 Research article
Research article
How Warm Eddies Affect Microbial Communities in the Tropical Pacific Ocean
Patrichka Wei-Yi Chen1,2, Madeline Olivia1,2, and An-Yi Tsai1,2,3*
1Institute of Marine Environment and Ecology, National Taiwan Ocean University, Keelung, Taiwan
2Doctoral Degree Program in Ocean Resource and Environmental Changes, National Taiwan Ocean University, Keelung, Taiwan
3Center of Excellence for the Oceans, National Taiwan Ocean University, Keelung, Taiwan
An-Yi Tsai, Institute of Marine Environment and Ecology, National Taiwan Ocean University, Keelung, Taiwan
Received Date: August 27, 2024; Published Date:September 06, 2024
Abstract
Mesoscale eddies are one of the most common processes driving water mixing and nutrient supply in the upper ocean globally. However, the impacts of the physical forcing of warm eddies on microbial communities and microzooplankton grazing largely remained to be explored. In terms of microbial responses by warm eddies, top-down control has been proposed to be the major process affecting microbial communities. To test the topdown hypothesis and investigate how microzooplankton grazers affect microbial communities, we designed an experiment to evaluate the grazing pressure between the eddy core (EC) and the outside eddy (OE). Successive seven-day microcosm experiments were conducted in the core and outside regions. We found that Synechococcus spp. was the most abundant picophytoplankton in the surface water at the core. Its abundances in the outside samples were higher than in the core samples at other depths, with maximum values at 60 m above the deep chlorophyll maximum (DCM).
Prochlorococcus spp. dominated in EC samples from the surface to 100 m, with the highest abundance at 100 m. The vertical variations of nanoflagellate, bacterial, and viral abundances also showed that the core surface water samples had higher abundances than OE samples. In the OE microcosm experiments indicate that regenerated nutrients and organic carbon availability regulate bacteria and picophytoplankton growth. Furthermore, the convergence effect drives nanoflagellates toward the core, elevating picophytoplankton grazing rates, dissolved organic carbon concentrations, and bacteria biomass. This study demonstrated that top-down control regulated by warm eddies would affect microbial communities, by converging with the water.
Keywords:Warm eddies; nanoflagellates; bacteria; picophytoplankton; viruses
Introduction
In the ocean, mesoscale eddies are ubiquitous [1,2], with important implications for biogeochemistry and productivity. Mesoscale eddies commonly occur in oligotrophic open ocean so that the physical mixing process may significantly affect the dynamics of nutrients and the subsequent changes in phytoplankton and productivity [3,4]. Generally, primary and secondary production would increase during cold cyclonic eddies and decreased in warm anticyclonic eddies [5]. The biological response to anticyclonic eddies is spatially varied in the eddies. At the edge of a warm eddy, nutrient pumping would enhance phytoplankton biomass [6]. However, the other study showed that warm-core anticyclonic eddies is usually associated with relatively high chlorophyll concentrations within the eddies, probably resulting from the transport and concentration of phytoplankton patches in them [7]. Biogeochemical carbon cycles in the water column are controlled by phytoplankton, heterotrophic bacteria, viruses, and protozoa [8].
It has been observed that picophytoplankton communities (Synechococcus spp., Prochlorococcus spp., and picoeukaryotes) constitute the majority of phytoplankton, accounting for 50%–90% of the total chlorophyll in oligotrophic waters [9]. Additionally, heterotrophic bacteria are major consumers of dissolved organic matter (DOM) and contribute significantly to carbon and nutrient cycling [10]. Picophytoplankton (0.2 to 2 μm) are the smallest phytoplankton cells and are the most abundant primary producers in the oceans [11]. Physical processes, especially mesoscale eddies [12,13], the lower nutrient requirements of picophytoplankton cause them to dominate during anticyclonic eddies. However, cyclonic eddies may result in large phytoplankton species accumulating in oligotrophic waters [4,12]. The spatiotemporal distribution of picophytoplankton is also influenced by light irradiance [14,15]. The maximum abundance of picophytoplankton occurs in the subsurface because of the high sunlight intensity on the surface [16].
There are different light adaptations among picophytoplankton groups, and thus different niches [17,18]. Prochlorococcus spp. can adapt better to low light than Synechococcus spp. [19]. Synechococcus spp. typically inhabits the upper euphotic layer, while Prochlorococcus spp. is still found at 200 m [16]. Additionally, Prochlorococcus spp. prefers warm oligotrophic eddies [20-22], while Synechococcus spp. was found to be significantly less abundant in warm eddies [6,21]. Even with these general findings, less is known about the dynamics of trophic interactions within microbial food webs, and how these changes are caused by warm eddy influence. Nanoflagellates are major grazer of bacteria and picophytoplanktons in the ocean [23,24]. As an additional benefit of grazing, macronutrients like P and N are recycled by nanoflagellate grazing [25]. Some studies reported that predators benefit from warmer, oxygen-rich waters in the center of anticyclonic eddies as they find prey more easily [26,27].
The convergence water flow from the outer to the core region drive microzooplankton and nanoflagellates toward to eddy core so that the grazing rates of microzooplankton and nanoflagellates on bacteria and picophytoplankton would increase in the core, respectively [28]. The purpose of the current study was to study how anticyclonic eddies affect the trophic interactions in microbial food webs. We hypothesize that microzooplankton grazing pressure are in the core region than the outside region of warm eddies and affects microbial interactions accordingly. The effects of microzooplankton grazing on microbial communities (viruses, bacteria, Prochlorococcus spp., Synechococcus spp., and nanoflagellates) were examined by four time-series experiments conducted in surface and deep chlorophyll maximum (DCM) of inside and outside warm eddies in the west Pacific Ocean.
