 Research article
Research article
The Role of Seaweeds in Carbon Sequestration
Nicola Cantasano*
Institute for Agriculture and Forestry Systems in the Mediterranean (ISAFoM) CNR, Italy
Nicola Cantasano, Institute for Agriculture and Forestry Systems in the Mediterranean (ISAFoM) CNR, Italy
Received Date:August 14, 2024; Published Date:September 06, 2024
Abstract
Global warming is the result of an increasing trend in the concentration of Green House Gases, especially of carbon dioxide, in the terrestrial atmosphere. To date, there is a great interest by science and policy in the potential of marine vegetation, as a carbon sink, to remove part of the organic carbon soluted in ocean waters. This important role could be realized by wild and farming seaweed that could sequester, altogether, large amounts of carbon dioxide in ocean sediments, within a blue carbon strategy. Amongst vegetated coastal ecosystems, the meadows of marine phanerogams, tidal salt marshes and mangroves are hot spots for carbon storage. Side by side, seaweed farming is increasing worldwide and represents an important storage of organic carbon buried in coastal sediments. So, ocean afforestation could be a viable pathway for the mitigation of climate changes. However, there are some constraints that must be solved to extend farming seaweed in the deep ocean. In conclusions, wild and farming seaweeds are a potential solution to reduce the concentration of carbon dioxide in the terrestrial atmosphere but further studies are necessary to quantify the amount of carbon sequestration stored, from wild and farming seaweeds, in deep ocean sediments.
Keywords:Carbon dioxide; wild seaweeds; farming seaweeds; carbon sequestration; ocean sediments
Introduction
The main reason of global warming is the remarkable increase in the concentration of Green House Gases (hereafter, GHG) in the terrestrial atmosphere. Today, there is an urgent need to reduce the global emissions of GHG, so to control the thermal increase of the planet within +1.5 oC above pre-industrial levels [1]. Carbon dioxide (hereafter, CO2) is the most important GHG released by human activities, being the main responsible of global warming. Really, carbon emissions show an increasing trend, causing a lot of negative impacts not only on natural ecosystems, but also on human health [2]. CO2 presents an atmospheric concentration of 416 ppm [3] much higher than pre-industrial levels, as 280 ppm, leading to serious environmental consequences on biodiversity levels and, more generally, on human health [4-8]. In the period 1990-2010, GHG reached a total value of 46 × 103 Tons [9]. United States, China, European Union and India are the four continents’ leaders in GHG emissions with respective percentages of 30%, 15%, 10% and 6.5% of the world production [10].
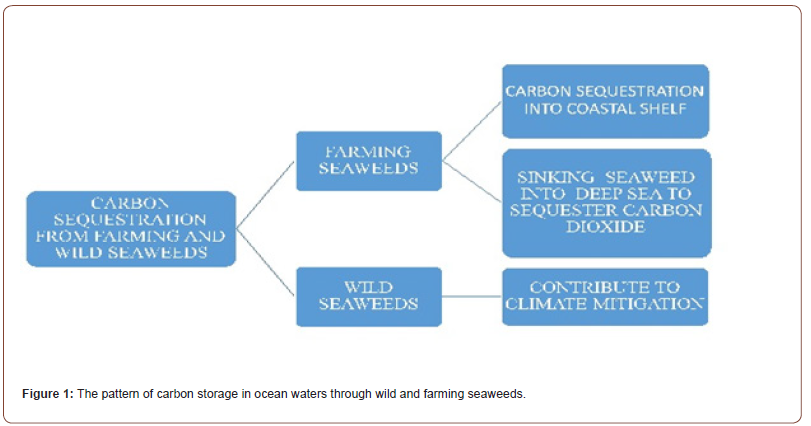
Worldwide, there is an increasing interest by science and policy in the potential of marine vegetation as a possible carbon sink able to fix CO2 through photosynthesis. In this way, some marine ecosystems such as tidal saltmarshes, mangroves and seagrass beds, together with artificial seaweed farmings, represent an important tool for carbon storage in their biomass and, also, for its long-term sequestration in sediment deposition [11], so contributing to climate change mitigation (Figure 1). Recently, it has been estimated that natural seaweed could sequester altogether 173 million metric tons. of CO2 per year [12]. The carbon stored in the ocean biomass is named “blue carbon” and represents an important short-term sequestration, being remineralized in carbon dioxide by bacterial metabolism [13-15]. In fact, a large amount of this carbon is released back to terrestrial atmosphere through respiration and oxidative processes but partly it is exported in deep ocean waters [16]. So, a meaningful fraction of the carbon stored by wild and farming seaweeds is sequestered for a long time in oceanic sediments leading to an effective Carbon Dioxide Removal (hereafter, CDR). In this review, it is summarized the potential role of wild and farming seaweeds in CDR, suggesting, also, a potential outlook for the future.
