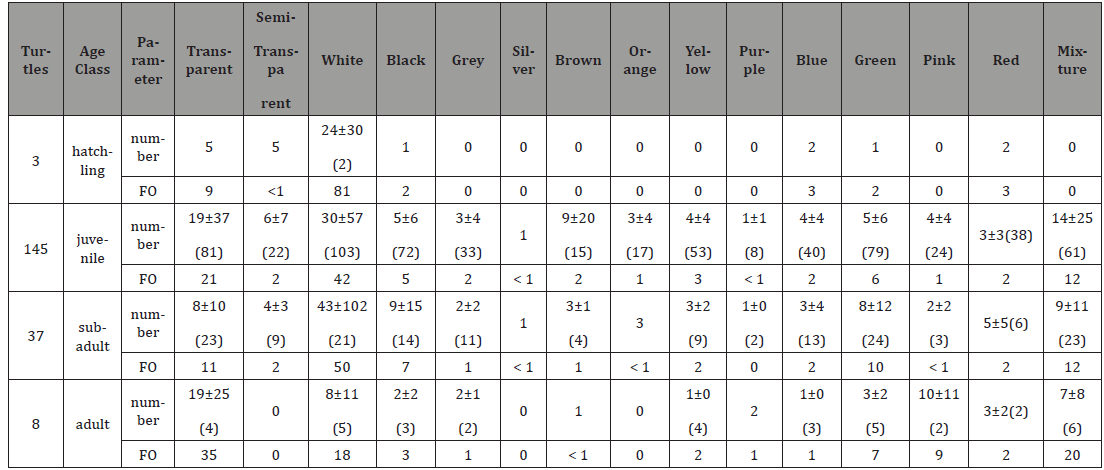
female turtles (p = 0.013). Three hatchlings, 143 juveniles, 37 sub adults and 8 adults were
recorded. The study found that the soft plastic was the common
while unimportant ingested debris of hatchling, juvenile and adult
turtles. Plastic rope was either the common or most common and
either important or unimportant ingested debris of juvenile, sub
adult and adult turtles. Hard plastic was either common or most
common and either important or unimportant ingested debris
of both hatchling and juvenile turtles. Foam plastic was common
while unimportant ingested debris of both juvenile and sub adult
turtles. Unidentified material was the common while unimportant
ingested debris of both juvenile and adult turtles (Table 5). Table 3:Number of turtles, number- and weight-specific, FO (%) and IRI of each ingested debris type of different gender of green turtles. Unit is mg for weight. Table 4:Number of turtles, number-specific and FO (%) of ingested different color debris by different genders of green turtles.
Table 5:Number of turtles, number- and weight-specific, FO (%) and IRI of ingested different debris type of different age class of green turtles. Unit: mg in weight. Statistical analysis showed that the juvenile turtles ingested
more hard plastic and foam plastic than the metal, rubber, textile
and cotton thread. They also ingested more soft plastic than the
rubber and textile (p < 0.001). Compare among different age classes
showed that juvenile ingested more types of debris than the other
age classes. Statistical analysis showed that juvenile ingested more
pieces of debris than adult turtle (p= 0.039). In term of ingested
color, white was either the common or most common ingested color of all age classes. Transparent and mixture were the common
ingested color of juvenile, sub adult and adult turtles (Table 6). The number of either SCL or debris ingestion of both hatchling
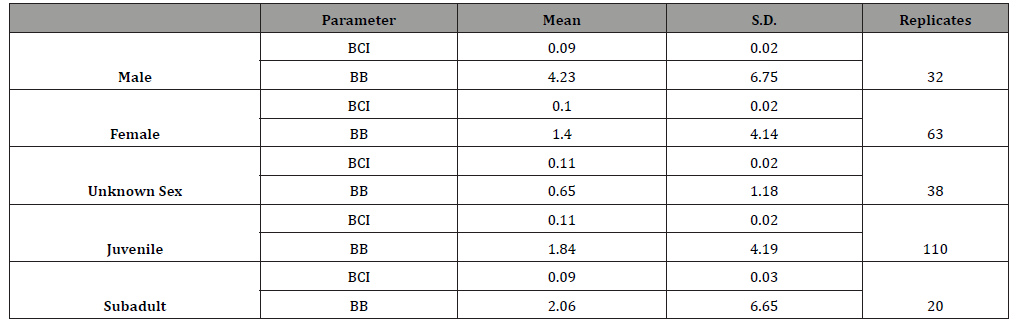
and adult turtles were less than 2, these two parameters were
excluded from analyses. The mean BCI ranged from 0.09 (male
and subadult) to 1.84 (juvenile), and mean BB ranged from 0.65
(unknown sex) to 4.23 (male) (Table 7). Analysis showed that the BCI of the genders and age classes
were not influenced by the ingested debris weight (p = 0.57 for
male turtles, p = 0.982 for female turtles, p = 0.393 for unknown
sex turtles, p = 0.962 for juvenile turtles, p = 0.626 for subadult
turtles). Except female turtle, BB was also not influenced by the
ingested debris abundance (p = 0.63 for male turtles, p = 0.578
for unknown sex turtles, p = 0.175 for juvenile turtles, p = 0.903
for subadult turtles). For the female turtle, body burden (BB) was
found increased with the straight carapace length of the turtles (BB
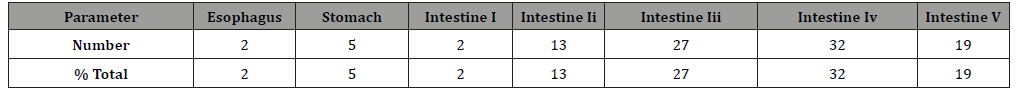
= -2.921+0.0899(SCL), n = 63, r = 0.276, p = 0.029). (Table 8) Fifty-night turtles were available for determining the
position of debris within the digestive tracts. A total of 100 pieces
of debris were collected. Analyzed showed the debris contained in