Materials and Methods
Study Site and Samplings
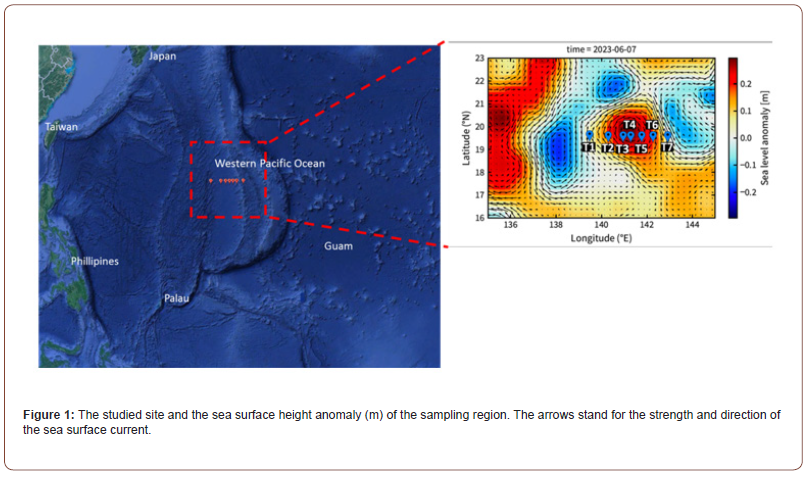
The field investigation was conducted at 7 stations in the west Pacific Ocean during the cruise period from May 29 and June 10 in 2023 by R/V Thompson (Figure 1). Based on the sea level anomaly obtained from AVISO (http://www.aviso.altimetry.fr) oceanographic satellite sea-level anomaly data, we divided the study area into eddy core (EC), eddy edge (EE), and out-of-eddy (OE) regions (Figure 1). Stations T3, T4, and T5 were in the EC, whereas stations T2 and T6 were in the EE, and stations T1 and T7 were in the OE. Temperature and salinity data were also analyzed for clustering (Figure 2). A surface depth of 2-5 m and 130-140 m DCM depths were evaluated for the incubation experiments at St. T1 (OE) and St. T3 (EC) (Figure 1). Teflon-coated Go-Flo bottles were used to collect seawater samples. The SBE 9/11plus CTD (Sea-Bird Scientific) was used to obtain temperature and salinity vertical profiles. For the detection of Chl a in water samples, a 25 mm GF/F filter and an in vitro fluorometer (Turner Design 10-AU-005) were used [29].
Nutrient samples were collected then stored in -20℃ freezer onboard for spectrophotometry analysis at land-based laboratory. Nitrite concentrations were determined by the classic Griess assay. Nitrate concentrations were determined by a new method using vanadium reduction with the Griess assay [30]. Phosphate and silicate concentrations were measured by the phosphomolybdenum blue or silicomolybdenum blue/yellow methods, respectively [31]. The detection limits of nitrite, nitrate, phosphate, and silicate were 0.002, 0.01, 0.002, and 0.01 μΜ, respectively. Low level concentration samples collected in the mixed layer waters were determined by 5 cm wide cell. The accuracy of the analysis was validated by using certified reference material (Kanso, Japan), with the deviations of phosphate, silicate, and nitrate from the certified value mostly ranging from 0.2 to 1.4, 0.1-2.0, and 0.2-3.0 %, respectively.
Size-Fractionation Experiments
The effects of microzooplankton grazing on microbial communities (viruses, bacteria, Prochlorococcus spp., Synechococcus spp., and nanoflagellates) were examined in this study. We performed incubations with and without microzooplankton grazers following a 2 μm filtering treatment to remove microzooplankton and other larger zooplankton. Four consecutive 7-d microcosm experiments were conducted in surface and deep chlorophyll maximum (DCM) of EC (T3) and OE (T1) region. Firstly, 10 L pre-filtered (2 μm poresize) seawater. In comparison to the control treatment, in which the samples were not filtered. We incubated all microcosms for seven days on board under solar radiation and at in situ temperatures. The experiments were conducted at the same temperature under 1% natural illumination in a flow-through tank on the deck covered with light screening mesh during DCM incubation. During the study period, subsamples were collected twice a day, among the mornings, between 08:00 and 09:00 h, and the evenings, between 20:00 and 21:00 h (local time). A subsample of 1 mL was collected from each incubation, fixed in paraformaldehyde (1% final concentration), and frozen in liquid nitrogen. Viral, bacterial, Prochlorococcus spp., Synechococcus spp. and nanoflagellate samples were preserved at -80°C before flow cytometry (FCM) analysis.
Flow Cytometric Analyses
We collected 2 ml seawater samples from each treatment, preserved them in 0.5% paraformaldehyde (final concentration), flash-frozen, and stored them in liquid nitrogen for enumerating nanoflagellates, picophytoplankton and heterotrophic bacteria. Samples were frozen at −80°C until analysis in the laboratory a CytoFLEX S flow cytometer (Beckman Coulter, Indianapolis) equipped with a 488 nm air-cooled argon-ion laser, a standard 525 nm filter, and an SYBR signal trigger. To minimize interference from high particle density, viral samples were diluted 1:10 in TE buffer (pH 8.0, EM grade) prior to staining. SYBR Green I (final concentration 1:50,000 commercial stock) was stained onto the diluted samples and incubated in the dark for 10 minutes at 80°C. Following the staining process, samples were cooled to 25 °C in an ice bath and analyzed by FCM according to Brussaard [32]. To detect and eliminate buffer noise, blank controls of TE buffer stained with SYBR Green I were used. According to Hammes and Egli [33], bacteria samples were stained with SYBR Green I (final concentration 1:10,000) for 15 minutes in the dark, then processed by FCM.
Based on flow cytometric analysis, on the basis of their red fluorescence from chlorophyll (>650 nm) and orange fluorescence from phycoerythrin (578 nm) and light scatter signals (SSC), picophytoplankton from the area were separated into two groups (Synechococcus spp. and Prochlorococcus spp.) according to Calvo-Díaz and Morán (2006). Furthermore, in this study, heterotrophic and pigmented nanoflagellates enumeration was also performed using flow cytometer according to Rose et al [34].
Results
Environmental Dynamics and Vertical Distribution of Nanoflagellates, Bacterial, Picophytoplankton and Viral Abundance
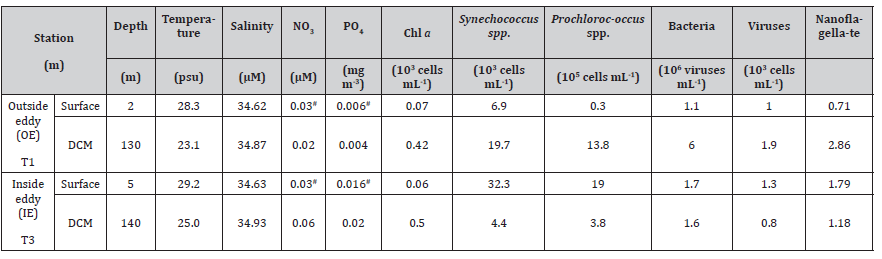
Figure 2 displays a vertical section of a warm-core eddy observed in this study. Sea surface temperatures (SST) at the anticyclonic eddy (29.2°C) indicated that core temperatures were at least 1°C higher than nearby temperatures (Sta. T1 and T7) (Figure 2A). Warm eddies also affected vertical distributions of temperature and salinity (Figure 2). The core station (Sta. T3) measured 29.2℃ at the surface and 3.7℃ at 130 m, while the outside station recorded 28.3℃ to 3.9℃ (Figure 2A). There was a pronounced dome-like distribution of cold and low salinity water in the 200–600 m water layer at the junction of the NEC and the STCC at 15–20°N (Figures 2B-2D). Chlorophyll a concentration in the surface water was similar within the eddy compared to outside station. However, at OE, the deep chlorophyll maximum (DCM) was centered at nearly 130 m, while within the eddy the DCM was deeper, 140 m (Table 1) (Figure 3A).
