 Research Article
Research Article
Embryonic Mortality Across the Developmental Stages of the Green Turtle (Chelonia Mydas) on Lanyu Island, Taitung County, Taiwan
Hou-Chun Chou and I Jiunn Cheng*
Institute of Marine Biology, National Taiwan Ocean University, Keelung, Taiwan
I Jiunn Cheng, Institute of Marine Biology, National Taiwan Ocean University, Keelung, Taiwan, 202-24, ROC.
Received Date: October 18, 2021; Published Date: November 30, 2021
Abstract
Green turtle (Chelonia mydas) embryogenesis can be separated into 31 stages, each with a unique set of morphological characters. During embryonic development, the environment in which embryos develop can influence their mortality. However, little is known about which embryonic stages are particularly sensitive to environmental factors, and which environmental factors significantly influence mortality.
In 2017 and 2018, we relocated and monitored 33 recently oviposited C. mydas nests, using data loggers to record nest temperature, air temperature, and precipitation. Upon hatchling emergence, we sampled nests to estimate stages at which embryonic mortality occurred. Three environmental factors were measured—nest temperature, net nest temperature (defined as the rise of nest temperature by the metabolic heat produced during embryogenesis) and precipitation- and net nest temperature was found to have the greatest impact on mortality. Net nest temperature can reach the highest value of incubation, sometimes even exceeding the safe incubation temperature range. The highest mortality was probably caused by high temperatures because the greatest mortality and highest temperatures coincided at the end of incubation, which may exceed the capacity of heat shock protein of embryos.
Keywords:Green turtle; Embryogenesis; Mortality; Nest temperature; Net nest change
Introduction
Embryonic development is the series of morphological changes that a fertilized egg undergoes to become a hatchling. Oviparous animals, like sea turtles, do not have parental care. Thus, the success of embryonic development depends solely on the incubation environment [1,2]. The physical characters in the sand effect the thermal conductivity, humidity and gas exchange between the egg and surrounding environment [3-7]. Gas exchange, on the other hand, is influenced by grain size and water content in the sand layer [8-14]. Insufficient gas exchange caused by the large clutch size or higher environmental temperature may slow down embryonic development, lengthen incubation period and even decrease hatching success [12].
Nest temperature is another influential factor. Bell et al, [15] found that, in leatherback turtles (Dermochelys coriacea), sex dif ferentiation and organ growth occur during the first 22 stages of embryonic development and is thermally dependent. Basically, the temperature influences the development of development rate, especially for the ectotherm animal. Variations in temperature and humidity during these stages, especially towards the extreme end of tolerance, of the sea turtles can delay or stop development and cause the embryo to deform [16-18]. Billett et al, [19] found that embryos of loggerhead turtles incubated at 33-34℃ caused serious embryo deformation and suggested that individuals develop normally when incubating at 25-33℃.
During incubation, embryos face not only daily variations in sand temperature, but also the metabolic heat produced during the second half of incubation. This may result in the nest temperature exceeding the thermal tolerance range [20], and consequently deformity or death [2,21,22]. Metabolic heating can affect sex ratios. Excess precipitation and flooding, on the other hand, may decrease the sand temperature and oxygen content and influence hatching success [13,22-25]. These suggest that the nest environment is the key factor influencing embryo development, hatching success, hatchling size and locomotion and recruitment [26-28].
Previous research has identified periods during which embryos may be particularly susceptible to mortality. Many studies found that the eggs incubated artificially under constant-temperature scenarios, most embryonic mortality occurred during the early to middle stage of development; prior to the develop of dorsal carapace and mainly determined by the external environment [15, 29-31]. Mortality during the late stage of development was mainly related to the poor health and facial deformities of hatchlings that could not get out of their shell [30].
Previous studies in Taiwan have investigated natural nest environments of Chelonia. mydas. The studies found that, the nest oxygen content decreases as the incubation period progresses and the nest temperature increases substantially during the middle and late stages of incubation (after stage 25) [32-37]. Chen, et al. found that the clutch size during embryogenesis was the most influential factor affecting mortality on Wan-an Island, Penghu Archipelagos. The biological and non-biological factors that influence each stage of development were also found to differ. However, none of the above studies determined the relationship between mortality during embryogenesis and the nest environment.
Cheng, et al. [38] found that the warm surface-water temperature around the Lanyu Island tends to result in shorter inter-nesting intervals and larger females tended to deposit larger clutch sizes. The average clutch size of green turtle on this island ranged from 73 to 116 eggs per clutch [38]. In this study, we further examined dead embryos of green turtles in each stage of embryogenesis to determine which stage(s) have the highest mortality rate. Because the weather, especially the fluctuate temperature and precipitation can have major influence on the embryogenesis, we also determine whether these embryo mortalities are related to these environmental factors. Furthermore, the climate change can influence the air temperature and precipitation on both global and local scales. Most embryogenesis studies up to now are focus on constant incubation temperature. The present study thus can help us to understand the effect of natural environment on the embryogenesis.