Methodologies
To select the most important scientific publications in the field of CDR, literature research has been conducted in the Scopus database, updated on 09/07/2024. The keywords searched are seaweed, macroalgae, wild seaweed, farming seaweed, aquaculture, blue carbon, carbon storage, carbon sink and afforestation. The search highlights the presence of 52 different articles, excluding duplicates and works drawn by grey literature. The article is organized into three sections. The first step regards the contribution of wild seaweeds in sequestering CO2 from seawaters. The second stage studies the role of farming seaweed, as carbon sink, in the depths below the marine factories. This paragraph analyzes, also, the pathway of dissolved and particulate organic carbon towards the deep ocean where this kind of biological debris is sequestered into deep sediments for a long time. Finally, the third section of the research suggests a new outlook for the future, speaking about the possibility to realize a potential afforestation of the oceans so to realize into their sediments an effective sequestration of CO2, as a potential tool for a blue carbon strategy.
Wild Seaweeds
Marine macroalgae include about 16.000 species distinguished in three phyla as Chlorophyta (green algae), Rhodophyta (red algae) and Ochrophyta (brown algae). Amongst marine macroalgae, the brown ones can reach larger sizes than red and green seaweeds and, therefore, can capture more carbon per surface area. Therefore, some Ochrophyta species could be ideal patterns for ocean afforestation. Phytobenthos is widespread in the intertidal and subtidal coastal zones and represents the main primary producer of marine biota, being one of the most productive areas of the Earth [17,18]. These vegetated ecosystems cover an overall marine area valued between 2.0 and 6.8 million Km2 of continental shelf [18] with a global Net Primary Production (NPP) of 1.5 Pg C per year [19]. This process of carbon sequestration is realized by some coastal ecosystems such as marine phanerogams, mangroves and tidal saltmarshes able to capture CO2 through photosynthesis. Beyond seaweeds, there are also, some marine plants able to fix a lot of organic carbon through their photosynthetic processes, as the meadows of marine phanerogams representing key blue carbon habitats [20-23].
Globally, seaweed and marine plants can store a large amount of organic carbon as 173 Tg C per year on a global marine surface area of 3.5 million Km2 [18]. In this way, it has been estimated that all the wild seaweeds of marine areas can store about 70% of CO2 widespread in ocean waters [13,23], being able to remove a great amount of carbon from seawaters throughout their life spans [24,25]. The carbon cycle into the oceans begins from the biological carbon pump through which CO2 is stored in the macroalgal biomass via photosynthesis (Figure 2). Part of the carbon produced by macroalgal metabolism is exported as Dissolved Organic Carbon (DOC 52%) while the other fraction is released as Particulate Organic Carbon (POC 48%). A share of DOC remains in coastal seawaters, as labile DOC and it is metabolized by heterotrophic marine bacteria, returning in terrestrial atmosphere. Another part is exported down-hill, as marine snow, and it is stored in coastal sediments [26]. This fraction represents a short-term carbon storage, within a quick carbon cycle, because the carbon stored in surface sediments is rapidly remineralized to CO2 by bacterial metabolism [27].