Two cyanobacterial species (Figures 3B&3C) had different vertical distributions inside and outside of the eddy. At surface waters, Synechococcus spp. was the most abundant picophytoplankton in EC samples (Figure 3B). Synechococcus spp. abundances in OE samples were higher than in EC samples at other depths, with maxi mum values at 60 m above the DCM (Figure 3B). As compared with Synechococcus spp., Prochlorococcus spp. dominated in EC samples from the surface to 100 m, with the highest abundance at 100 m (Figure 3C). Moreover, the vertical variation in nanoflagellate, bacterial, and viral abundances exhibited similar trends during the study period. The abundance of these communities was significantly higher in EC surface water samples than in OE samples, but lower between 10 and 200 m in EC samples than in OE samples (Figures 3D-3F).
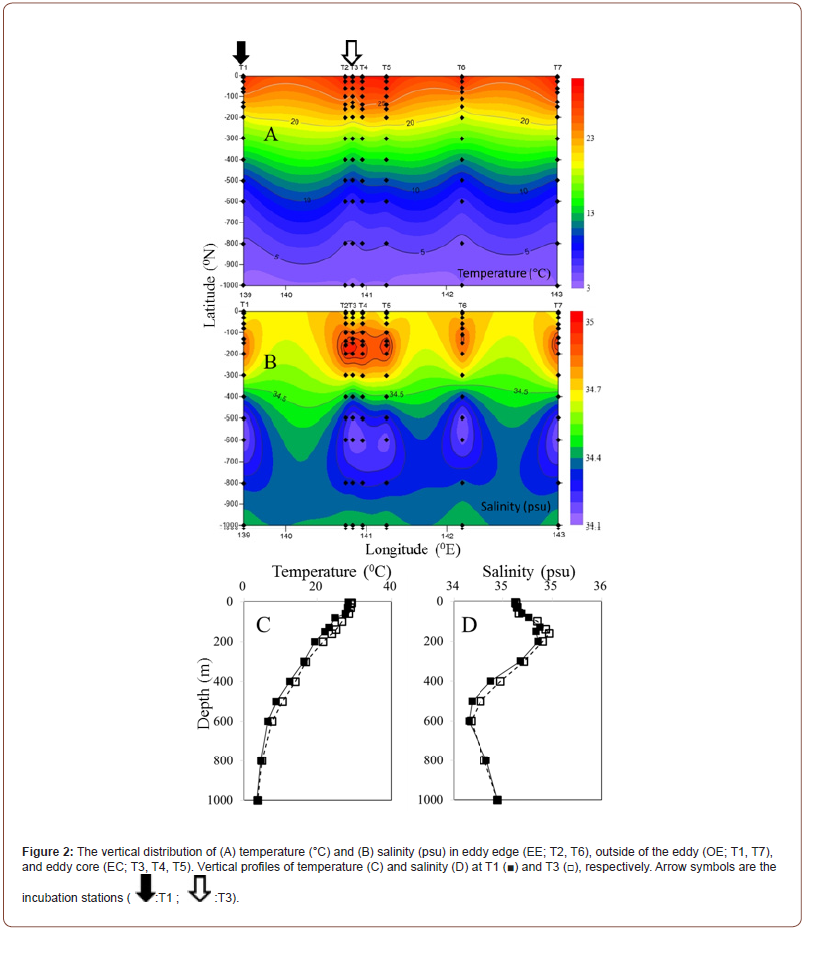
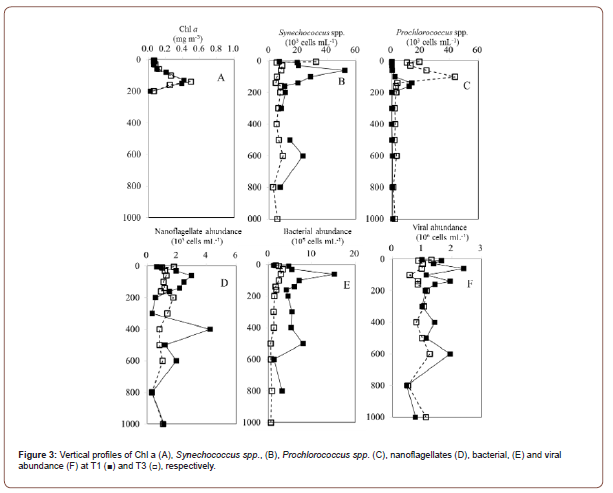
Table 1:Temperature, salinity, nutrients, chlorophyll α concentration, picophytoplankton, bacterial, viral and nanoflagellate abundance at the sampling stations and depths. #: Analyzing nutrient data at a depth of 10 m.
Time-Series of Microbial Communities’ Abundance in Surface Waters
In our time-series incubation experiments, the total abundance of nanoflagellate (the sum of heterotrophic nanoflagellate and pigmented nanoflagellate) ranged from 0.5 to 3.1 x 103 cells mL−1 and 0.1 to 1.4 x 103 cells mL−1 in the control treatments and 2 μm filtration in the surface waters in OE, respectively (Figure 4A). As for EC surface station, the total abundance of nanoflagellate ranged from 1.0 to 4.9 x 103 cells mL−1 in the 2 μm filtration waters (Figure 4F). In OE surface water, bacterial abundance showed a small fluctuation during the study period. However, in EC, there was a significantly higher abundance of bacteria filtered by 2 μm after 2 days of incubation than the unfiltered treatment (Figures 4B-4G). Similarly, to bacterial abundance, Synechococcus spp. abundance increased in 2 μm filtered treatment between 2 and 4 days of incubation at the core station (Figure 4H). Furthermore, Prochlorococcus spp. abundance declined gradually in unfiltered surface waters of OE from 1.9 to 0.2 x 104 cells mL−1 during the study period (Figure 4D). There was a significantly higher viral abundance in surface waters in OE than in EC in both treatments (Figures 4E-4J).