Materials and Methods
Study site
Lanyu Island (22°00’ to 08’ N, 121°50’ to 60’ E) is in the Pacific Ocean, southwest of Taitung, Taiwan. The island is approximately 90 km from mainland Taiwan and has an area of 45.74 km2 (Figure 1), making it the second largest offshore island in Taiwan (after Penghu Island). Lanyu Island is volcanic in origin and has typical tropical rainforest weather, with 1490 hours of sunshine per year on average, an annual average air temperature of 22.4 °C, annual precipitation exceeding 3077 mm, and an annual relative humidity of approximately 90% (Natural Conservation Society, 1988). There are three nesting beaches on the island: Don-Chin Beach, facing the Pacific Ocean, approximately 675 m in length; Small Bai-Dai Beach, facing mainland Taiwan, approximately 256 m in length; and Big Bai-Dai Beach, also facing mainland Taiwan, approximately 1500 m in length. Big Bai-Dai Beach is seriously impacted by light pollution. Small Bai-Dai Beach is the main nest site on the island, but it is small and overcrowded with the nesting females. Don-Chin Beach is the largest and widest beach, but hosts fewer turtle nests; thus, we chose this last beach as our experimental site because it is the least disturbed by humans.
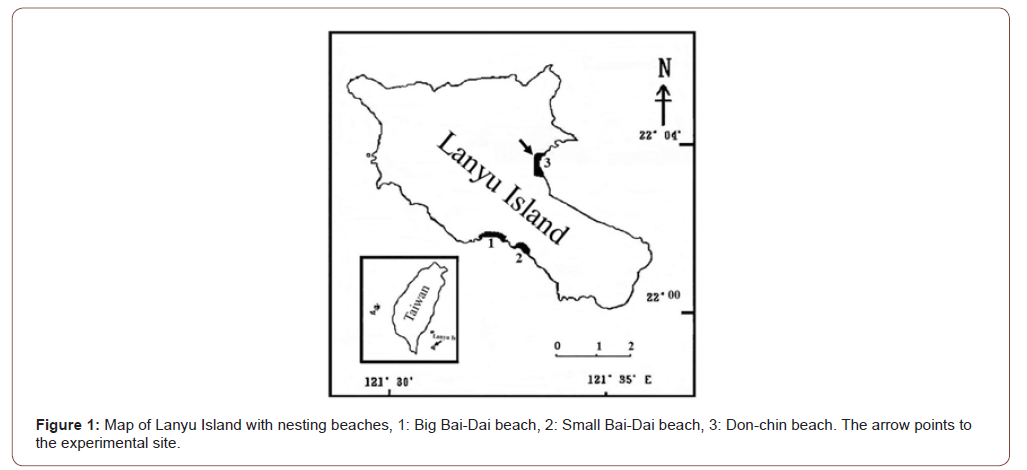
Experimental procedures
Experiments were carried out from July to September 2017 and 2018. During the experimental period, all three beaches were patrolled every 2 hours from 19:00 to 3:00 next morning. After female oviposition, nests were relocated to the experimental site on Don-Chin Beach within four hours in plastic cooler on the back of motorbikes and reburied in an artificial nest about 70 cm deep. The diameter and weight of 30 randomly chosen eggs were measured prior to reburial. Egg size was determined by measuring with a Vernier slide caliper (±0.5 mm) and weight was measured with an electronic balance (Kang-Jen Model KH, ±0.01 g). In addition, both the nesting and hatchling emergence date and time were recorded. From previous studies (unpublished data), it was determined that the average hatching period in Taiwan was about 50 days, a plastic net with diameter of 1m was set up around the nest to prevent the hatchling escape from the nest. After the setup of the plastic net, we patrolled the nest site every two hours each night until hatchling was found in the net and determine the hatchling emergence date. Chen, et al. found that, the alive hatchlings can still be found emerge from the nest. Thus, we wait for three days after the major emergence date of the hatchlings. Then, we dug up the nest in the late afternoon of the fourth day with hand to determine the nest depth, save the hatchling remains in the nest and determine the number of unhatched eggs, dead hatchlings and hatchlings remain in the nest. A temperature logger (HOBO Pro v2 Onset) was placed in the relocated nest. The logger recorded in situ nest temperature every 30 minutes. Another logger was buried 1 m from the nest at the same depth to record the background sand temperature. The record time was the same as the one in the nest.
Embryo development stage determination
Two to five days after the majority of the hatchlings emerged, each nest was dug up and the number of unhatched eggs, embryos died during the incubation, dead hatchling and alive hatchlings remain in the nest were determined. We opened the unhatched eggs and determined the development stage based on the morphological characters described in Miller [14] . In order to determine the developmental stage with higher accuracy, the unhatched eggs were brought back to the field station and the morphology of the dead embryos were carefully examined by using a dissecting microscope. In this study, we classified a turtle as hatched if it reached the sea, died at stage 31, or was still alive but had failed to climb out of the nest.
The criteria for each incubation stage were based on Miller [14], who conducted 50-day-long incubation tests in a laboratory under a constant temperature of 29 ℃. These criteria cannot be applied to the field directly, so we adapted a method described in Chen, et al. to determine each developmental stage in situ. Briefly, studies on Wan-an Island [39] found that the incubation duration decreases by 3 days for every 1 ℃ increases in nest temperature above 29℃- a 6% change in the incubation period. In order to determine the incubation duration for each stage at our study site, an adjusted incubation duration was calculated as: first, the adjusted incubation duration of each stage of development (%) was calculated as the average temperature of each stage minus 29 ℃, then multiple 6% and the percentage duration of each stage of the whole incubation period. Then, the percentage of actual incubation period of each stage in the whole incubation was calculated as the percentage of adjusted incubation period subtracted from the percentage of each stage estimated from Miller [14].