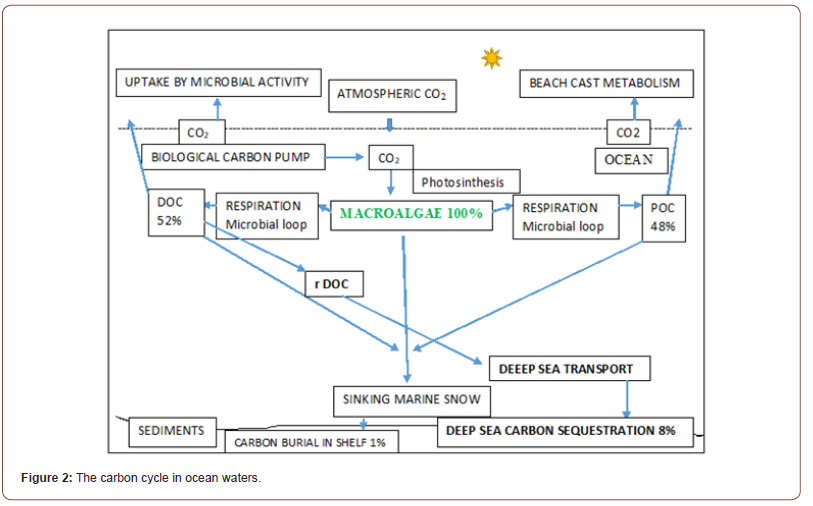
Finally, the third portion of DOC remains refractory to degradation process, and it is exported, as r DOC, to deep ocean sediments where it forms a long-term storage [28]. As regards POC, a great amount of organic carbon is recycled in shelf areas, as beach cast material. Another part is exported beyond the continental shelf towards ocean depths, being sequestered in deep sediments, below 1.000 meters depth [26]. In conclusion, r DOC and a residual part of POC are exported to deep oceans and buried in sedimentary stores, as a slow carbon cycle, so representing a long-term storage, valuable for thousand years, and so removed from terrestrial atmosphere.
Farming Seaweeds
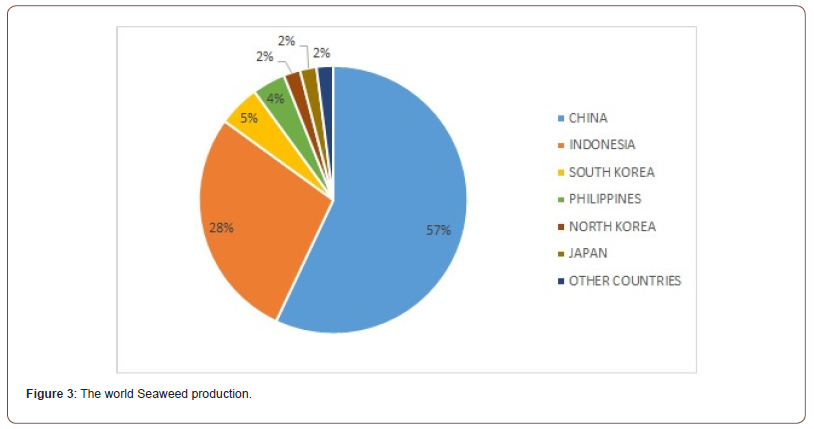
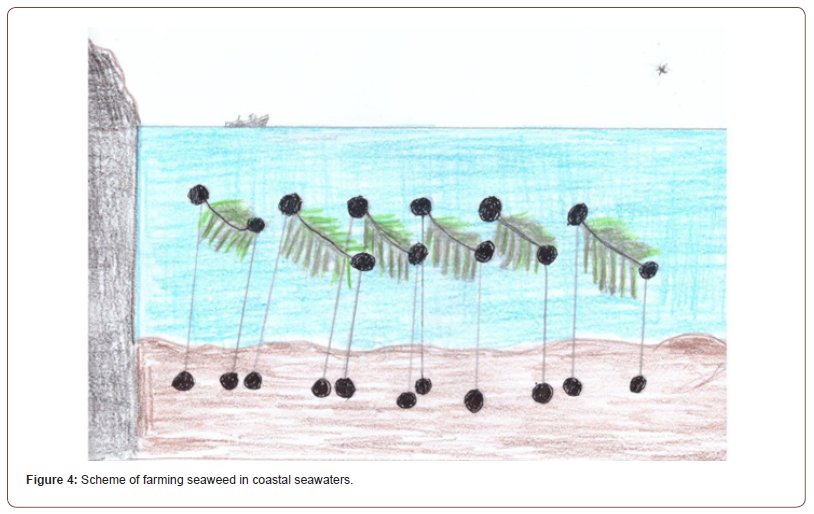
About one hundred macroalgal species are actually cultivated in seaweed farmings for commercial and industrial purposes. However, 98% of macroalgal production derives from seven genera, as are: Saccharina, Pyropia, Euchema, Kapparophycus, Gracilaria, Porphyra and Undaria [29]. Most of the production derives from the coastal seawaters of Asiatic continent, accounting for 97.38% of the global seaweed aquaculture output. More in detail, China is the first nation in algal trade as the 57% of the whole production. The second country is Indonesia accounting for 28% of the global breeding. South Korea is third as 5% of the whole, followed by Philippines (4%), North Korea (2%), Japan (2%), and other countries as 2% (Figure 3) [30]. Generally, seaweed farmings are located in sheltered bays of coastal seawaters over soft sediments providing a depositional environment where the carbon produced by cultivated macroalgae is buried into the sediments just below the farms (Figure 4) [31].
Most of the organic carbon is stored for a short time in coastal sediments just below the farms and it is rapidly remineralized to CO2 through bacterial metabolism. At the same time, a residual part of the carbon produced by cultivated macroalgae is exported towards ocean depths where it is sequestered into deep sediments for a long time valuable in thousand years. It has been estimated that seaweed farmings can remove from seawaters 0.24 Pg CO2 per year [31]. In these last decades, this commercial sector is increasing at a rate of 7.3% per year representing 