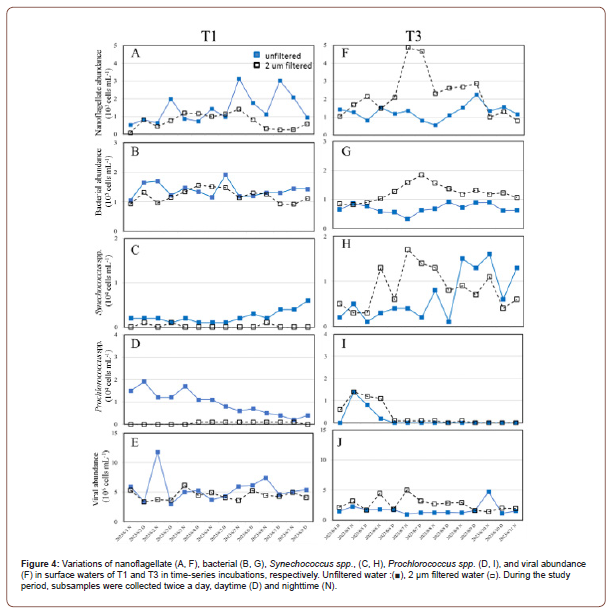
Time-Series of Microbial Communities’ Abundance in DCM Waters
Nanoflagellates were examined for unfiltered eddy-affected and eddy-outer samples, and a different pattern was observed in DCM waters (Figures 5A-5F). The results in Figure 5A illustrate an obvious difference in nanoflagellate abundance between unfiltered and 2 μm filtered treatments in OE, and that bacterial abundance in OE generally increased from 1.1 to 5.6 x 103 cells mL−1 after 3 days incubation in DCM waters. Time-series experiments show that the eddy-affected area in DCM water has lower bacterial abundance than the eddy-outer area in unfiltered treatments (t-test, p< 0.05) (Figures 5A-5F). However, the nanoflagellate abundance was almost constant in both incubations in DCM of EC waters (Figure 5F). As for bacteria, its abundance increased gradually in unfiltered surface waters of OE in DCM waters from 1.1 to 3.4 x 105 cells mL−1 during the study period (Figure 5B). However, in DCM region, small temporal variability is observed for bacterial abundance in both treatments in EC (Figure 5G).
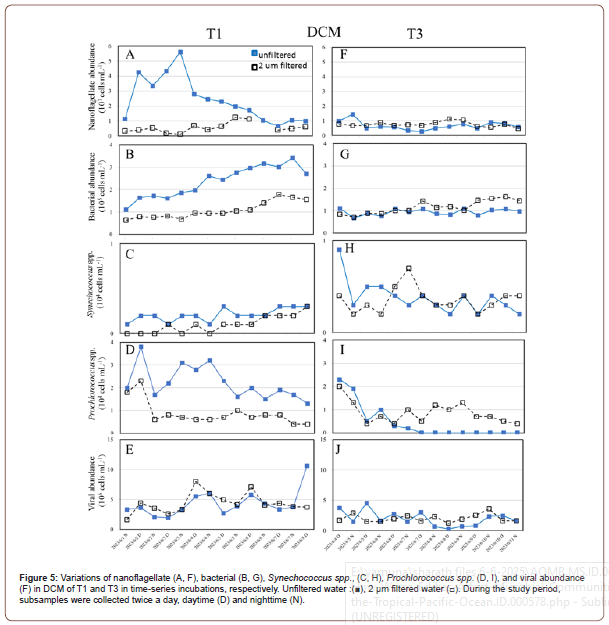
Furthermore, in OE incubation, the abundance of Synechococcus spp. and Prochlorococcus spp. in unfiltered treatment showed the higher value than the 2 μm filtered treatment (Figures 5C&5D). However, in EC experiment, there was a significantly higher abundance of Prochlorococcus spp. filtered by 2 μm after 3 days of incubation than the unfiltered treatment (Figure 5I). Overall, in DCM samples, viral abundance ranged from 1.9 to 10.6 x 105 viruses mL−1 and 1.6 to 8.0 x 105 viruses mL−1 in unfiltered and 2 μm filtered treatment, respectively, with no statistical difference in OE samples (t-test, p > 0.05) (Figure 5E). In EC samples, small temporal variability is observed for viral abundance in both treatments, with <5 x 105 viruses mL−1 during the study period (Figure 5J).
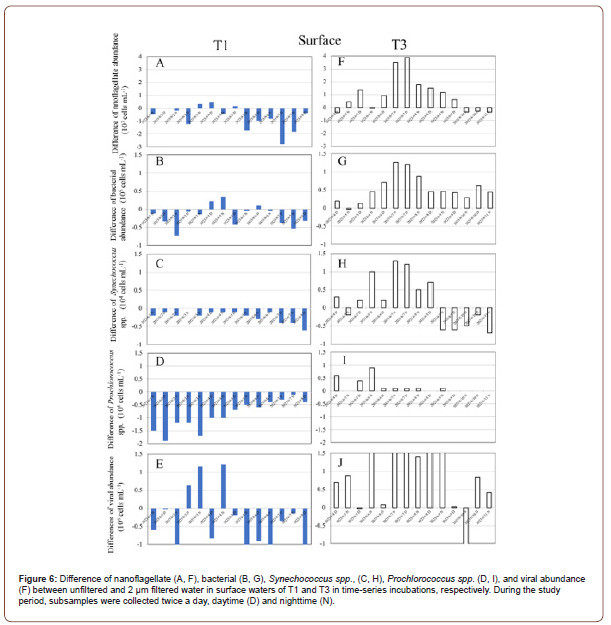
Differences in The Microbial Communities’ Abundance with Changed in Unfiltered and 2 μm Filtered Waters
In this study, to reveal the difference in abundance of microbial communities between the unfiltered and 2 μm filtered treatments influenced by warm eddies, we explored the value in both treatments (difference values=2 μm filtered waters – unfiltered waters). In surface waters samples, most of negative values of mi crobial communities (nanoflagellate, bacteria, Synechococcus spp., Prochlorococcus spp. and viruses) occurred in OE samples (Figure 6). In contrast to OE samples, higher values of microbial communities were mostly observed in 2 μm filtered waters during the study period in EC samples (Figure 6). Thus, most of positive values of microbial communities (nanoflagellate, bacteria, Synechococcus spp., Prochlorococcus spp. and viruses) were observed in EC samples (Figure 6). Furthermore, in DCM samples, similar trends were measured in abundance of nanoflagellate, bacteria, Synechococcus spp., and Prochlorococcus spp. in OE samples compared with the surface waters (Figure 6). In the case of DCM samples, all of negative values of microbial communities occurred in OE samples (Figure 7). However, most of the positive values of these microbial communities were observed in EC samples (Figure 7).