Determining the nest temperature, net nest temperature, incubation period, hatching and emergence rates, and mortality of each stage
Incubation period is usually defined as the date of egg deposition to the emergence of the first hatchling (Broderick et al. 2000). However, it takes several days for the hatchling to climb onto the beach. Thus, the real hatching date is earlier than the determined date [40]. Wang [37] analyzed the nest oxygen content on Wan-an Island, Taiwan, and found that the hatching date was 4 days earlier than the hatchling emergence. Thus, we determined the incubation period by subtracting 4 days from the hatchling emergence date.
Hatching rate (%) = (number of hatchling/ total eggs)* 100%.
Nest emergence success (%) = [(number of eggs in nest at laying - (number of eggs died during incubation + number of hatchlings that did make it out of nest))/(number of eggs in nest at laying) X 100%
Mortality rate in each development stage = (dead eggs in each development stage /total eggs) * 100%
Nest temperature was defined as the temperature in the nest during incubation. Net nest temperature was defined as the difference in temperature between the nest temperature and the background sand temperature. This difference is produced by the metabolic heat during incubation. The statistics program used in this study is SPSS 18.0.
Climate data
The daily air temperatures and precipitation on Lanyu Island were available for 2017 and 2018. The data were purchased from the Central Weather Bureau of the ROC (Central Weather Bureau, 2017–2018).
Statistical analysis
Non-parametric Kruskal-Wallis and Dum posthoc tests were used to determine if there was a significant difference in mortality rate among different development stages. Each percentage was divided by 100 and arcsine transfer was performed prior to analysis. Equal variance and normality tests were carried out prior to the test. One-way ANOVA was used to analyze the nest temperature among stages during incubation [41]. If variations were homogenous, then the Scheffe post hoc test was used for the analyses. If the variations were heterogeneous, then the Games-Howell post hoc test was used for the analyses. Linear and multiple linear analyses were used to determine whether the sand temperature was influenced by air temperature and precipitation. They were also used to determine the relationship between and among environmental factors (air and nest temperature and precipitation) and mortality rate of each developmental stage and hatching and emergence rate [41].
Results
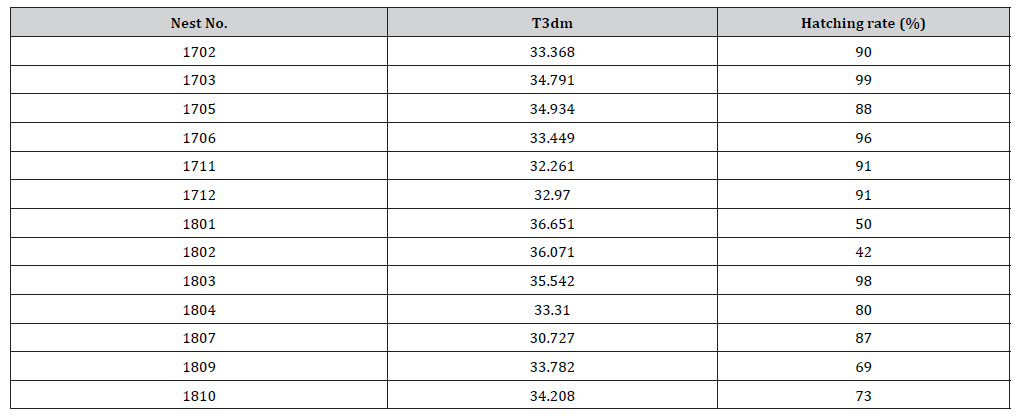
In Taiwan, green turtle deposits 4 to 5 nests per season (Cheng unpublished data). In this study, females deposited nine nests in the season of 2017. Clutch sizes ranged from 41 to 118 eggs, averaging 79 eggs. Average egg diameter ranged from 4.1±0.1 to 4.5±0.1 cm; average egg weight ranged from 41.3±0.9 to 49.8±1.6 g. Incubation period ranged from 45 to 53 days, averaging 50 days. Hatching rate ranged from 71 to 99%, averaging 91%. Nest emergence success ranged from 68 to 100%, averaging 93%.
Eleven females deposited eggs into 24 nests in 2018. Clutch size ranged from 52 to 141 eggs, averaging 97 eggs. Average egg diameter ranged from 4±0.1 to 4.6±0.1 cm; average egg weight ranged from 35.4±1.8 to 49.2±3.6 g. Incubation period ranged from 48 to 55 days, averaging 51 days. Hatching rate ranged from 15 to 98%, averaging 64%. Nest emergence success ranged from 87 to 100%, averaging 96% (Table 1).
Table 1:The highest mean 3 days in a row temperature (T3dm;oc) and the corresponding hatching rate (%) of the nests in this study.