each section of digestive tract were 2% in the esophagus, 5% in the
stomach, 2% in stomach I, 13% in stomach II, 23% in stomach III,
32% in stomach IV and 19% in stomach V. Table 6:Number of turtles, number-specific and FO (%) of ingested different color by different age class of green turtles. Table 7:BCI and BB of different gender and age class of green turtles. Table 8:Number and proportion of debris contained in each section of the digestive tract. Marine debris in total, represented by four major types: plastics,
ropes, Styrofoam and monofilament lines [30]. In this study, except
the hatchlings, soft plastic and plastic rope were the common and
either important or unimportant ingested debris. Soft plastic was
the common while unimportant ingested debris of hatchling. Hard
plastic and foam plastic were the common while unimportant
ingested debris, mainly of male, unknown sex, hatchling and juvenile
turtles. Unidentified material was the common while unimportant
ingested debris, mainly of juvenile and adult turtles. Thus, different
gender and age class might select ingest different debris types, the
major ingested types were similar. Overall, soft and hard plastics,
plastic rope, foam plastic and unidentified material were the major
ingested debris of green turtles in Northern Taiwan. Plastic ropes
includes mainly the fishing lines such as monofilaments rope, and
synthetic ‘soft bait’ lures rope for wrap up bags, etc. They are the
marine debris originate mainly from the fishing vessels [4,8,31].
Results of this study suggested that the marine debris is a major
source of ingested debris in the nearshore waters of Taiwan.
High fishing activities may responsible for this result. In addition,
plastic rope can also entangle marine debris [20], or macroalgae
[2] which also ingest by accident. Both soft and hard plastic were
either the common or most common ingested debris. Soft plastics
(i.e. synthetic debris) include sheetlike plastic, single-use plastics
such as food packaging and bags. Hard plastic includes plastic cap,
piece of plastic bottles etc. [1,6,8,32]. These debris mainly derived
from land sources of high human activities or dumpsters, and
transport to the sea as garbage. Sea turtles feed on soft and hard
plastics because they mistake for the natural foods [3,13,14,33].
Plastic may also become the base for the growth of plankton or
algae [13,34], results in the ingestion by accident [2]. Unidentified
material was the common while unimportant ingested debris for
green turtles. Green turtle is an omnivorous feeder, they are less
selective compare to the carnivorous sea turtles [2,23]. Thus, it
may include unidentified material as the common ingested debris.
The statistical analysis showed that the green turtles ingested least
amount of cotton thread and rubber. These two debris may be least
expose to the animal. Turtles were found ingested less amount of
metal, textile, cotton thread, and rubber than the major ingested
debris such as soft and hard plastics, plastic rope and foam plastic.