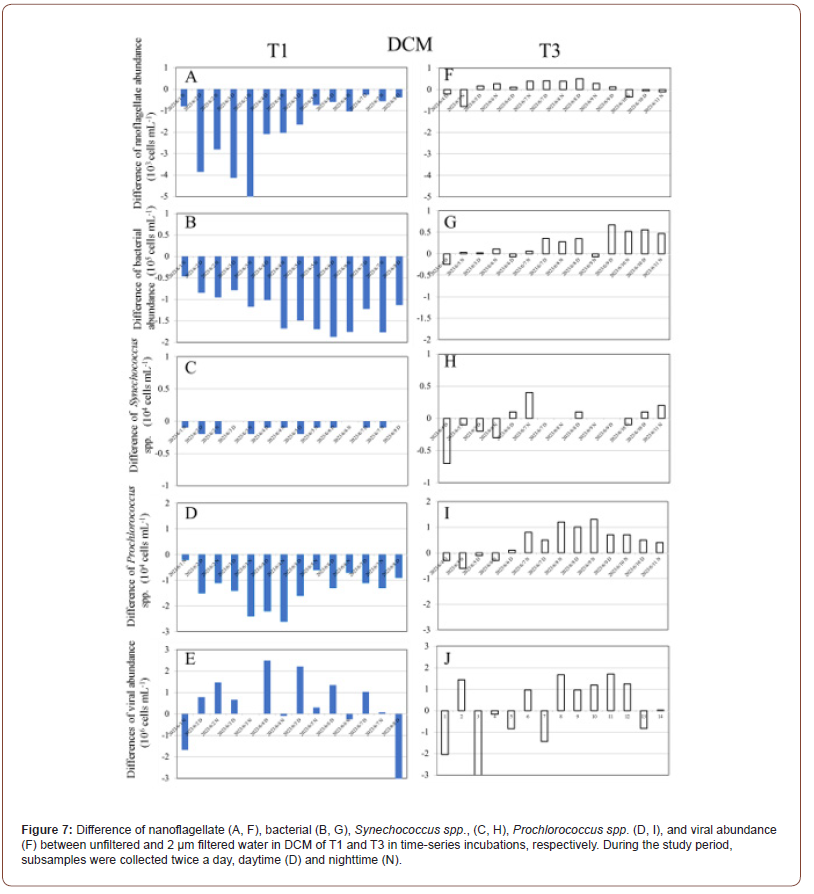
Discussion
Previous studies reported that picophytoplankton was dominate in warm eddies as picophytoplankton may survive in nutrient deplete water. Relatively, cyclonic cold eddies would result in an accumulation of large phytoplankton species in pico-dominated waters [4]. Moreover, although some studies have reported bacterial communities’ variations in mesoscale eddies [35,36], no studies have described their trophic interactions in microbial communities within and outside anticyclonic eddies.
Vertical variations of microbial communities
Variations in nutrients, light, and temperature driven by mesoscale eddies are widely recognized as the major process influencing phytoplankton distributions [37,38]. Several studies have shown that Prochlorococcus spp. prefers oligotrophic warm eddies [21,22] and can be used as a bioindicator of the Kuroshio Current [39,40], while the abundance of Synechococcus spp. in warm eddies was significantly lower than that of Prochlorococcus spp. [21]. In this study, for picophytoplankton community structures in the OE and EC samples, different picophytoplankton communities were found; Prochlorococcus spp. was 7 times more abundant in EC than Synechococcus spp. above 100 m depth (Figures 3B&3C). In nutrient- limited water, Prochlorococcus spp. and Synechococcus spp. are highly competitive [41], and Prochlorococcus spp. may outgrow Synechococcus spp. when heterotrophic bacteria are present [42].
Further, the uptake rate of bioavailable nutrients may affect the spatial dynamics of Synechococcus spp. and Prochlorococcus spp. According to Zubkov et al. [43], Prochlorococcus spp. takes up depleted phosphate rapidly, while Synechococcus spp. occupies a minor role in direct phosphate uptake. Therefore, at the EC location, higher phosphate concentrations were found (Table 1) and this may have been beneficial to Prochlorococcus spp. growth. There is a growing understanding that mortality factors are important regulators of picophytoplankton abundance and diversity [44], but these factors are rarely considered as factors affecting picophytoplankton biogeography. As a result of our analysis, we found a relationship between Synechococcus spp. abundance and nanoflagellate abundance in the EC. There may be a top-down effect of microzooplankton grazing pressure on Synechococcus spp. that contributed to the lower abundance of Synechococcus spp. in warm eddies compared to Prochlorococcus spp. (Figures 3B&3C).
Furthermore, viruses also play a vital role in the microbial loop, but their distribution and activity (lysis of prokaryotes) in oceanic eddies are unknown. We found that viral abundance was significantly higher in OE than in EC above 200 m depth. A possible explanation for this spatial pattern is that higher host (such as bacteria or Synechococcus spp.) were found in the OE region (Figure 3). There has been an established correlation between viruses and bacteria in the water column, as demonstrated by previous studies [45,46], indicating that bacteriophages are a significant fraction of the viral community. In addition, viruses of photosynthetic organisms may also make up a significant fraction of viruses in the epipelagic layer because viruses and bacteria are positively correlated with Chl a [45]. There is an alternative explanation for these data, which is that different viral infections could cause them. Changing balances between lytic and lysogenic infections are dynamic phenomena that rapidly respond to changes in environmental conditions [47]. There was a greater frequency of lysogenic infection at anticyclonic stations, where bacteria were under higher pressure from protists [45].
Grazing pressure experiments
Applying filtration treatment, microzooplankton grazing pressure was supposed to be negligible in filtered treatments. It is expected to observe an increase in nanoflagellate and picoplankton abundance in filtrated treatments during 7-d time series incubations. However, that we observed higher abundances of nanoflagellates, bacteria, Synechococcus spp., and Prochlorococcus spp. in unfiltered OE samples compared to samples filtered with 2 μm (Figures 4&5). Major nutrients and DOC are the two most important factors regulating picoplankton, and nanoflagellates abundance, which are also controlled by prey supply (picoplankton) in this study. A similar result was also reported by Ferrier and Rassoulzadegan [48] who demonstrated that protozoan grazing can increase in situ picoplankton- specific growth rates by more than a factor of 2. Several studies suggest that regenerated iron can provide up to 90% of the demand in a limited area [49], and regenerated iron can also be found from microzooplankton grazing [50]. Also, in oligotrophic regions, nutrient cycling by viral lysis has been observed [51], with picophytoplankton able to directly absorb viral lysis products, including organic nitrogenous compounds [52].
