 Research article
Research article
Comparison of Fish Assemblage in Mooring Scars and Adjacent Seagrass, Studland Bay, Dorset
Hayley Roberts, Ken Collins* and Antony Jensen
School of Ocean and Earth Science, University of Southampton, UK
Ken Collins, School of Ocean and Earth Science, University of Southampton, UK
Received Date:August 28, 2024; Published Date:September 12, 2024
Abstract
Seagrass provides vital ecosystem services from carbon cycling to hosting an abundance of fish species. However, anthropogenic impacts like mooring scars are causing a decline in seagrass and this is influencing a change in the habitat’s fish assemblage. The present study has set out to investigate the fish assemblage in mooring scars and the adjacent seagrass in Studland, Dorset, UK. Underwater video systems were set up for an hour at 14 sites (7 seagrass, 7 mooring scars). Overall, 13 marine fish species from 9 families were identified and the 2 main species present included Pomatoschistus minutus for mooring scar sites and Pollachius pollachius for seagrass sites. Non-parametric statistical analysis of the results showed a clear difference between the fish assemblage within seagrass and mooring scar sites. These results are essential for the conservation and management of seagrass in Studland Bay and the fish species that it hosts.
Keywords: Fish assemblage; seagrass; community; ecosystem; mooring; niche; habitat; diet; fragmentation
Introduction
Approximately 60 species of seagrass are flowering plants, that have adapted to inhabit marine environments. Across their ranges, they provide vital services, including, coastal defence, nutrition and carbon cycling [1-5]. To add to this, seagrasses are a significant source of detrital material, which is the main source of food for numerous species [6]. Globally, seagrass is a major carbon sink and can capture carbon 35 times faster than rainforests [7]. Past literature has estimated a global seagrass spatial distribution range from 177,000 to 600,000 km2 [8,9]. Recent research shows that since 1936, 44% of the UK’s seagrass has been lost (cover estimated to be 16,524 ha in 1936) [10]. Humans are the major contributors to this degradation of seagrass through pollution, Urbanisation, and boat moorings [11]. Globally seagrasses are known for hosting a high biodiversity of fish species [12,13], with Z. marina said to have one of the richest varieties and abundance of marine life in the sea [14]. In addition to support this biodiversity of fish, seagrasses provide an abundance of food including small crustaceans [15,16] and infauna species [17].
Studland Bay in Dorset hosts seagrass, Zostera marina meadows. It was designated as a Marine Conservation Zone (MCZ) in May 2019, covering an area of approximately 4 km2, the seagrass beds cover approximately a quarter of that area. The bay is a key seagrass habitat site as it has desirable characteristics for seagrass growth including shelter from southwest winds and coarse sediment. Conservation was prompted by its seagrass beds and its iconic spiny seahorse, Hippocampus guttulatus population [18]. The seagrass abundance is threatened by mooring chains and intensive boat anchoring, both these are destructive, as when moored boats move around in the wind and tide, the mooring chain churns up the seagrass when it is dragged, and anchor recovery dislodges the plants. Following this, the sediment becomes less cohesive and more mobile and has been reported to have less organic material and lower silt fractions resulting in conditions that do not support seagrass [19]. Studies of such impacts have shown that seagrass vegetative reproduction cannot withstand such ongoing disturbance [20-22].
Past studies have revealed that seagrass holds a greater number of fish than other coastal habitats such as sandy seabed’s [23]. Pihl et al. [17] detected a greater number of fish species in Z. marina seagrass in Sweden (n = 28) compared to areas with seagrass loss (n = 19). Research focusing specifically on seagrass loss from boat moorings and fish presence has been dominated by seine net surveys [23-27]. Previous studies looking at fish assemblages in situ have used video systems as this is a cost-effective and non-destructive method [28,29] and is desirable in the context of conservation efforts especially when monitoring in Marine Protected Areas. Most video analyses studying fish utilize bait, and this is great for attracting them to the camera, however, this also creates a bias and attracts selected species [28]. Un-baited videos remove the attractive effect of bait and allow normal behaviour to occur in the video [28]. In addition, using bait may attract fish from the seagrass into the mooring scar videos that would not naturally be there in undisturbed conditions. This study aimed to determine if there were differences between the fish assemblage within bare sediment mooring scars and the adjacent healthy seagrass using un-baited video monitoring.