51.3% of the whole production by mariculture [32]. So, seaweed farming is valued as an effective blue carbon option [33]. In this way, the development of this kind of aquaculture could represent an effective tool for the mitigation of global warming and for the control of climate changes. The potential role of seaweed farmings to draw down anthropogenic CO2, widespread in terrestrial atmosphere, could be very important in the next future [34]. So, macroalgal aquaculture shows a remarkable role in carbon storage although the present distribution of marine farms covers only sheltered bays located in coastal areas.
However, the marine areas cultivated with seaweed farmings is just 1.600 Km2, as 0.004% of the whole marine areas covered by wild populations [35,36]. It has been valued that about 48 million Km2 could be suitable for the cultivation of macroalgae [37] while only 0.01% of the whole ocean surface is used for marine farms, all located in coastal seawaters [34]. These data suggest that the sector of seaweed farmings for carbon storage could be extended towards the open ocean and greatly developed in time.
Constraints and Opportunities for The Future
The issue of carbon storage, as one of the main benefits deriving from seaweed farmings has, recently, drawn a great interest in scientific, political and industrial sectors [9,34,38]. The rapid growth of seaweed farming industry has suggested the attractive theory that macroalgal cultivation in the open ocean could be realized on a large scale, in pelagic seawaters where organic carbon could be sequestered, for a long time, into deep sediments within a blue carbon strategy [39]. In this way, carbon afforestation could be a promising and viable tool for the removal of CO2 from terrestrial atmosphere and for the mitigation of climate changes, but it is necessary to select the most suitable seaweed species for a successful cultivation. Brown macroalgal species, belonging to the orders of Fucales and Laminariales, are the ideal patterns for ocean afforestation because they are able to reach larger sizes than red and green macroalgae, so sequestering more carbon per surface area [40]. Indeed, to meet the growing need for effective carbon storage by farming seaweed, macroalgal cultivation must be extended towards deep ocean waters, well beyond the continental shelf.