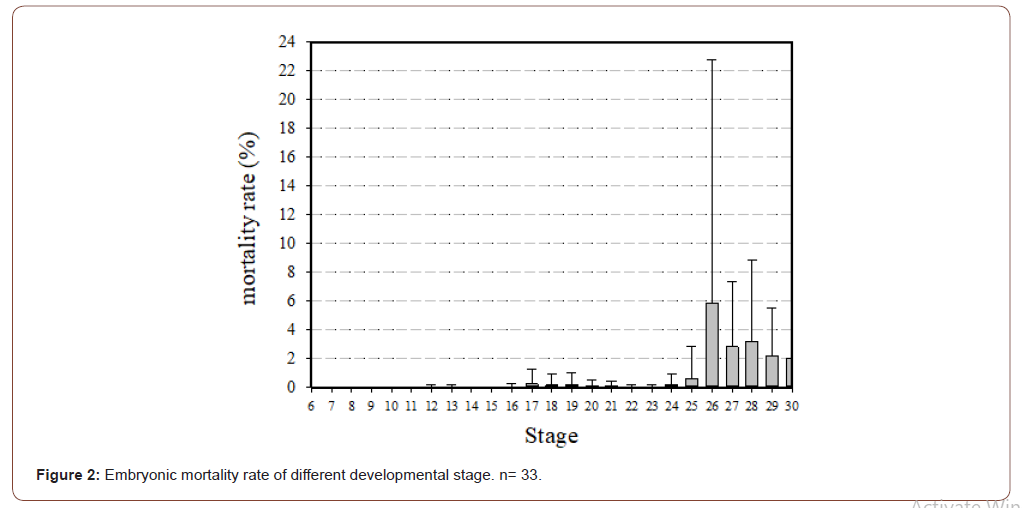
Major mortality occurred after stage 26; it was highest at stage 26, followed by stages 27 and 28 (Figure 2). Forty-three embryos belonged to the undetermined stage.
Environment data
Air temperature from July 5 to September 11, 2017, ranged from 22.3 to 30 ℃, averaging 26.3±1.3 ℃ (n= 69); air temperature from July 5 to September 16, 2018, ranged from 22.2 to 30.3 ℃, averaging 25.9±1.4℃ (n=74). Air temperature was significantly higher in 2017 than in 2018 (p < 0.001). Sand temperature from July 5 to September 11, 2017, ranged from 26.6 to 34.3 ℃, averaging 30.6±1.6 ℃ (n=1653); sand temperature from July 5 to September 16, 2018, ranged from 25.8 to 31.2 ℃, averaging 29.1±1.5 ℃ (n=1731). Sand temperature was also significantly higher in 2017 than in 2018 (p < 0.001).
Total precipitation was 898.8 mm in 2017 and 797.3 mm in 2018. Total precipitation during the nest incubation period ranged from 268.3 to 785.6 mm in 2017, averaging 530.3 mm (n = 9), and 232.3 to 672.3 mm in 2018, averaging 433 (n = 24).
Due to the fact that both air and sand temperatures were higher in 2017 than 2018, the influence of air temperature and precipitation on sand temperature was determined separately for the two years. In 2017, sand temperature was positively influenced by air temperature and negatively by precipitation (sand temperature = 24.016+0.251 (air temperature) - 0.017 (precipitation), n = 1653, r = 0.206, p < 0.001). Of the two, air temperature was the major factor (p < 0.001), while precipitation was not a significant factor (p = 0.234). In 2018, sand temperature was also positively influenced by air temperature and negatively by precipitation (sand temperature = 15.999+0.505 (air temperature) - 0.0279 (precipitation), n = 1731, r = 0.49, p < 0.001). Between the two, air temperature was the major factor (p < 0.001), and precipitation again was not a significant factor (p = 0.072). Thus, precipitation was not used to analyze either year in this study.
Nest temperature
Five loggers malfunctioned—three in 2017 and two in 2018. Thus, six nest temperatures were available in 2017 and eight were available in 2018. In 2017, the average nest temperature ranged from 31.6 to 32.2 ℃, and the highest nest temperature range was 34 to 35.9 ℃. In 2018, the average nest temperature ranged from 30.5 to 32.9 ℃, and the highest nest temperature ranged from 32.9 to 36.7 ℃.
Mortality rate at different stages of development
Analyses of mortality rate of different stage in 2017 (n = 8) and 2018 (n = 24) showed that this value was higher at stages 27 and 28 than stages 6 to 25 (Kruskal–Wallis test, p<0.001). It was also higher at stage 26 than stages 6 to 24; at stage 29 than stages 6 to 19 and 21 to 24; and at stage 30 than stages 6 to 16, 19, 22 and 23 (Dunn Post Hoc test, p<0.05).
Effect of 3-day highest nest temperature on the hatching rate
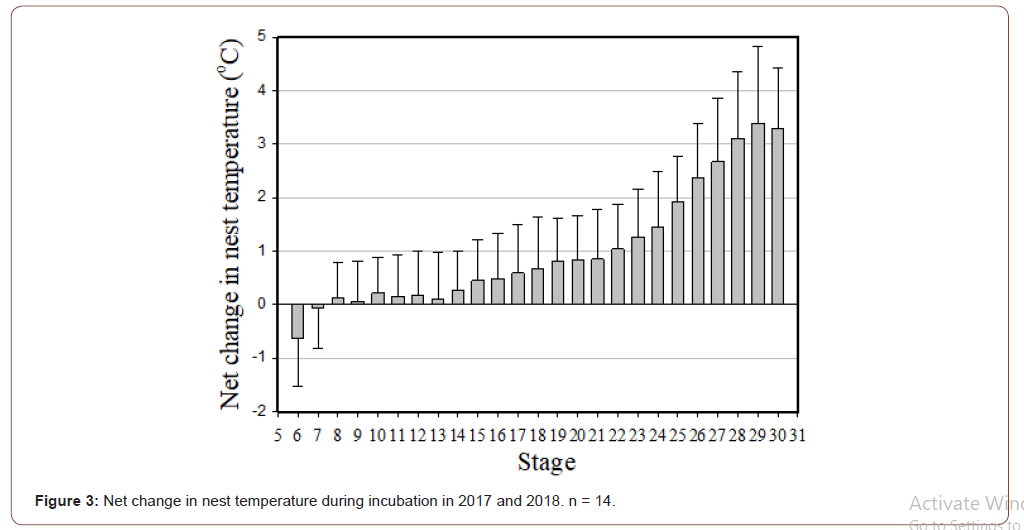
Both the highest temperature and duration of exposure to this high temperature are important to the survivorship of hatchling. This is due to the highest nest temperature occurs towards the end of incubation when the metabolic heat produce reaches its highest value (Figure 3). Thus, we used the highest mean 3 days in a row temperature (T3dm) [2] as an index to determine the highest nest temperature to the survivorship of the hatchling. Results showed that the hatching rate decrease to 40% or less when 3DT rose above 36 ℃, which were nests 1801 and 1802 (Table 1).