Methods
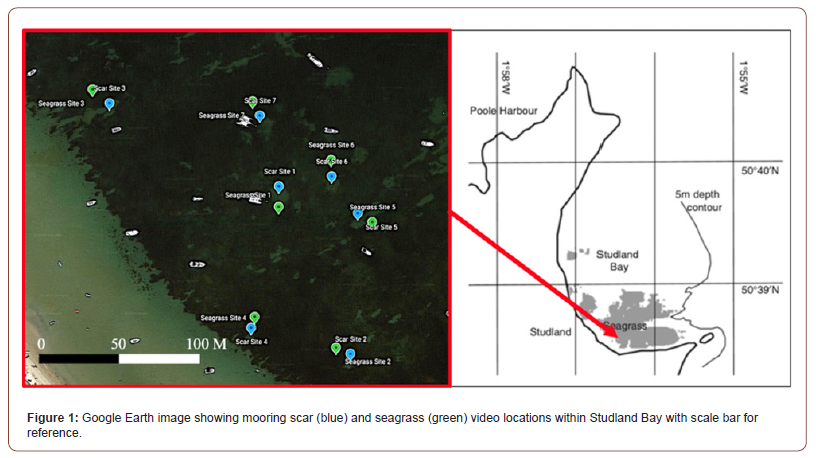
Collection of data in the field was undertaken over 3 days: 19th, 20th, and 21st July 2021 between 09:00 and 18:00. The remote underwater video (RUV) system was an un-baited GoPro Hero 4 camera (1080p, 30fps) attached to a weighted stand set to record for 1 hour. Camera deployment was undertaken by paddle boarding out to mooring buoys where position and the time were recorded. Once at the site, 2 cameras were deployed: one in the mooring scar and the other in the adjacent seagrass (Figure 1). Each 1-hour video was split into five 10-minute videos with 2-minute gaps. Two minutes were excluded from the beginning and end of each video as this allowed time for settlement of the suspended matter and to reduce disturbance from deployment and retrieval. Analysing videos was undertaken using Video VLC software (Version 3.0.16, https://www.videolan.org). Many shoals of minuscule fish were present in the videos and therefore they were named altogether as ‘Unidentified Fish 1’ as there is no clear way of differentiating these fish without capture and inspection. After this, the videos were analysed again recording species’ presence or absence, the time they were seen in the video, how often and any notable behaviour.
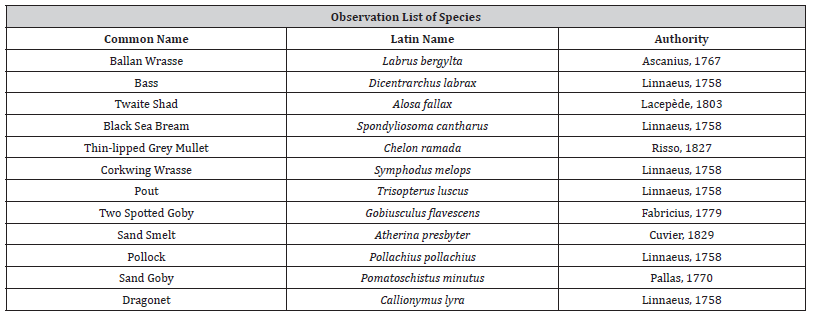
Results
A notable finding in this study has been the identification of the Twaite shad (Alosa fallax) (Figure 2) in a seagrass site. A member of the Clupeiformes (herrings) family, this species spawns in a few rivers of Wales and England and is classed as ‘Least Concern’ by the IUCN in the UK [30,31]. Overall, 14 videos were taken from this study, 7 in the seagrass and 7 in the mooring scar sites. From these videos, 13 species from 9 families were identified. The species identified are as follows in Table 1 which excludes the unidentified shoals of small fish. From the video analysis, the results show that there were differences in the species composition in the videos at seagrass sites and mooring scar sites, however, the diversity of species is equal in both sites (n = 11) presented in Figure 3. For analysis of the results, an ANOSIM was performed. The fish assemblage within seagrass and mooring scar sites was found to separate into notably different groups (P <0.001, R = 0.697). Therefore, there were more differences than similarities in fish assemblage between mooring scar and seagrass sites i.e. mooring scar and seagrass sites had notably different fish assemblages. A 2D MDS plot (Figure 4) using PRIMER v6 shows the seagrass and mooring scar sites to be separated to some extent, as there is overlap in the middle of the two groups. Seagrass has 8 sites that are more dissimilar to the other sites and mooring scar sites have 5 dissimilar sites. There is natural variance within both sites which is expected.