By contrast, in surface EC, there was a significantly higher abundance of bacteria, Synechococcus spp. and nanoflagellates filtered by 2 μm than the unfiltered treatment (Figures 4F-4H). In this situation, grazing pressure by microzooplankton (top-down control) on nanoflagellate and picophytoplankton should be important in the surface layers of warm eddy (Figure 5). Additionally, we observed a relationship between the abundance of Synechococcus spp. and bacteria in 2 μm filtrated treatment and nanoflagellated in surface EC (Figures 8A&8B). In this situation, bacteria and Synechococcus spp. are considered major prey for nanoflagellates in the surface EC layer in this study. Moreover, in EC, Prochlorococcus spp. exhibited a positive relationship with nanoflagellate abundance in DCM (Figure 8F). There have been few comparison analyses comparing microzooplankton grazing rates between different mesoscale eddies [53], despite recent reports of microzooplankton feeding rates in mesoscale eddies. The top-down effect of grazing pressure by microzooplankton on the driving mechanisms of mesoscale physical processes is lacking.
Impacts of Warm Eddy on Microbial Communities’ Variables
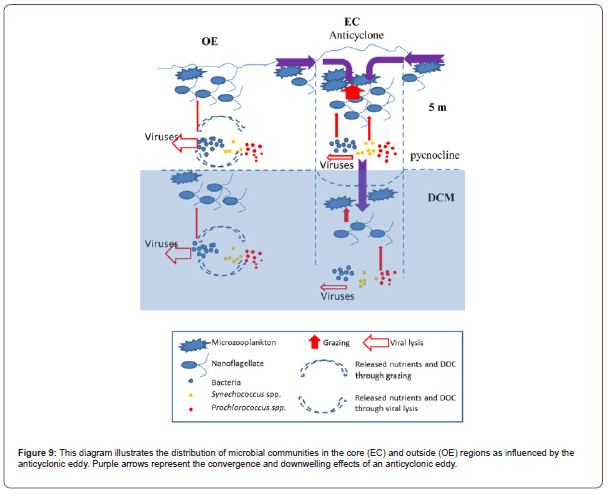
Temperature increase may increase grazing pressure in the anticyclonic eddy [54]. The effects of eddies on phytoplankton communities are either directly or indirectly influenced by bottom-up or top-down controls [54]. Similarly, Boras et al. [45] observed high grazing activity at stations subjected to anticyclonic eddies, which may be related to the higher abundance of nanoflagellates. It has also been shown that warmer and more oxygen-rich water in the center of anticyclonic eddies provide predators with a metabolic advantage and facilitates the search for suitable prey [27]. The convergence effect in anticyclonic eddies drives the microzooplankton and nanoflagellates towards the eddy core. In turn, this increases the amount of microzooplankton and nanoflagellates in the surface waters, causing a marked increase in grazing rates of bacteria and picophytoplankton in warm eddy cores [28]. Here, we propose a new conceptual model to explain the differences in microbial communities between the outside and core of an anticyclonic eddy based on the data observed in this study (Figure 9).
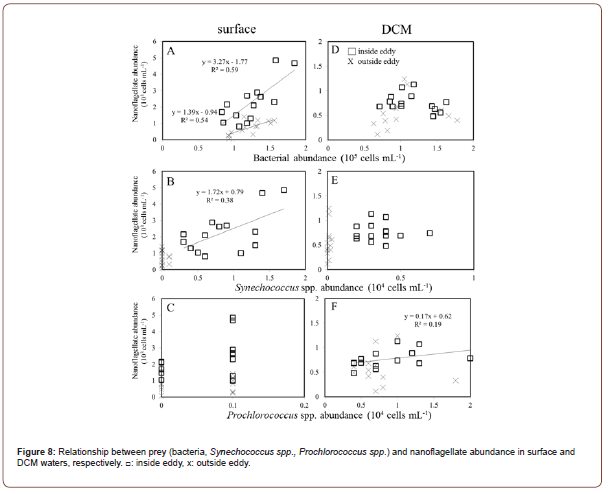
It may help explain why the abundance of bacteria and picophytoplankton are lower in surface OE than EC (Figure 9). Major nutrients were almost depleted in the DCM layer, regenerating nutrients and carbon is the most limiting factor for bacteria and phytoplankton growth in OE region (Figure 9). The convergence effect drives microzooplankton and nanoflagellates from the periphery of the eddy toward the core (54), which explain the increased amounts of microzooplankton and nanoflagellates in the surface waters of EC and results in increasing grazing rates on bacteria and picophytoplankton (Figure 8). Our study provides plausible observational evidence to confirm that the abundance of bacteria and Synechococcus spp. in the center of an anticyclonic eddy is the dominant prey for predators. This aggregation mechanism promotes the formation of microbial communities from the surface to deep waters, then nanoflagellate grazing significantly contributes to Prochlorococcus grazing (Figure 9). A lower abundance is seen in the EC region due to the lower growth rates of microbial communities under the top-down controlled conditions of DCM (Figure 9). In OE, the viral abundance was significantly higher than in EC (Figure 4E-4J), suggesting that higher hosts (such as bacteria or Synechococcus spp.) were found in the OE region.
Conclusion
In summary, our study confirms that two cyanobacterial species had different vertical distributions EC and OE. At surface waters, Synechococcus spp. was the most abundant picophytoplankton in EC samples. Synechococcus spp. abundances in OE samples were higher than in EC samples at other depths, with maximum values at 60 m above the DCM. As compared with Synechococcus spp., Prochlorococcus spp. dominated in EC samples from the surface to 100 m, with the highest abundance at 100 m. Applying filtration treatment, we found that grazing pressure by microzooplankton (top-down control) on nanoflagellate and picophytoplankton should be important in the surface layers of warm eddy. Moreover, major nutrients were almost depleted in the DCM layer, regenerating nutrients and carbon is the most limiting factor for bacteria and phytoplankton growth in OE region.
Conflict of Interest
Authors declare that is no conflict of interest in this research study.
Acknowledgments
This study was supported by a grant (MOST 111-2119-M- 019-002) from the Ministry of Science and Technology, ROC. We appreciate the language editing and helpful comments from Choice Language Service Co., Ltd. on this manuscript.
References
- Hans Van Haren, Claude Millot, Isabelle Taupier Letage (2006) Fast deep sinking in Mediterranean eddies. Geophysical Research Letters 330(4).
- Federico Baltar, Javier Arístegui, Josep M Gasol, Itziar Lekunberri, Gerhard J Herndl (2010) Mesoscale eddies: hotspots of prokaryotic activity and differential community structure in the ocean. Isme J 4(8): 975-988.