Discussion
This study determined the hatching mortality of each development in the nature. Results found that the hatching mortality was low during the early stage of development, which may relate to the relocation of nests within four hours of oviposition. The high mortality occurred during the late stage of development might due mainly to a combination of both increase in environmental temperatures at nesting site accompanied with the metabolic heating generated during those stages which may exhaust the heat shock protein produced by the embryos.
Mortality rate at different development stages
Blanck and Sawyer [29] found that 52% of loggerhead turtle embryos died in the precarapace phase (before stage 20). Whitmore and Dutton [31] found that embryonic mortality mainly occurred prior to the eye coloring, which occurs before stage 19. In this study, embryo mortality was 3.6% prior to stage 20, dropped to < 1% at stage 22, and increased to 9% at stage 30. Only the mortality rate at one stage in the present study, stage 30, was similar to that in Bell et al, [15].
There are several possible reasons for this difference. Studies found that the hatching success of green or loggerhead embryos might decrease if they are disturbed within a mere few hours after oviposition [31,42,43]. The mortality in the early stage of development in this study were low because we relocated the green turtle eggs within four hours of oviposition when mortality occurs under natural environmental conditions of Lanyu Island.
Bell, et al. [15] compared the hatching condition of leatherback embryos between the laboratory and field conditions. They found that the embryo mortality rate during the first period of development (stages 6 to 10) in the field group was 1.5 times lower than that of the laboratory group, and three times lower than that of the laboratory group during the middle period of development (stages 11 to 20). Mortality during undetermined stages of the field group, however, was 17 times higher than that of the laboratory group. The mortality rates during the late period of development (stages 21 to 30) were similar between the field and laboratory groups. Bell, et al. [15] showed that the field study misidentified dead embryos in the early development as unfertilized eggs. The decay of the embryo made the judgement more difficult. Care should be taken when using morphology to identify a turtle’s embryonic stage in early embryos.
The results of our study were similar to those of Ko [33], who found that the highest mortalities occurred at stages 28 and 29. Ko suggested that the high mortalities during these two stages were mainly caused by the increase in metabolic heat from the outflow of water vapor [44,45]. Embryos might die from not absorbing enough water.
Relationship between embryonic mortality and nest temperature
Embryo produces heat shock protein under the high temperature, especially during the last period of embryogenesis, when both the surrounding sand temperature and generate metabolic reaches the maximum values. It may enable the embryo to adjust its physiological status and withstand the high temperature [16]. However, the prolong exposure to the high temperatures may exhaust this protein and increase the hatchling mortality across turtle species.
Some studies exposed the embryos to 34 to 36 ℃ for several days during the last two weeks of incubation and found that many embryos still hatched successfully: consequently. The authors argue that embryos have a higher temperature tolerance by producing heat shock protein during the last period of incubation [2,16,46- 49]. However, this mechanism may fail if the high temperature is maintained too long. Maulany, et al. [2] found that the hatching success of olive ridley turtle embryos decreased when exposed to 34℃ for more than 3 days. They suggested that the chance of abnormality in embryonic development increases when embryos are incubated under the high temperature for long period [49-51]. In this study, the hatching success of nests N1801 and N1802 were lower than the other nests. The 3Tdm of these two nests were all above 36 ℃. Mortality rates thus were high in these two nests (Table 1).