It is possible that these materials were occasionally encountered by
the green turtles. Except the male turtles, white was the common and most
important ingested color debris. Except the hatchling turtles,
transparent was the important ingested color and except female
and hatchling, mixture was the important ingested color debris.
Thus, white, transparent and mixture were the major ingested
color debris. Turtles ingested less purple and red debris than white
and transparent debris. It is possible that the encounter of these
color debris was less frequent than the major ingested color. Again,
green turtles of different gender and age class might select ingest
different color. However, the major ingested types were similar.
Overall, white, transparent and mixture were the common ingested
color for gre White and transparent seem the most widespread
colors of floating bags, thus the highest frequent ingested debris
[4,5,11,19]. The other possibility is that sea turtles can distinguish
colors [35] and prefer certain colors when feeding [23]. Sea turtles
in the nearshore shallow waters have stronger color selection than
the ocean species. A large part of the nearshore species’ diets is
jellyfish or cephalopods [36]. They may thus mistake white and
transparent debris for food and actively ingest them [3,4,10,23].
Other colors were also commonly ingested by sea turtle may relate
to the mixture of these colors with the predominant colors or
encrusted by natural preys [10,12]. Turtles select ingest mixture
color may be due to the mixture of marine debris with the food they
ingested. This study found that the major ingested debris changed with
the age class. For the hatchlings, soft and hard plastic was either the
common or most common and either the important or unimportant
ingested debris. For the juvenile turtles, soft plastic, plastic rope,
foam plastic and unidentified material was the common or most
common and either the important or unimportant ingested debris.
For the subadult turtles, plastic rope and foam plastic were the
common while unimportant ingested debris. For the adult turtles,
plastic rope and unidentified material were the common or most
common and either the important or unimportant ingested debris.
Thus, it is clear that juvenile turtles ingested more debris types
than the other age classes. However, there was no trend of change
in color debris ingested among different age classes. Ingestion can
occur at all stages of a sea turtle’s lifecycle; however, it appears to
be most frequent in juvenile and pelagic stages [12,17,23]. Ocean
plastics are a combination of floating plastics (subject to wind
stress) and neutrally buoyant plastics in the mixed layer [12,20,23].
Oceanic life-stage turtles were significantly more likely to ingest
debris (plastic and proportionally more sheets and line) than the
neritic life-stage turtles [17,20,28]. Juvenile turtles experienced
the shift of foraging habitat from pelagic oceanic to benthic neritic
habitats. Thus, they foraged more debris type than the other age
classes [37]. Relationship between BB and ingested debris weight
and between BCI and body size (SCI) of different gender and age
class Sea turtle increase debris ingestion as they grow [3,15,33].
Sea turtles, however, may decrease debris ingestion through
naivety and/or ontogenetic shifts in diet or decrease the chance
of contact marine debris by shift their habitat with age [28,37]. In
this study, except the body burden (BB) of female turtle increased
slightly with the body size, no relationship was found between BB
and body size and between BCI and number of debris ingested in all genders and age classes. It is possible that the major size range of
this study was too narrow; 75% was juvenile (35 to 55 cm in SCL).
Thus, no significant relationship can be detected. This study found that 78% of the ingested debris was in the
large intestine (section III to V in Table 8). Similar results were
found in the other studies [9,28,34]. Most turtles consumed debris
several weeks to months prior to egestion or be found [28,34].
Marine debris thus can affect sea turtles through dietary dilution as
it passes throughout the digestive tract and is ultimately excreted
[4,9]. Marine debris might remain in the digestive tract for several
weeks or longer in this study. The above arguments indicate that
the ingestion of anthropogenic debris does not kill sea turtles in
Taiwan directly, but a sublethal effect cannot be ruled out [28,38].
More research on this topic is warranted if we wish to understand
this problem and find a proper solution to it. Anthropogenic debris is a “tragedy of the commons” that has
become a major threat to the marine ecosystem [1,2,39]. The recent
prevalence of the novel coronavirus (SARS-CoV-2) is expected
to substantially increase the numbers of disposal facial masks
being used; this will in turn increase the amount of plastic and
microplastic in the ocean in the near future. It is thus important
to determine how debris, especially plastic pollutants, affect the
health of coastal environment. Sea turtles are a flagship animal,
and thus the study of the marine debris they ingest can reflect the
detrimental impact to the marine fauna in the region. We can also use
the flagship character of sea turtles to conduct marine environment
awareness campaigns to reduce the input of debris into the marine
environment. There are not many studies on marine debris
ingestion by sea turtles in Asia [40], and this is the first such study
to be carried out in Taiwan. Taiwan has a population of approximate
23 million people concentrated in a few major cities, most of which
are located near the coastal areas. Thus, the anthropogenic debris
that leak into nearshore waters may not directly cause mortality in
Taiwan’s sea turtles, but it can still kill these organisms over time.