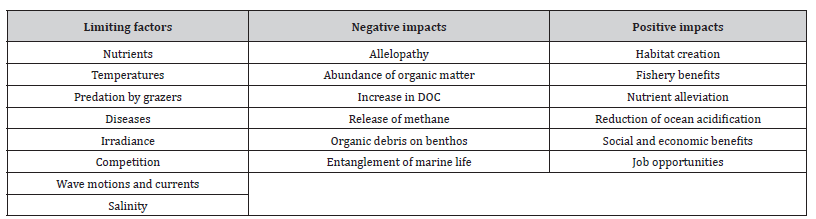
In this way, it could be possible to increase the removal of atmospheric CO2 and to sequester, for a long time, a large amount of organic carbon, so to mitigate the increasing trend of global warming [25,34,41-43]. However, macroalgal growth and the process of carbon sink by cultivated seaweeds could be limited by some biotic and abiotic factors, having also many negative and positive impacts (Table 1). Really, the potential of cultivated seaweed to sequester organic carbon is very debated for some logistic problems and for the financial feasibility to expand seaweed farmings towards the open ocean [44-48]. Finally, the enlargement of seaweed aquaculture is limited by herbivore pressures, by the potential impact of alien species, by hydrodynamic forces and by the reduced nutrient supply [49,50]. The complex interactions in the osmotic exchange of CO2 between terrestrial atmosphere and ocean waters and vice versa, the rapid turnover of algal biomass and the lateral export of DOC/POC mean that the expansion of farming seaweeds towards the deep ocean remains a challenging enterprise in a general context of climate change.
Table 1:Limiting factors, negative and positive impacts in the macroalgal growth of cultivated seaweeds
Conclusions
Earth is covered by seawater as 71% of its surface and, therefore, it could be realized a large seaweed afforestation all around the world so to uptake part of the CO2 concentrated in terrestrial atmosphere. However, a great care should be taken in case of a large-scale seaweed cultivation towards the pelagic marine areas of the ocean. In fact, some farming sites could be unsuitable for wave motion, for nutrient shortage and for unfavorable levels of salinity and temperature. So, the fascinating hypothesis of widespread ocean afforestation seems unrealistic and very debated by the scientific community [37]. Great uncertainty remains as to the fate of macroalgal-sourced organic and remineralized carbon after its export to the deep sea. Indeed, many doubts remain about the ecological impacts of vegetal biomass on the deep ocean floor [51]. Anyway, the suggestion to naturalize and restore seaweed beds could be a useful means of carbon storage because thousand kilometers of coastal floor could be afforestated with marine macroalgae [38]. Seaweeds could play an important role in carbon sequestration only if the carbon, produced by phytobenthos as POC and DOC, could enter in the slow carbon cycle sinking on the bottom of deep ocean waters.
Therefore, it is necessary to extend the marine areas suitable for farming purposes, currently used as 0.04% of the whole surface marine areas [52]. The potential carbon storage by seaweed farming and wild seaweed is considerable but it is timely to extend ocean afforestation as a climate mitigation option. Worldwide, there is an urgent need for prompt solutions not only to reduce the anthropogenic GHG emissions but also to remove part of CO2 from the terrestrial atmosphere. Some scientific projects and social foundations promote the use of seaweed to limit the effects of climate changes. Amongst them, Oceans 2050 (https://www.oceans2050. com) has recently launched a novel planning to value the amount of CO2 stored in twenty-three farming seaweeds widespread in ocean waters, suggesting, also, some actions to promote seaweed aquaculture for climate restoration [36]. In conclusion, wild and farming seaweeds could be a potential solution to reduce the atmospheric concentration of CO2, but further studies are necessary to fully understand the nutrient reallocation, the real quantification of carbon sequestration by natural and farming seaweeds and the potential of cost-effective infrastructures for ocean afforestation.