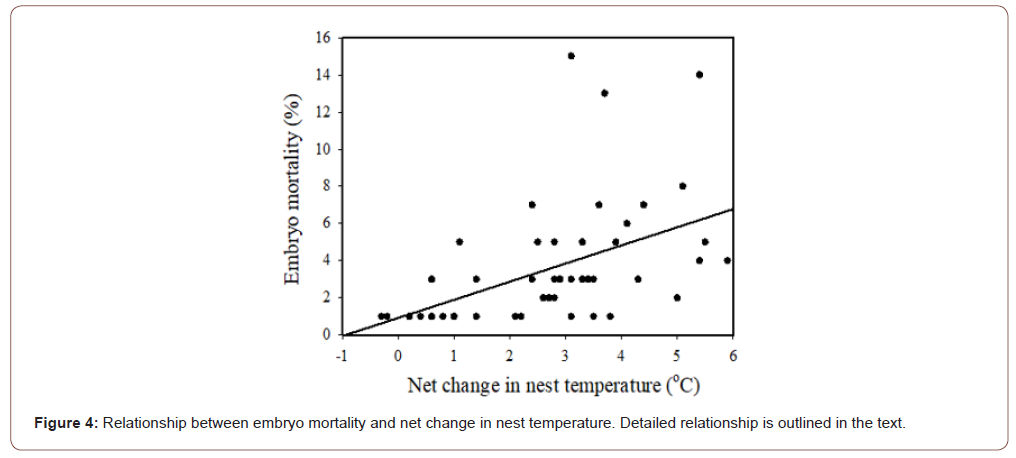
In this study, net nest temperature (the difference from sand to nest) increased gradually with incubation and peaked from stage 28 to 30 (Figure 3). A previous study on Wan-an and Lanyu Islands also found that the combination of environmental temperatures and metabolic heating peaked in the final stage of incubation [35- 37]. Similar results were also found in other studies [40,52,53]. Previous studies found that the net nest temperature produced during the middle and final periods of incubation can cause the nest temperature to exceed the suitable incubation range. The factors such as heat shock that influencing the embryonic development as well as possibly limited oxygen to supply the increased metabolic demands of the embryo, may kill the embryos [2,21,22].
Matsuzawa, et al. [40] found that the net nest temperature was related to the hatchling mortality rate during the hatching stage (i.e., stage 30). They proposed three reasons for this. First, net nest temperature peaks during the last few days of incubation, resulting in the death of the embryos. Second, studies found that the high temperature may inhibit muscle coordination, resulting in a decrease in hatchling activities [40,54-56]. They believed that this mechanism also applies to the embryos during the hatching stage and may prevent the embryos from hatching. Third, high temperature can speed the metabolic rate of the embryo, causing an exhaustion of oxygen. This would limit the gas exchange in the nest, possibly suffocating the embryos (Figure 4).
Conclusion and conservation implications
This study found that the embryonic mortality in our study was highest from stage 26 to 30. Net nest temperature peaked towards the end of incubation, sometimes exceeding the safe temperature range. A combination of both increases in environmental temperatures at the nesting site on Lanyu Island and the metabolic heating that might exhaust heat shock protein and contributed to the high embryonic mortality during the last stages of incubation.
It is known that the global warming does increase the nesting site temperature. This will skrews the hatchling sex ratio towards the female (e.g., Blechschmidt, et al. [57]). Furthermore, the higher sand temperature can also increase the embryonic mortality, especially during the middle to late development period [58-63]. In order to decrease the deteriorate effect of higher temperature, one can install the cooling system, such as sprinkler or shading device to decrease the nest temperature, thus increase hatching success [48].
Acknowledgement
The authors thank Mr. Ko, Z-Y, Ms. Jo, P-J, and the field volunteers for their assistance. This project was partly supported by a grant from the 109 Marine Wildlife Ecology and Conservation of Taitung County (#109H32404) from the Marine Conservation Council. Mr. Noah Last of Third Draft Editing edited the manuscript’s English language.
Conflict of Interest
None.
References
- Koch AU, Guinea ML, Whiting SD (2007) Effects of sand erosion and current harvest practices on incubation of the flatback sea turtle (Natator depressus). Australian J Zool 55(2): 97-105.
- Maulany RI, Booth DT, Baxter GS (2012) Emergence success and sex ratio of natural and relocated nests of olive ridley turtles from Alas Purwo National Park, East Java, Indonesia. Copeia 2012(4): 738-747.
- Mortmer JA (1990) The influence of beach sand characteristics on the nesting behavior and clutch survival of green turtles (Chelonia mydas). Copeia 1990(3): 802-817.
- Ackerman RA (1997) The nest environment and the embryonic development of sea turtles. In: Lutz PL, and Musick JA (eds) The biology of sea turtles. Vol. 1, CRC Press, Boca Raton, FL, pp. 83-106.
- Hewavisenthi S, Parmenter CJ (2002) Incubation environment and nest success of the flatback turtle (Natator depressus) from a natural nesting beach. Copeia 2002 (2): 302-312.
- Ackerman RA, Lott DB (2004) Thermal, hydric andrespiratory climate of nests. In: Deeming DC (ed) Reptilian incubation: environment, evolution and behaviour. Nottingham, England: Nottingham University Press, UK, pp. 15-43.
- Miller JD, Mortimer JA, Limpus CJ (2017) A field key to the developmental stages of marine turtles (Cheloniidae) with notes on the development of Dermochelys. Chelonian Conserv Biol 16(2): 111-122.
- Ragotzkie R (1959) Mortality of loggerhead turtle eggs from excessive rainfall. Ecology 40(2): 303-305.
- Prange HD, Ackerman RA (1974) Oxygen consumption and mechanisms of gas exchange of green turtle (Chelonia mydas) eggs and hatchlings. Copeia 1974(3): 758-763.
- Ackerman RA (1980) Physiological and ecological aspects of gas exchange by sea turtle eggs. Amer Zool 20(3): 575-583.
- Ackerman RA (1981a) Growth and gas exchange of embryonic sea turtles (Chelonia, Caretta). Copeia 1981(4): 757-765.
- Ackerman RA (1981b) Oxygen consumption by sea turtle (Chelonia mydas) during development. Physiol Zool 54(3): 316-324.