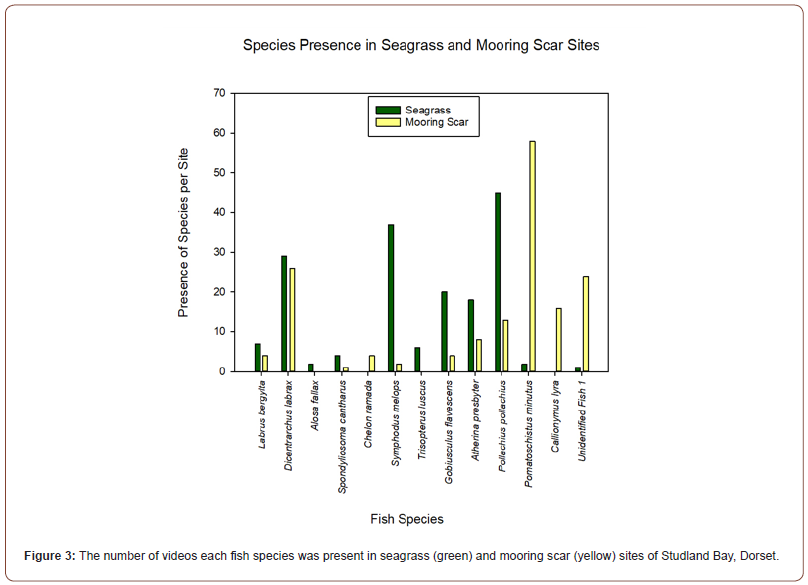
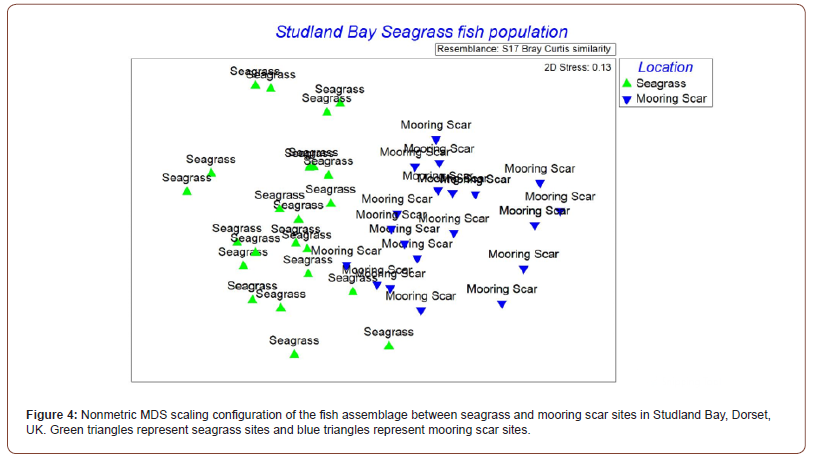
Table 1:List of the 13 identified species in the seagrass and mooring scar videos in Studland Bay, Dorset, UK.