- Robert R Bidigare, Claudia Benitez Nelson, Carrie L Leonard, Paul Quay, Michael L Parsons, et al. (2003) Influence of a cyclonic eddy on microheterotroph biomass and carbon export in the lee of Hawaii. Geophys Res Lett 30(3): 1318.
- Robert D Vaillancourt, John Marra, Michael P Seki, Michael L Parsons, Robert R Bidigare (2003) Impact of a cyclonic eddy on phytoplankton community structure and photosynthetic competency in the subtropical North Pacific Ocean. Deep Sea Research Part I: Oceanographic Research Papers 50(7): 829-847.
- Qingyou He, Haigang Zhan, Jie Xu, Shuqun Cai, Linbin Zhou, et al. (2019) Eddy‐Induced Chlorophyll Anomalies in the Western South China Sea. Journal of Geophysical Research: Oceans 124(12): 9487-9506.
- Lei Wang, Bangqin Huang, Edward A. Laws, Kuanbo Zhou, Xin Liu, et al. (2018) Anticyclonic Eddy Edge Effects on Phytoplankton Communities and Particle Export in the Northern South China Sea. J Geophys Res Oceans 123.
- Antonio Bode, Barquero S, Cardoso M, Braun JG, Demetrio de Armas (2001) Pelagic bacteria and phytoplankton in oceanic waters near the Canary Islands in summer. Mar Ecol Prog Ser 209: 1-17.
- Paul del Giorgio, Peter Williams (2005) The global significance of respiration in aquatic ecosystems: from single cells to the biosphere. Respiration in Aquatic Ecosystems 14: 267-303.
- Nona Sheila Romualdo Agawin, Carlos M Duarte, Susana Agustí (2000) Nutrient and temperature control of the contribution of picoplankton to phytoplankton biomass and production (Errata). Limnol and Ocanogr 45(3): 591-600.
- Azam F, Fenchel T, Field JG, Gray JS, Meyer Reil LA, et al. (1983) The Ecological Role of Water-Column Microbes in the Sea. Mar Ecol Prog Ser 10(3): 257-263.
- Robert JW Brewin, Gavin H Tilstone, Thomas Jackson, Terry Cain, Peter I Miller, et al. (2017) Modelling size-fractionated primary production in the Atlantic Ocean from remote sensing. Prog in Oceanogr 158: 130-149.
- Shigeto Nishino, Motoyo Itoh, Yusuke Kawaguchi, Takashi Kikuchi, Michio Aoyama (2011) Impact of an unusually large warm-core eddy on distributions of nutrients and phytoplankton in the southwestern Canada Basin during late summer/early fall 2010. Geophys Res Lett 38(16).
- Arya Mohan, Abraham Biju (2021) Influence of warm core eddy on the vertical distribution of autotrophic pico- and nanoplankton in the Bay of Bengal. Mar Bio Res 16(93): 1-12.
- Pedro Flombaum, José L Gallegos, Rodolfo A Gordillo, José Rincón, Lina L Zabala, et al. (2013) Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus. Proc Natl Acad Sci U S A 110(24): 9824-9829.
- Agusti S, Lubián LM, Moreno-Ostos E, Estrada M, Duarte CM (2019) Projected Changes in Photosynthetic Picoplankton in a Warmer Subtropical Ocean. Front Mar Sci 5.
- Yu Ming Cai, Xiu Ren Ning, Cheng Gang Liu, Qiang Hao (2007) Distribution Pattern of Photosynthetic Picoplankton and Heterotrophic Bacteria in the Northern South China Sea. J Integr Plant Biol 49(3): 282-298.
- Pedro Flombaum, Wei Lei Wang, Francois W Primeau, Adam C Martiny (2020) Global picophytoplankton niche partitioning predicts overall positive response to ocean warming. Nat Geosci 13(2): 116-120.
- Coello Camba A, Agustí Susana (2021) Picophytoplankton Niche Partitioning in the Warmest Oligotrophic Sea. Front in Mar Sci 8.
- Susana Agustí, Sánchez MC (2002) Cell viability in natural phytoplankton communities quantified by a membrane permeability probe. Limnol and Oceanogr 47(3): 818-828.
- Tiegang Li, Jingtao Zhao, Rongtao Sun, Fengming Chang, Hanjie Sun (2010) The variation of upper ocean structure and paleoproductivity in the Kuroshio source region during the last 200kyr. Mar Micropaleontol 75(1-4): 50-61.
- Andréa da Consolação de Oliveira Carvalho, Carlos Rafael B Mendes, Rodrigo Kerr, José Luiz Lima de Azevedo, Felippe Galdino, et al. (2019) The impact of mesoscale eddies on the phytoplankton community in the South Atlantic Ocean: HPLC-CHEMTAX approach. Mar Environ Res 144: 154-165.
- Belkin N, Guy Haim T, Rubin Blum M, Lazar A, Sisma Ventura G, et al. (2022) Influence of cyclonic and anticyclonic eddies on plankton in the southeastern Mediterranean Sea during late summertime. Ocean Sci 18(3): 693-715.
- Robert W Sanders, Berninger UG, Lim EL, Kemp PF, Caron DA (2000) Heterotrophic and mixotrophic nanoplankton predation on picoplankton in the Sargasso Sea and on Georges Bank. Mar Ecol Prog Ser 192: 103-118.
- Tsai An Yi, Chiang Kuo Ping, Jeng Chang (2005) Seasonal diel variations of picoplankton and nanoplankton in a subtropical western Pacific coastal ecosystem. Limnol and Oceanogr 50(4): 1221-1231.
- Twiss M, Campbell Peter (1995) Regeneration of trace metals from picoplankton by nanoflagellate grazing. Limnol and Oceanogr 40(8): 1418-1429.
- Peter Gaube, Camrin Braun, Gareth L Lawson, Dennis J McGillicuddy, Alice Della Penna, et al. (2018) Mesoscale eddies influence the movements of mature female white sharks in the Gulf Stream and Sargasso Sea. Sci Rep 8(1): 7363.
- Camrin D Braun, Peter Gaube, Tane H Sinclair-Taylor, Gregory B Skomal, Simon R Thorrold (2019) Mesoscale eddies release pelagic sharks from thermal constraints to foraging in the ocean twilight zone. Proc Natl Acad Sci U S A 116(35): 17187-17192.
- Wang Y, Wang W (2023) Latitudinal and meridional patterns of picophytoplankton variability are contrastingly associated with Ekman pumping and the warm pool in the tropical western Pacific. Ecology and Evolution 13(10): e10589.