It is important to identify such threats to the nearshore marine
environment so that proper management strategies can be carried
out to combat them. The other solution to the plastic problem is
to transition straight economy into a circulatory economy by
shifting “from a produce, use, dispose approach to a design, use,
re-design/re-use approach” [39-44]. This can not only decrease
the amount of trash in the environment, recycle them in a smart
way, but enhance the relevant industries. For example, a high-tech
company in Taiwan; LITEON Inc. has turned foam Styrofoam boxes
into laptop mouses and keyboards, and turned plastic bottles into
T-shirts in recent years (Personal communication). Even though
they only demonstrate the ability to convert the trash into useful
tools without become part of the business. They do, however, open
a road to find “gold mine in the trash”. The authors thank Ms. N-J Chou, Ms. C-H Chen, Ms. I-T Chan, J-H
Jen, and the volunteers in the research teams for their assistance
with the field work. This project was partly supported by a grant
from the 2019 and 2020 Rescue of Stranding and Bycatch sea
turtles in Northern Taiwan (#108-C-2 and 109-C-12) from the
Marine Conservation Council to I-J. Cheng. No conflict of interest.Statistical analyses
Result
Total debris ingestion
Debris ingestion among different sex
Debris ingestion among different age class
BCI and BB of all species and the relationship with the
body length (SCL) and ingested debris abundance
Proportion of debris in each section of the intestine tract
Discussion
Marine debris selection of green turtle in the nearshore
waters of Northern Taiwan
Marine debris color ingested by green turtles
Marine debris selection changes with the age classes
Distribution of marine debris in different section of the
intestine
Conservation implications
Acknowledgements
Conflict of Interest
References
- Campani T, Baini M, Giannetti M, Cancelli F, Mancusi C, et al. (2013) Presence of plastic debris in loggerhead turtle stranded along the Tuscany coasts of the Pelagos Sanctuary for Mediterranean Marine Mammals (Italy). Marine Pollution Bulletin 74(1): 225-230.
- Colferai AS, Silva-Filho RP, Martins AM, Bugoni L (2017) Distribution pattern of anthropogenic marine debris along the gastrointestinal tract of green turtles (Chelonia mydas) as implications for rehabilitation. Marine Pollution Bulletin 119(1): 231-237.
- Domenech F, Aznar FJ, Raga JA, Tomás J (2019) Two decades of monitoring in marine debris ingestion in loggerhead sea turtle, Caretta caretta, from the western Mediterranean. Marine Pollution Bulletin 244: 367-378.
- Yaghmour F, Bousi MA, Whittington-Jones B, Pereira J, García-Nuñez S, et al. (2018) Marine debris ingestion of green sea turtles, Chelonia mydas, (Linnaeus, 1758) from the eastern coast of the United Arab Emirates. Marine Pollution Bulletin 135: 55-61.
- Camedda A, Marra S, Matiddi M, Massaro G, Coppa S, et al. (2014) Interaction between loggerhead sea turtles (Caretta caretta) and marine litter in Sardinia (Western Mediterranean Sea). Marine Environment Research 100: 25-32.
- Leite AS, Santos LL, Costa Y, Hatje V (2014) Influence of proximity to an urban center in the pattern of contamination by marine debris. Marine Pollution Bulletin 81: 242-247.
- Vegter AC, Barletta M, Beck C, Borrero J, Burton H, et al. (2014) Global research priorities to mitigate plastic pollution impacts on marine wildlife. Endangered Species Research 25: 225–247.
- Godoy DA, Stockin KA (2018) Anthropogenic impacts on green turtles Chelonia mydas in New Zealand. Endangered Species Research 37: 1–9.
- Pham CK, Rodríguez Y, Dauph A, Carriço R, Frias JPGL, et al. (2017) Plastic ingestion in oceanic-stage loggerhead sea turtles (Caretta caretta) off the North Atlantic subtropical gyre. Marine Pollution Bulletin 121(1-2): 222-229.