Discussion
Analysis of the videos showed an equal number of species in seagrass sites and mooring scar sites, which appears to be inconsistent with past studies. Studies comparing fish assemblage in seagrass and sand habitats have provided evidence of a greater number of species in seagrass compared to sandy habitats [32-34]. Similarly, Collins et al. [19], found there was a three times greater number of infauna in seagrass compared to the mooring scars in Studland Bay. Bertelli & Unsworth [23] found motile macro-fauna in Z. marina in seagrass (n = 26) and sand sites (n=23) in North Wales which is greater than the present study of n =11 in both the seagrass and mooring scar sites. However, their methodology utilized a beach seine net which is known to capture a greater number of species than a video system [35]. Theories for their higher species number in seagrass included the seagrass’s physical structure that creates food and shelter [34,36].
The fish assemblage in both sites was contrasting and likely each species utilizes different ecological niches. The species that were found in only one of the habitats include Callionymus lyra (seagrass) and mooring scar sites Alosa fallax and Trisopterus luscus.
These species show specialist species characteristics as they require specific environmental and habitat conditions [37]. As a mooring chain destroys the seagrass habitat species that were originally inhabiting the seagrass decrease and an increase of species that display generalist traits like the Dicentrachus labrax, Labrus bergylta, Atherina presbyter are seen as they can tolerate a wider range of environmental conditions [37]. In Studland Bay Pomatoschistus minutus dominated the mooring scar sites, this is possibly due to P. minutus being a demersal species and the 1m high camera placement in seagrass sites is most likely the reason the P. minutus sightings were fewer. However past studies have shown them to be a more generalist species [38]. Changes in habitat and species composition can cause cascading effects, as the original predation or food source changes, which in turn may create a change in community structure [39]. Previous studies have found that seagrass hosts juvenile commercial species, acting as a nursery [23,29]. The presence of P. pollachius in this study indicates that Studland Bay seagrass provides a nursery ground for this commercial species [40].
It has been suggested that seagrass provides a lower-energy environment that is of importance for pollack fry [41]. The results and analysis suggest there was a notable difference in the fish community between seagrass and mooring scar sites within Studland Bay. One major difference is the habitat types, mooring scars are described as sandy bottoms and seagrass is characterised as a three-dimensional habitat [41]. For example, G. flavescens was most frequently found in seagrass compared to the mooring scars and this supports past literature that suggests this species prefers complex three-dimensional seascapes [42]. Furthermore, the change between the seagrass and sandy patches (mooring scars) produces a boundary with a change in environmental variables like water flow and sediment size that may be specific requirements for fish species [43,44]. The mooring scars in Studland Bay can be characterised as fragmentation; described by Fahrig [45], as the process where a habitat is disturbed, creating smaller areas of that habitat like a matrix. Furthermore, the results in Figure 3 present a lower presence of small juvenile fish including P. pollachius and S. cantharus in the mooring scars compared to the seagrass, which supports the findings of Jackson et al [16].
Their argument explaining these results is that the seagrass has a stable environment, and the fragments lack protection from predators. Fragmentation of the Studland Bay seagrass habitat is hopefully being averted by the declaration of a voluntary no-anchor zone [46] and an increasing installation of eco-moorings with elastic rodes overseen by the Studland Bay Marine Partnership [46].
References
- Christianen M. J. A., van Belzen J., Herman P. M. J., van Katwijk M. M., Lamers L. P. M., van Leent P. J. M., Bouma T. J. (2013) Low-Canopy Seagrass Beds Still Provide Important Coastal Protection Services. PLoS ONE, 8, e62413. DOI: https://doi.org/10.1371/journal.pone.0062413
- Costanza R, d’Arge R, de Groot R, Farber S, Grasso M, Hannon B, Limburg K, Naeem S, O’Neill RV, Paruelo J, Raskin RG, Sutton P, van den Belt M. (1997) The value of the world’s ecosystem services and natural capital. Nature, 387, pp. 253–260. DOI: https://doi.org/10.1038/387253a0
- Orth R. J., Carruthers T. J. B., Dennison W. C., Duarte C. M., Fourqurean J. W., Heck K. L., Hughes A. R., Kendrick G. A., Kenworthy W. J., Olyarnik S., Short F. T., Waycott M., Williams S. L. (2006) A global crisis for seagrass ecosystems. Bioscience, 56, pp. 987–996. DOI: https://doi.org/10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2
- Short F. T., Carruthers T., Dennison W., Waycott M. (2007) Global seagrass distribution and diversity: a bioregional model. Journal of Experimental Marine Biology and Ecology, 350, pp. 3-20. DOI: https://doi.org/10.1016/j.jembe.2007.06.012
- Yap, H. T. (2000). The case for restoration of tropical coastal ecosystems. Ocean Coast. Manag. 43, pp.841–851. DOI: 10.1016/S0964-5691(00)00061-2
- Wood E. J F., Odum W. E., Zieman J. C. (1969) Influence of seagrasses on the productivity of coastal lagoons. Coastal lagoons, a symposium, Universidad Nacional Autonoma de Mexico, Cuidad Universitaria, pp. 495-502.