References
- Christianen M. J. A., van Belzen J., Herman P. M. J., van Katwijk M. M., Lamers L. P. M., van Leent P. J. M., Bouma T. J. (2013) Low-Canopy Seagrass Beds Still Provide Important Coastal Protection Services. PLoS ONE, 8, e62413. DOI: https://doi.org/10.1371/journal.pone.0062413
- Costanza R, d’Arge R, de Groot R, Farber S, Grasso M, Hannon B, Limburg K, Naeem S, O’Neill RV, Paruelo J, Raskin RG, Sutton P, van den Belt M. (1997) The value of the world’s ecosystem services and natural capital. Nature, 387, pp. 253–260. DOI: https://doi.org/10.1038/387253a0
- Orth R. J., Carruthers T. J. B., Dennison W. C., Duarte C. M., Fourqurean J. W., Heck K. L., Hughes A. R., Kendrick G. A., Kenworthy W. J., Olyarnik S., Short F. T., Waycott M., Williams S. L. (2006) A global crisis for seagrass ecosystems. Bioscience, 56, pp. 987–996. DOI: https://doi.org/10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2
- Short F. T., Carruthers T., Dennison W., Waycott M. (2007) Global seagrass distribution and diversity: a bioregional model. Journal of Experimental Marine Biology and Ecology, 350, pp. 3-20. DOI: https://doi.org/10.1016/j.jembe.2007.06.012
- Yap, H. T. (2000). The case for restoration of tropical coastal ecosystems. Ocean Coast. Manag. 43, pp.841–851. DOI: 10.1016/S0964-5691(00)00061-2
- Wood E. J F., Odum W. E., Zieman J. C. (1969) Influence of seagrasses on the productivity of coastal lagoons. Coastal lagoons, a symposium, Universidad Nacional Autonoma de Mexico, Cuidad Universitaria, pp. 495-502.
- McLeod E., Chmura G. L., Bouillon S., Salm R., Bjork M., Duarte C. M., Lovelock C. E., Schlesinger W. H., Silliman B. R. (2011) A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 9, pp. 552–560. DOI:https://doi.org/10.1890/110004
- Duarte C. M., Marba N., Gacia E., Fourqurean J. W., Beggins J., Barron C., Apostolaki E. T. (2010) Seagrass community metabolism: assessing the carbon sink capacity of seagrass meadows. Global Biogeochem. Cycles 24, pp. 8. DOI: 10.1029/2010GB003793.
- Green E. P., and Short, F. T. (2003) World Atlas of Seagrasses. University of California Press, Berkeley, USA. pp. 324.
- Green A. E., Unsworth R. K. F., Chadwick M. A., Jones P. J. S. (2021) Historical Analysis Exposes Catastrophic Seagrass Loss for the United Kingdom. Font. Plant Sci. 12:629962. DOI: 10.3389/fpls.2021.629962
- Gamble C., Bertelli C., Debney A., Glover A., Hendy I., Lilley R., Nuuttila H., Potouroglou M., Ragazzola F., Unsworth R., Preston J. (2021) Seagrass Restoration Handbook. pp. 3-13.
- Duffy J.E. (2006) Biodiversity and the functioning of seagrass ecosystems. Mar. Ecol.-Progr. Ser., 311, pp.233-250. DOI:10.3354/meps311233
- Orth R. J, Heck K. L., van Montfrans J. (1984) Faunal communities in seagrass beds: a review of the influence of plant structure and prey characteristics o predator-prey relationships. Estuaries, 7, pp. 339-350. DOI: https://doi.org/10.2307/1351618
- Adams S. M. (1976) The ecology of eelgrass, Zostera marina (L.), fish communities. I. Structural analysis. Journal of Experimental Marine Biology and Ecology, 22(3), pp. 269-291. DOI: https://doi.org/10.1016/0022-0981(76)90007-1
- Beck M. W., Heck K. L. Jr., Able K. W., Childers D. L., Eggleston D. B., Gillanders B. M., Halpern B, Hays C. G, Hoshino K., Minello T. J. (2001) The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 51, pp. 633–641. https://doi.org/10.1641/0006-3568(2001)051[0633:TICAMO]2.0.CO;2
- Jackson E. L., Attrill M. J., Rowden A. A., Jones M. B. (2006) Seagrass complexity hierarchies: Influence on fish groups around the coast of Jersey (English Channel). Journal of Experimental Marine Biology and Ecology, 330(1), pp. 38-54. DOI: 10.1016/j.jembe.2005.12.016
- Pihl L., Baden S., Kautsky N., Ronnback P., Soderqvist T., Troell M., Wennhage H. (2006) Shift in fish assemblage structure due to loss of seagrass Zostera marina habitats in Sweden. Estuarine, Coastal and Shelf Science, 67, pp. 123-132. DOI10.1016/j.ecss.2005.10.016
- Marine Management Organisation. (2022) Managing marine non-licensable activity in Studland Bay Marine Conservation Zone. [Online] Available at <https://www.gov.uk/government/publications/managing-marine-non-licensable-activities-studland-bay-next-steps> [Accessed 18th April 2022].