- Casale P, Freggi D, Paduano V, Oliverio M (2016) Biases and best approaches for assessing debris ingestion in sea turtles, with a case study in the Mediterranean. Marine Pollution Bulletin 110: 238-249.
- Nelms SE, Duncan EM, Broderick AC, Galloway TS, Godfrey MH, et al. (2016) Reviews. Plastic and marine turtles: a review and call for research. ICES Journal of Marine Science 73(2): 165–181.
- Schuyler QA, Wilcox C, Townsend KA, Wedemeyer-Strombel KR, Balazs GH, et al. (2016) Risk analysis reveals global hotspots for marine debris ingestion by sea turtles. Global Change Biology 22(2): 567-576.
- Abreo NGS, Macusi ED, Blatchley DD, Cuenca GC (2016) Ingestion of Marine Plastic Debris by Green Turtle (Chelonia mydas) in Davao Gulf, Mindanao, Philippines. Philippine Journal of Science 145(1): 17-23.
- Hoarau L, Ainley L, Jean C, Ciccione S (2014) Ingestion and defecation of marine debris by loggerhead sea turtles, Caretta caretta, from by-catches in the South-West Indian Ocean. Marine Pollution Bulletin 84(1-2): 90-96.
- Lynch JM (2018) Quantities of marine debris ingested by sea turtles: global metaanalysis highlights need for standardized data reporting methods and reveals relative risk. Environment Science Technology 52(21): 12026-12038.
- Santos RG, Andrades R, Boldrini MA, Martins AS (2015) Debris ingestion by juvenile marine turtles: An underestimated problem. Marine Pollution Bulletin 93(1-2): 37-43.
- Wilcox C, Puckridge M, Schuyler QA, Townsend K, Hardesty BD (2018) A quantitative analysis linking sea turtle mortality and plastic debris ingestion. Science Report 8, 12536.
- Isangedighi IA, David GS, Obot OI (2018) Plastic waste in the aquatic environment: impacts and management. Cites 2:1-31.
- Rizzia M, Rodrigues FL, Medeiros L, Ortega I, Rodrigues L, et al. (2019) Ingestion of plastic marine litter by sea turtles in southern Brazil: abundance, characteristics and potential selectivity. Marine Pollution Bulletin 140: 536-548.
- Vélez-Rubio GM, Teryda N, Asaroff PE, Estrades A, Rodriguez D, et al. (2018) Differential impact of marine debris ingestion during ontogenetic dietary shift of green turtles in Uruguayan waters. Marine Pollution Bulletin 127: 603-611.
- Santos RG, Andrades R, Demetrio GR, Kuwai GM, Sobral MF, et al. (2020) Exploring plastic-induced satiety in foraging green turtles. Environment Pollution 265: 114918.
- García-Besné G, Valdespino C, Rendón-von Osten J (2015) Comparison of organochlorine pesticides and PCB residues among hawksbill (Eretmochelys imbricata) and green (Chelonia mydas) turtles in the Yucatan Peninsula and their maternal transfer. Marine Pollution Bulletin 91(1): 139-148.
- Schuyler Q, Hardesty BD, Wilcox C, Townsend K (2013) Global Analysis of Anthropogenic Debris Ingestion by Sea Turtles. Conservation Biology 28(1): 129–139.
- Wyneken J (2001) The Anatomy of Sea Turtles. NOAA Tech. Memorandum NMFS-SEFSC-470, p. 172.
- Ferreira B, Garcia M, Jupp BP, Al-Kiyumi A (2006) Diet of the Green Turtle (Chelonia mydas) at Ra's Al Hadd, Sultanate of Oman. Chelonian Conservation Biology 5(1): 141–146.
- von Brandis RG, Mortimer JA, Reilly BK, van Soest RWN, Branch M (2014) Diet Composition of Hawksbill Turtles (Eretmochelys imbricata) in the Republic of Seychelles. Western Indian Ocean. Journal of Marine Science 13(1): 81-91.
- Keller JM, Pugh R, Becker PR (2014) Biological and Environmental Monitoring and Archival of Sea Turtle Tissues (BEMAST): rationale, protocols, and initial collections of banked sea turtle tissues. In: U.S. Department of Commerce (Ed.), NIST Internal Report 7996, Gaithersburg, MD.