- Gong G-C, Shiah F-K, Liu K-K, Wen Y-H, Ming-Hsin L (2000) Spatial and temporal variation of chlorophyll a, primary productivity and chemical hydrography in the southern East China Sea. Continental Shelf Research 20(4-5): 411-436.
- Pai S-C, Su Y-T, Lu M-C, Chou Y, Ho T-Y (2021) Determination of Nitrate in Natural Waters by Vanadium Reduction and the Griess Assay: Reassessment and Optimization. ACS ES&T Water 1(6): 1524-1532.
- Pai S-C, Chung-Cheng Y, Riley JP (1990) Effects of acidity and molybdate concentration on the kinetics of the formation of the phosphoantimonylmolybdenum blue complex. Analytica Chimica Acta 229: 115-120.
- Brussaard CPD (2004) Optimization of Procedures for Counting Viruses by Flow Cytometry. Appl Environ Microbiol 70(3): 1506-1513.
- Hammes F, Egli T (2010) Cytometric methods for measuring bacteria in water: advantages, pitfalls and applications. Anal Bioanal Chem 397(3): 1083-1095.
- Rose J, Caron DA, Sieracki ME, Poulton N (2004) Counting heterotrophic nanoplanktonic protists in cultures and aquatic communities by flow cytometry. Aquatic Microbial Ecology 34(3): 263-277.
- Zhang Y, Sintes E, Chen MN, Dai M, Jiao N, et al. (2009) Role of mesoscale cyclonic eddies in the distribution and activity of Archaea and Bacteria in the South China Sea. Aquatic Microbial Ecology 56(1): 65-79.
- Nelson CE, Carlson CA, Ewart CS, Halewood ER (2014) Community differentiation and population enrichment of Sargasso Sea bacterioplankton in the euphotic zone of a mesoscale mode-water eddy. Environ Microbiol 16(3): 871-887.
- Gaube P, McGillicuddy D, Chelton DB, Behrenfeld JM, Strutton PG (2014) Regional variations in the influence of mesoscale eddies on near-surface chlorophyll. Journal of Geophysical Research Oceans 119(12): 8195-8220.
- Kang J, Wang Y, Huang S, Pei L, Luo Z (2022) Impacts of Mesoscale Eddies on Biogeochemical Variables in the Northwest Pacific. Journal of Marine Science Engineering 10(10): 1451.
- Huang Y, Laws E, Chen B, Huang B (2019) Stimulation of Heterotrophic and Autotrophic Metabolism in the Mixing Zone of the Kuroshio Current and Northern South China Sea: Implications for Export Production. Journal of Geophysical Research Biogeosciences 124(9): 2645-2661.
- Zhao Y, Yu R-C, Kong F-Z, Wei C-J, Liu Z, et al. (2019) Distribution Patterns of Picosized and Nanosized Phytoplankton Assemblages in the East China Sea and the Yellow Sea: Implications on the Impacts of Kuroshio Intrusion. Journal of Geophysical Research Oceans 124(2): 1262-1276.
- Tsiola A, Pitta P, Fodelianakis S, Pete R, Magiopoulos I, et al. (2016) Nutrient Limitation in Surface Waters of the Oligotrophic Eastern Mediterranean Sea: an Enrichment Microcosm Experiment. Microb Ecol 71(3): 575-588.
- Calfee BC, Glasgo LD, Zinser ER (2022) Prochlorococcus Exudate Stimulates Heterotrophic Bacterial Competition with Rival Phytoplankton for Available Nitrogen. mBio 13(1): e0257121.
- Zubkov MV, Mary I, Woodward EMS, Warwick PE, Fuchs BM, et al. (2007) Microbial control of phosphate in the nutrient-depleted North Atlantic subtropical gyre. Environ Microbiol 9(8): 2079-2089.
- Breitbart M, Bonnain C, Malki K, Sawaya NA (2018) Phage puppet masters of the marine microbial realm. Nat Microbiol 3(7): 754-766.
- Boras J, Montserrat Sala M, Baltar F, Aristegui J, Duarte CM, et al. (2010) Effect of viruses and protists on bacteria in eddies of the Canary Current region (subtropical northeast Atlantic). Limnology and Oceanography 55(2): 885-898.
- Tsai A-Y, Gong G-C, Liu H (2018) Seasonal variations in virioplankton and picoplankton in semi-enclosed and open coastal waters. Terrestrial Atmospheric and Oceanic Sciences 29(4): 465-472.
- Long A, McDaniel LD, Mobberley J, Paul JH (2008) Comparison of lysogeny (prophage induction) in heterotrophic bacterial and Synechococcus populations in the Gulf of Mexico and Mississippi River plume. Isme j 2(2): 132-144.
- Ferrier-Pagès C, Rassoulzadegan F (1991) Density-dependent effects of protozoans on specific growth rates in pico- and nanoplanktonic assemblages. Limnology and Oceanography 36: 657-669.
- Poorvin L, Rinta-Kanto JM, Hutchins DA, Wilhelm SW (2004) Viral release of iron and its bioavailability to marine plankton. Limnology and Oceanography 49(5): 1734-1741.
- Hutchins DA, Bruland KW (1994) Grazer-mediated regeneration and assimilation of Fe, Zn and Mn from planktonic prey. Marine Ecology Progress Series 110(2-3): 259-269.
- Suttle CA (2005) Viruses in the sea. Nature 437(7057): 356-361.
- Zubkov MV, Fuchs BM, Tarran GA, Burkill PH, Amann R (2003) High Rate of Uptake of Organic Nitrogen Compounds by Prochlorococcus Cyanobacteria as a Key to Their Dominance in Oligotrophic Oceanic Waters. Appl Environ Microbiol 69(2): 1299-1304.
- Chen B, Liu H, Landry MR, Dai M, Huang B, et al. (2009) Close coupling between phytoplankton growth and microzooplankton grazing in the western South China Sea. Limnology and Oceanography 54(4): 1084-1097.
- An L, Liu X, Xu F, Fan X, Wang P, et al. (2024) Different responses of plankton community to mesoscale eddies in the western equatorial Pacific Ocean. Deep Sea Res Part I Oceanogr Res Pap 203: 104219.
-
Patrichka Wei-Yi Chen, Madeline Olivia, and An-Yi Tsai*. How Warm Eddies Affect Microbial Communities in the Tropical Pacific Ocean. Ad Oceanogr & Marine Biol. 4(1): 2024.AOMB.MS.ID.000578.
-
Warm eddies; nanoflagellates; bacteria; picophytoplankton; viruses; iris publishers; iris publisher’s group
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