- McLeod E., Chmura G. L., Bouillon S., Salm R., Bjork M., Duarte C. M., Lovelock C. E., Schlesinger W. H., Silliman B. R. (2011) A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 9, pp. 552–560. DOI:https://doi.org/10.1890/110004
- Duarte C. M., Marba N., Gacia E., Fourqurean J. W., Beggins J., Barron C., Apostolaki E. T. (2010) Seagrass community metabolism: assessing the carbon sink capacity of seagrass meadows. Global Biogeochem. Cycles 24, pp. 8. DOI: 10.1029/2010GB003793.
- Green E. P., and Short, F. T. (2003) World Atlas of Seagrasses. University of California Press, Berkeley, USA. pp. 324.
- Green A. E., Unsworth R. K. F., Chadwick M. A., Jones P. J. S. (2021) Historical Analysis Exposes Catastrophic Seagrass Loss for the United Kingdom. Font. Plant Sci. 12:629962. DOI: 10.3389/fpls.2021.629962
- Gamble C., Bertelli C., Debney A., Glover A., Hendy I., Lilley R., Nuuttila H., Potouroglou M., Ragazzola F., Unsworth R., Preston J. (2021) Seagrass Restoration Handbook. pp. 3-13.
- Duffy J.E. (2006) Biodiversity and the functioning of seagrass ecosystems. Mar. Ecol.-Progr. Ser., 311, pp.233-250. DOI:10.3354/meps311233
- Orth R. J, Heck K. L., van Montfrans J. (1984) Faunal communities in seagrass beds: a review of the influence of plant structure and prey characteristics o predator-prey relationships. Estuaries, 7, pp. 339-350. DOI: https://doi.org/10.2307/1351618
- Adams S. M. (1976) The ecology of eelgrass, Zostera marina (L.), fish communities. I. Structural analysis. Journal of Experimental Marine Biology and Ecology, 22(3), pp. 269-291. DOI: https://doi.org/10.1016/0022-0981(76)90007-1
- Beck M. W., Heck K. L. Jr., Able K. W., Childers D. L., Eggleston D. B., Gillanders B. M., Halpern B, Hays C. G, Hoshino K., Minello T. J. (2001) The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 51, pp. 633–641. https://doi.org/10.1641/0006-3568(2001)051[0633:TICAMO]2.0.CO;2
- Jackson E. L., Attrill M. J., Rowden A. A., Jones M. B. (2006) Seagrass complexity hierarchies: Influence on fish groups around the coast of Jersey (English Channel). Journal of Experimental Marine Biology and Ecology, 330(1), pp. 38-54. DOI: 10.1016/j.jembe.2005.12.016
- Pihl L., Baden S., Kautsky N., Ronnback P., Soderqvist T., Troell M., Wennhage H. (2006) Shift in fish assemblage structure due to loss of seagrass Zostera marina habitats in Sweden. Estuarine, Coastal and Shelf Science, 67, pp. 123-132. DOI10.1016/j.ecss.2005.10.016
- Marine Management Organisation. (2022) Managing marine non-licensable activity in Studland Bay Marine Conservation Zone. [Online] Available at <https://www.gov.uk/government/publications/managing-marine-non-licensable-activities-studland-bay-next-steps> [Accessed 18th April 2022].