- Collins K. J., Suonpää A. M., Mallinson J. J. (2010) The impacts of anchoring and mooring in seagrass, Studland Bay, Dorset, UK. Underwater Technology: The International Journal of the Society for Underwater, 29, pp. 117-123. DOI:10.3723/ut.29.117
- Greve T. M., Krause-Jensen D., Rasmussen M. B., Christensen P. B. (2005) Means of rapid eelgrass (Zostera marina L.) recolonisation in former dieback areas. Aquat. Bot., 82, pp. 143-156. DOI:10.1016/J.AQUABOT.2005.03.004
- Jarvis J. C., Moore K. A. (2010) The role of seedlings and seed bank viability in the recovery of Chesapeake Bay, USA, Zostera marina populations following a large-scale decline. Hydrobiologia, 649, pp. 55-68. DOI:10.1007/s10750-010-0258-z
- Plus M., Deslous-Paoli J-M., Dagault F. (2003) Seagrass (Zostera marina L.) bed recolonisation after anoxia-induced full mortality. Aquat. Bot., 77, pp. 121-134. DOI:10.1016/S0304-3770(03)00089-5
- Bertelli C. M., Unsworth R. K. F. (2014) Protecting the hand that feeds us: Seagrass (Zostera marina) serves as commercial juvenile fish habitat. Marine Pollution Bulletin, 82(2), pp. 425-429. DOI: 10.1016/j.marpolbul.2013.08.011
- Connolly R. M. (1994a) A comparison of fish assemblages from seagrass and unvegetated areas of southern Australian estuary. Marine and Freshwater Research, 45, pp. 1033-1044. DOI:10.1071/MF9941033
- Connolly R. M. (1994b) Removal of seagrass canopy: effects on small fish and their prey. Journal of Experimental Marine Biology and Ecology 184, pp. 99-110. https://doi.org/10.1016/0022-0981(94)90168-6
- Leif P., Susanne B., Nils K., Patrik R., Tore S., Max T., Håkan W. (2006) Shift in fish assemblage structure due to loss of seagrass Zostera marina habitats in Sweden. Estuarine, Coastal and Shelf Science, 67, pp. 123-132. DOI: 10.1016/j.ecss.2005.10.016.
- McCloskey R. M., Unsworth R. (2015) Decreasing seagrass density negatively influences associated fauna. PeerJ, 3(6), e1053. DOI: 10.7717/peerj.1053
- Mallet D., Pelletier D. (2014) Underwater video techniques for observing coastal marine biodiversity: A review of sixty years of publications (1952–2012). Fisheries Research, 154, pp. 44-62. DOIhttps://doi.org/10.1016/j.fishres.2014.01.019
- Sheaves M., Bradley M., Herrera C., Mattoe C., Lennard C., Sheaves J., Konovalov D. A. (2020) Optimizing video sampling for juvenile fish surveys: using deep learning and evaluation of assumptions to produce critical fisheries parameters. Fish and Fisheries, 21(6), pp. 1259-1276. DOI: 10.1111/faf.12501
- Aprahamian M. W., Baglinière J-L., Sabatiè M. R., Alexandrino P., Thiel R., Aprahamian C. D. (2003) Biology, Status, and Conservation of the Anadromous Atlantic Twaite Shad Alosa fallax fallax. American Fisheries Society Symposium, 35, pp. 103-124.
- Connolly R. M. (1997) Differences in comparison of small motile invertebrate assemblages from seagrass and unvegetated habitats in a southern Australian estuary. Hydrobiologia, 346, pp. 137-148. DOI:10.1023/A:1002970100662
- Currás A., Sánchez-Mata A., Mora J. (1993) Estudio omparative de la macrofauna benthonica de un fondo de Zostera marina y un fondo arenoso libre de cubierta vegetal. Biol. Mar, 35, pp. 99-112.