- Kraemer JE, Bell R (1980) Rain-Induced Mortality of Eggs and Hatchlings of Loggerhead Sea Turtles (Caretta caretta) on the Georgia Coast. Herpetologica 36(1): 72-77.
- Miller JD (1985) Embryology of marine turtles. In: Gans C, Billett F, Maderson PF (eds) Biology of the reptilia Vol. 14, John Wiley and Sons Press, New York, NY: pp. 269-328.
- Bell BA, Spotila JR, Paladino FV, Riena RD (2003) Low reproductive success of leatherback turtles, Dermochelys coriacea, is due to high embryonic mortality. Biol Conser 115(1): 131-138.
- Massey MD, Hutchings JA (2020) Thermal variability during ectotherm egg incubation: A synthesis and framework. J Exp Zool Part A Ecol Integr Physiol 335(1): 59-71.
- Morris KA, Packard GC, Boardman TJ, Paukstis GL, Packard MJ (1983) Effect of the hydric environment on growth of embryonic snapping turtles (Chelydra serpentina). Herpetol 39(3): 272-285.
- Packard GC, Packard MJ (1988) The physiological ecology of reptilian eggs and embryos. In: Gans C, Huey RB (eds) Biology of the reptilia: defense and life history, Ecology B Vol. 16, Alan R. Liss Press, New York, NY, USA, pp: 523-605.
- Billett FS, Collins P, Goulding DA, Sutherland J (1992) The development of Caretta caretta, at 25–34°C, in artificial nests. J Morphol 213(2): 251-263.
- Ackerman RA (1994) Temperature, time, and reptile egg water exchange. Israel J Zool 40(3-4): 293-306.
- Howard R, Bell I, Pike D (2014) Thermal tolerances of sea turtle embryos: current understanding and future directions. Endang Species Res 26(1): 75-86.
- van Lohuizen S, Rossendell J, Mitchell NJ, Thums M (2016) The effect of incubation temperatures on nest success of flatback sea turtles (Natator depressus). Mar Biol 163(7): 1-12.
- Kam YC (1994) Effects of simulated flooding on metabolism and water balance of turtle eggs and embryos. J Herpetol 28(2): 173-178.
- Fuentes M, Bateman B, Hamann M (2011) Relationship between tropical cyclones and the distribution of sea turtle nesting. J Biogeogr 38(10): 1886-1896.
- Pike D, Roznik E, Bell I (2015) Nest inundation from sea-level rise threatens sea turtle population viability. R Soc Open Sci 2(7).
- Booth D, Astill K (2001) Temperature variation within and between nests of the green sea turtle, Chelonia mydas (Chelonia: Cheloniidae) on Heron Island, Great Barrier Reef. Australian J Zool 49(1): 71-84.
- Tomillo PS, Saba VS, Blanco GS, Stock CA, Paladino FV, et al. (2012) Climate driven egg and hatchling mortality threatens survival of eastern Pacific leatherback turtles. PLoS One 7(5): e37602.
- Tedeschi J, Kennington W, Berry O, Whiting S, Meekan M, et al. (2014) Increased expression of Hsp70 and Hsp90 mRNA as biomarkers of thermal stress in loggerhead turtle embryos (Caretta caretta). J Thermal Biol 47: 42-50.
- Blanck CE, Sawyer RH (1981) Hatchery practices in relation to early embryology of the loggerhead sea turtle, Caretta caretta (Linné). J Experi Mar Biol Ecol 49(2-3): 163-177.
- Rafferty AR, Reina RD (2014) The influence of temperature on embryonic developmental arrest in marine and freshwater turtles. J Exp Mar Biol Ecol 450: 91-97.
- Whitmore CP, Dutton PH (1985) Infertility, Embryonic Mortality and Nest-Site Selection in Leatherback and Green Sea Turtles in Suriname. Biol Conserv 34(3): 251-272.
- Chen YS (2009) Factors that influence the nest water potential and variation of water potential during embryogenesis on Wan-an Island, Penghu Archipelago. MS Thesis, National Taiwan Ocean University.
- Ko PJ (2006) The horizontal change in water potential that influence the hatching success of green turtles on Small Bai-Dai Beach, Lanyu Island, Taitung County. MS Thesis, National Taiwan Ocean University.
- Lin JH (2013) Study of variation and influential factors of oxygen content during embryogenesis of green turtles on Lanyu Island, Taitung County. MS Thesis, National Taiwan Ocean University. Taiwan.
- Liu YI (2012) Influence of clutch size on the water potential and hatching success of the green turtles on Wan-an Island, Penghu Archipelagos, Taiwan MS Thesis, National Taiwan Ocean University, Taiwan.
- Kuo JW (2008) Oxygen changes in the nest during the embryogenesis of green turtles on Lanyu Island, Taitung County, Taiwan. MS Thesis, National Taiwan Ocean University, Taiwan.
- Wang SJ (2009) Biotic and abiotic factors that influence nest oxygen content and the variation of oxygen content during embryogenesis of green turtle on Wan-an Island, Penghu Archipelagos. MS Thesis, National Taiwan Ocean University, Taiwan.
- Cheng IJ, Huang CT, Hung PY, Ke BZ, Kuo CW, et al. (2009) A ten-year monitoring of the nesting ecology of the green turtle, Chelonia mydas, on Lanyu Island, Taiwan. Zool Stud 48(1): 83-94.