- Clukey KE, Lepczyk CA, Balazs GH, Work TM, Lynch JM (2017) Investigation of plastic debris ingestion by four species of sea turtles collected as bycatch in pelagic Pacific longline fisheries. Marine Pollution Bulletin 120: 117–125.
- Sokal RR, Rohlf FJ (1982) Biometry, 2nd Ed. W.H. Freeman and Co., San Francisco, California.
- Lazar B, Gracăn R (2011) Ingestion of marine debris by loggerhead sea turtles, Caretta caretta, in the Adriatic Sea. Marine Pollution Bulletin 62(1): 43-47.
- Derraik JGB (2002) The pollution of the marine environment by plastic debris: a review. Marine Pollution Bulletin 44: 842-852.
- Wedemeyer‑Strombel KR, Balazs GH, Johnson JB, Peterson TD, Wicksten MK, et al. (2015) High frequency of occurrence of anthropogenic debris ingestion by sea turtles in the North Pacific Ocean. Marine Biology.
- Schuyler Q, Hardesty BD, Wilcox C, Townsend K (2012) To Eat or Not to Eat? Debris Selectivity by Marine Turtles. PLOS ONE 7(7): e40884.
- Carman VG, Acha EM, Maxwell SM, Albareda D, Campagna C, et al. (2014) Young green turtles, Chelonia mydas, exposed to plastic in a frontal area of the SW Atlantic. Marine Pollution Bulletin 78(1-2): 56-62.
- Fritsches KA, Warrant EJ (2013) Chap. 2. Vision. Wyneken J, Lohmann KJ, Musick JA (eds.) The Biology of Sea Turtles, vol. III. Pp. 31 – 58.
- Narazaki T, Sato K, Abernathy KJ, Marshall GJ, Miyazaki N (2013) loggerhead turtles (Caretta caretta) use vision to forage on gelatinous prey in mid-water. PLOS ONE 8(6): e66043.
- Duncan EM, Arrowsmith JA, Bain CE, Bowdery H, Broderick AC, et al. (2019) Diet-related selectivity of macroplastic ingestion in green turtle (Chelonic mydas) in the eastern Mediterranean. Science Report 9(1): 11581.
- Schuyler QA, Wilcox C, Townsend K, Hardesty BD, Marshall NJ (2014) Mistaken identity? Visual similarities of marine debris to natural prey items of sea turtles. Behavior Physiology Ecology 14.
- Vince J, Hardesty BD (2018) Governance solutions to the tragedy of the commons that marine plastics have become. Frontal Marine Science 5: 214.
- Ng CKY, Ang PO, Russell DJ, Balazs GH, Murphy MB (2016) Marine macrophytes and plastics consumed by green turtles (Chelonia mydas) in Hong Kong, South China Sea region. Chelonian Conserv. Biol. 15(2), 289-292.
- Bugoni L, Petry MV (2001) Marine Debris and Human Impacts on Sea Turtles in Southern Brazil. Marine Pollution Bulletin 42(12): 1330-1334.
- Carson HS, Lamson M, Nakashima D, Toloumu D, Hafner J, et al. (2013) Tracking the sources and sinks of local marine debris in Hawaii. Marine Environment Research 84: 76-83.
- de Carvalho RH, Lacerda PD, Mendes S da S, Barbosa BC, Paschoalini M, et al. (2015) Marine debris ingestion by sea turtles (Testudines) on the Brazilian coast: an underestimated threat? Marine Pollution Bulletin 101(1):746-749.
- Fukuoka T, Yamane M, Kinoshita C, Narazaki T, Marshall GJ, et al. (2016) The feeding habit of sea turtle marine debris influences their reaction to artificial marine debris. PLOS ONE 6:28015.
-
I-Jiunn Cheng, Pin-Chun Chou, Ying-Tin Chan, Tsung-Hsien Li. Hawkins. Marine Debris in Green Sea Turtles along the Northern Coast of Taiwan. Ad Oceanogr & Marine Biol. 2(3): 2020. AOMB.MS.ID.000537.
-
Sea turtles, Marine debris, Plastic type, Plastic color, Digestive tract, Marine animals, Fishing, Recreational boats, Plastic pollution, Biodegradable, Gastrointestinal, Delayed ovulation, Enzyme secretion, Gross morphology
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