- Guidetti P. (2000) Differences Among Fish Assemblages Associated with Nearshore Posidonia oceanica Seagrass Beds, Rocky–algal Reefs and Unvegetated Sand Habitats in the Adriatic Sea. Estuarine, Coastal and Shelf Science, 50, pp. 515-529. DOI: 10.1006/ecss.1999.0584.
- Harmelin-Vivien M. L., and Francoeur P. (1992) Trawling or visual censuses? Methodological bias in the assessment of fish populations in seagrass beds. P.S.Z.N. I: Marine Ecology, 13, pp. 41–51.
- Bell J. D., Westoby M., Steffe A. F. (1987) Fish larvae settling in seagrass: do they discriminate between beds of different leaf density? Journal of Experimental Marine Biology and Ecology, 111, pp. 133-144. DOI: https://doi.org/10.1111/j.1442-9993.1991.tb01056.x
- Ramiadantsoa T., Hanski I., Ovaskainen O. (2018) Responses of generalist and specialist species to fragmented landscapes. Theoretical Population Biology, 124, pp. 31-40. DOI: 10.1016/j.tpb.2018.08.001
- Fonds M. (1973) Sand gobies in the Dutch Wadden Sea (Pomatoschistus, Go- biidae, Pisces). Netherlands Journal of Sea Research, 6, pp. 417-178. DOI: 10.1016/0077-7579(73)90001-X
- Attrill M. J., Strong J. A., Rowden A. A. (2000) Are macroinvertebrate communities influenced by seagrass structural complexity? Ecography 23, pp. 114–121. DOI: 10.1111/j.1600-0587.2000.tb00266.x
- Charrier G., Durand J., Quiniou L., Laroche J. (2006) An Investigation of the Population Genetic Structure of Pollack (Pollachius pollachius) Based on Microsatellite Markers. ICES J. Mar. Sci., 63, pp. 1705–1709. DOI : 10.1016/j.icesjms.2006.07.006
- Furness E., Unsworth R. K. F. (2020) Demersal Fish Assemblages in NE Atlantic Seagrass and Kelp. Diversity, 12(10), pp. 366-266. DOI: https://doi.org/10.3390/d12100366
- Perry D., Staveley T. A. B., Gullström M. (2018) Habitat Connectivity of Fish in Temperate Shallow-Water Seascapes. Front. Mar. Sci, 4, pp. 440. DOI: https://doi.org/10.3389/fmars.2017.00440
- Bologna P. A. X., Heck K. L. (2002) Impact of habitat edges on density and secondary production of seagrass- associated fauna. Estuaries 25, pp. 1033-1044. DOI:10.1007/BF02691350
- Fonseca M. S., Fisher J. S. (1986) A comparison of canopy friction and sediment movement between four species of seagrass with reference to their ecology and restoration. Mar Ecol Prog Ser, 29, pp. 15-22.
- Fahrig L. (2003) Effects of Habitat Fragmentation on Biodiversity. Annual Review of Ecology, Evolution, and Systematics, 34(1), pp. 487-515. DOI: https://doi.org/10.1146/annurev.ecolsys.34.011802.132419
- Marine Management Organisation. (2021) Studland Bay, Protecting our precious seagrass habitats together. Studland Leaflet No.9, pp. 4. [Online] Available at <https://assets. publishing.service.gov.uk/media/64f060a36bc96d000d4ed38a/Studland_Leaflet__9_.pdf> [Accessed January 2023].
- Studland Bay Marine Partnership. [Online] Available at <https://www.dorsetcoast.com /project/studland-bay-marine-partnership/> [Accessed September 2024].
-
Nicola Cantasano*.The Role of Seaweeds in Carbon Sequestration. Ad Oceanogr & Marine Biol. 4(1): 2024. AOMB.MS.ID.000579.
-
Carbon dioxide; wild seaweeds; farming seaweeds; carbon sequestration; ocean sediments; iris publishers; iris publisher’s group
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