- Chen JL (1998) Variation of nest temperature on the hatchling sex ratio and hatching success of green turtle on Wan-an Island, Penghu Archipelagos, Taiwan. MS Thesis, National Taiwan Ocean University, Taiwan.
- Matsuzawa Y, Sato K, Sakamoto W, Bjorndal KA (2002) Seasonal fluctuations in sand temperature: effects on the incubation period and mortality of loggerhead sea turtle (Caretta caretta) pre-emergent hatchlings in Minabe, Japan. Mar Biol 140: 639-646.
- Sokal RR, Rohlf FJ (1982) Biometry, 2nd Ed. W.H. Freeman and Co., San Francisco, California.
- Limpus JC, Baker V, Miller JD (1979) Movement induced mortality of loggerhead eggs. Herpetol 35(4): 335-338.
- Parmenter CJ (1980) Incubation of the eggs of the green sea turtle, Chelonia mydas, in Torres Strait, Australia: the effect of movement on hatchability. Australian Wild Res 7(3): 487-491.
- Ackerman RA, Seagrave RC, Dmi'el R, Ar A (1985) Water and Heat Exchange between Parchment-Shelled Reptile Eggs and Their Surroundings. Copeia 1985(3): 703-711.
- Booth D (2002) Incubation of rigid-shelled turtle eggs: do hydric conditions matter? J Compar Physiol B 172(7): 627-633.
- Booth DT, Feeney R, Shibata Y (2013) Nest and maternal origin can influence morphology and locomotor performance of hatchling green turtles (Chelonia mydas) incubated in field nests. Mar Biol 160: 127-137.
- Carthy RR, Foley AM, Matsuzawa Y (2003) Incubation environment of loggerhead turtle nests : effects on hatching success and hatchling characteristics. In: Bolten AB, Witherington BE (eds) Loggerhead Sea turtles, Smithsonian Press, Washington, DC, pp. 144-153.
- Valverde RA, Wingard S, Gómez F, Tordoir MT, Orrego CM (2010) Field lethal incubation temperature of olive ridley sea turtle Lepidochelys olivacea embryos at a mass nesting rookery. Endangered Spe Res 12: 77-86.
- Wood A, Booth DT, Limpus CJ (2014) Sun exposure, nest temperature and loggerhead turtle hatchlings: Implications for beach shading management strategies at sea turtle rookeries. J Exp Mar Biol Ecol 451: 105-114.
- Deeming DC, Ferguson MW (1991) Physiological effects of incubation temperature on embryonic development in reptiles and birds. In: Deeming DC, Ferguson MW (eds) Egg incubation: its effects on embryonic development in birds and reptiles. Cambridge University Press, Cambridge, England, pp. 147-171.
- Reid KA, Margaritoulis D, Speakmana JR (2009) Incubation temperature and energy expenditure during development in loggerhead sea turtle embryos. J Exp Mar Biol Ecol 378(1-2): 62-68.
- Kraemer JE (1979) Variation in incubation period of loggerhead sea turtle, Caretta caretta, clutches on the Georgia coast. MS. University of Georgia, Athens, GA.
- McGehee MA (1979) Factors affecting the hatching success of loggerhead sea turtle eggs (Caretta caretta caretta). MS. University of Central Florida, Orlando, FL.
- Mrosovsky N (1968) Nocturnal emergence of hatchling sea turtles: control by thermal inhibition of activity. Nature 220(5174): 1338-1339.
- O'Hara J (1980) Thermal influences on the swimming speed of loggerhead turtle hatchlings. Copeia 1980(4): 773-780.
- Moran KL, Bjorndal KA, Bolten AB (1999) Effects of the thermal environment on the temporal pattern of emergence of hatchling loggerhead turtles Caretta caretta. Mar Ecol Prog Ser 189: 251-261.
- Blechschmidt J, Wittmann MJ, Blüml C (2020) Climate change and green sea turtle sex ratio—preventing possible extinction. Genes 11(5): 588.
- Davenport J (1997) Temperature and the life-history strategies of sea turtles. J Thermal Biol 22(6): 479-488.
- Hughes GR (1974a) The sea turtles of South-East Africa I. Status. morphology and distributions. Durban, South Africa: Oceanographic Research Institute.
- Hughes GR (1974b) The Sea Turtles of South-East Africa II. The biology of the Tongaland Loggerhead Turtle Caretta caretta L. with comments on the Leatherback Turtle Dermochelys coriacea L. and the Green Turtle Chelonia mydas L. in the study region. Durban, South Africa: Oceanographic Research Institute.
- Miller JD (1982) Development of marine turtles. University of New England, Armidale, New South Wales, Australia.
- Natural Conservation Society (1988) Investigation and evaluation of the natural resources in Lanyu National Park. Taipei, Taiwan: Department of Interior, Executive Yuan.
- Simon MH, Ulrich GF, Parkes AS (1975) The green sea turtle (Chelonia mydas ): mating, nesting and hatching on a farm. J Zool 177(3): 411-423.
-
Hou-Chun Chou, I Jiunn Cheng. Embryonic Mortality Across the Developmental Stages of the Green Turtle (Chelonia Mydas) on Lanyu Island, Taitung County, Taiwan. Ad Oceanogr & Marine Biol. 3(1): 2021. AOMB.MS.ID.000554.
-
Green turtle, Embryogenesis, Mortality, Nest temperature, Net nest change
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