- Collins K. J., Suonpää A. M., Mallinson J. J. (2010) The impacts of anchoring and mooring in seagrass, Studland Bay, Dorset, UK. Underwater Technology: The International Journal of the Society for Underwater, 29, pp. 117-123. DOI:10.3723/ut.29.117
- Greve T. M., Krause-Jensen D., Rasmussen M. B., Christensen P. B. (2005) Means of rapid eelgrass (Zostera marina L.) recolonisation in former dieback areas. Aquat. Bot., 82, pp. 143-156. DOI:10.1016/J.AQUABOT.2005.03.004
- Jarvis J. C., Moore K. A. (2010) The role of seedlings and seed bank viability in the recovery of Chesapeake Bay, USA, Zostera marina populations following a large-scale decline. Hydrobiologia, 649, pp. 55-68. DOI:10.1007/s10750-010-0258-z
- Plus M., Deslous-Paoli J-M., Dagault F. (2003) Seagrass (Zostera marina L.) bed recolonisation after anoxia-induced full mortality. Aquat. Bot., 77, pp. 121-134. DOI:10.1016/S0304-3770(03)00089-5
- Bertelli C. M., Unsworth R. K. F. (2014) Protecting the hand that feeds us: Seagrass (Zostera marina) serves as commercial juvenile fish habitat. Marine Pollution Bulletin, 82(2), pp. 425-429. DOI: 10.1016/j.marpolbul.2013.08.011
- Connolly R. M. (1994a) A comparison of fish assemblages from seagrass and unvegetated areas of southern Australian estuary. Marine and Freshwater Research, 45, pp. 1033-1044. DOI:10.1071/MF9941033
- Connolly R. M. (1994b) Removal of seagrass canopy: effects on small fish and their prey. Journal of Experimental Marine Biology and Ecology 184, pp. 99-110. https://doi.org/10.1016/0022-0981(94)90168-6
- Leif P., Susanne B., Nils K., Patrik R., Tore S., Max T., Håkan W. (2006) Shift in fish assemblage structure due to loss of seagrass Zostera marina habitats in Sweden. Estuarine, Coastal and Shelf Science, 67, pp. 123-132. DOI: 10.1016/j.ecss.2005.10.016.
- McCloskey R. M., Unsworth R. (2015) Decreasing seagrass density negatively influences associated fauna. PeerJ, 3(6), e1053. DOI: 10.7717/peerj.1053
- Mallet D., Pelletier D. (2014) Underwater video techniques for observing coastal marine biodiversity: A review of sixty years of publications (1952–2012). Fisheries Research, 154, pp. 44-62. DOIhttps://doi.org/10.1016/j.fishres.2014.01.019
- Sheaves M., Bradley M., Herrera C., Mattoe C., Lennard C., Sheaves J., Konovalov D. A. (2020) Optimizing video sampling for juvenile fish surveys: using deep learning and evaluation of assumptions to produce critical fisheries parameters. Fish and Fisheries, 21(6), pp. 1259-1276. DOI: 10.1111/faf.12501
- Aprahamian M. W., Baglinière J-L., Sabatiè M. R., Alexandrino P., Thiel R., Aprahamian C. D. (2003) Biology, Status, and Conservation of the Anadromous Atlantic Twaite Shad Alosa fallax fallax. American Fisheries Society Symposium, 35, pp. 103-124.
- Connolly R. M. (1997) Differences in comparison of small motile invertebrate assemblages from seagrass and unvegetated habitats in a southern Australian estuary. Hydrobiologia, 346, pp. 137-148. DOI:10.1023/A:1002970100662
- Currás A., Sánchez-Mata A., Mora J. (1993) Estudio omparative de la macrofauna benthonica de un fondo de Zostera marina y un fondo arenoso libre de cubierta vegetal. Biol. Mar, 35, pp. 99-112.
- Guidetti P. (2000) Differences Among Fish Assemblages Associated with Nearshore Posidonia oceanica Seagrass Beds, Rocky–algal Reefs and Unvegetated Sand Habitats in the Adriatic Sea. Estuarine, Coastal and Shelf Science, 50, pp. 515-529. DOI: 10.1006/ecss.1999.0584.
- Harmelin-Vivien M. L., and Francoeur P. (1992) Trawling or visual censuses? Methodological bias in the assessment of fish populations in seagrass beds. P.S.Z.N. I: Marine Ecology, 13, pp. 41–51.
- Bell J. D., Westoby M., Steffe A. F. (1987) Fish larvae settling in seagrass: do they discriminate between beds of different leaf density? Journal of Experimental Marine Biology and Ecology, 111, pp. 133-144. DOI: https://doi.org/10.1111/j.1442-9993.1991.tb01056.x
- Ramiadantsoa T., Hanski I., Ovaskainen O. (2018) Responses of generalist and specialist species to fragmented landscapes. Theoretical Population Biology, 124, pp. 31-40. DOI: 10.1016/j.tpb.2018.08.001
- Fonds M. (1973) Sand gobies in the Dutch Wadden Sea (Pomatoschistus, Go- biidae, Pisces). Netherlands Journal of Sea Research, 6, pp. 417-178. DOI: 10.1016/0077-7579(73)90001-X
- Attrill M. J., Strong J. A., Rowden A. A. (2000) Are macroinvertebrate communities influenced by seagrass structural complexity? Ecography 23, pp. 114–121. DOI: 10.1111/j.1600-0587.2000.tb00266.x
- Charrier G., Durand J., Quiniou L., Laroche J. (2006) An Investigation of the Population Genetic Structure of Pollack (Pollachius pollachius) Based on Microsatellite Markers. ICES J. Mar. Sci., 63, pp. 1705–1709. DOI : 10.1016/j.icesjms.2006.07.006
- Furness E., Unsworth R. K. F. (2020) Demersal Fish Assemblages in NE Atlantic Seagrass and Kelp. Diversity, 12(10), pp. 366-266. DOI: https://doi.org/10.3390/d12100366
- Perry D., Staveley T. A. B., Gullström M. (2018) Habitat Connectivity of Fish in Temperate Shallow-Water Seascapes. Front. Mar. Sci, 4, pp. 440. DOI: https://doi.org/10.3389/fmars.2017.00440
- Bologna P. A. X., Heck K. L. (2002) Impact of habitat edges on density and secondary production of seagrass- associated fauna. Estuaries 25, pp. 1033-1044. DOI:10.1007/BF02691350
- Fonseca M. S., Fisher J. S. (1986) A comparison of canopy friction and sediment movement between four species of seagrass with reference to their ecology and restoration. Mar Ecol Prog Ser, 29, pp. 15-22.
- Fahrig L. (2003) Effects of Habitat Fragmentation on Biodiversity. Annual Review of Ecology, Evolution, and Systematics, 34(1), pp. 487-515. DOI: https://doi.org/10.1146/annurev.ecolsys.34.011802.132419
- Marine Management Organisation. (2021) Studland Bay, Protecting our precious seagrass habitats together. Studland Leaflet No.9, pp. 4. [Online] Available at <https://assets. publishing.service.gov.uk/media/64f060a36bc96d000d4ed38a/Studland_Leaflet__9_.pdf> [Accessed January 2023].
- Studland Bay Marine Partnership. [Online] Available at <https://www.dorsetcoast.com /project/studland-bay-marine-partnership/> [Accessed September 2024].
-
Hayley Roberts, Ken Collins* and Antony Jensen. Comparison of Fish Assemblage in Mooring Scars and Adjacent Seagrass, Studland Bay, Dorset. Ad Oceanogr & Marine Biol. 4(1): 2024. AOMB.MS.ID.000580.
-
Fish assemblage; seagrass; community; ecosystem; mooring; niche; habitat; diet; fragmentation; iris publishers; iris publisher’s group
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






