 Review Article
Review Article
What is the Relationship between Chiari I Malformation and Obesity?
Harold L Rekate*
Department of Neurosurgery, Zucker Hofstra Northwell School of Medicine, USA
Harold L Rekate, Department of Neurosurgery, Zucker Hofstra Northwell School of Medicine, USA.
Received Date: October 04, 2018; Published Date: October 24, 2018
Abstract
The purpose of this review is to assess what is known about the importance of body weight as it relates to the diagnosis, pathophysiology and treatment of symptomatic patients with Chiari I malformation. It will attempt to answer several important questions.
a. Does obesity cause Chiari I malformation?
b. Does obesity cause syringomyelia in patients who have tonsillar ectopia and who are obese?
c. What is the effect of weight loss or gain occurring in the signs and symptoms associated with this condition?
d. Are obese patients at greater risk of failed surgery or complications of surgery for CM1 than are the non-obese.
e. What is the relationship between CM1 and idiopathic intracranial hypertension (IIH) that was previously called pseudotumor cerebri?
f. What is the proper assessment of CM1 patients who are obese?
g. What should be the physician’s approach to these patients be?
Introduction
As a pediatric neurosurgeon for forty years and more recently as director of the Chiari Institute on Long Island New York I have been treating patients of all ages who present with symptoms related to a mechanical distortion of the cerebellum in which the cerebellar tonsils have herniated below the foramen magnum. Generally, if this descent of the cerebellar tonsils is at least 5 mm the condition is called the Chiari I malformation (CM1). The nomenclature here is quite controversial and attempts to obtain a consensus on the definition of the term have been unsuccessful and frustrating. For most neuroradiologists the diagnosis depends on a measurement of 5 mm of descent. This number derives from the work of Barkovich who noted that the majority of patients with this degree of descent were symptomatic [1].
Prior to the routine availability of magnetic resonance imagine (MRI) CM1 was considered a very rare condition. From the late 1980’s it has become clear that the majority of patients who have significant degrees of tonsillar descent are asymptomatic and that tonsillar ectopia is not rare. It is estimated that as many as 0.5% of individuals would have scans that would be read as having CM1 [2]. What makes the difference between individuals with cerebellar dystopia with incapacitating symptoms and others with identical imaging who are asymptomatic? Does obesity play a role?
In order to understand the relationship between obesity and symptomatic CMI a Boolean search was conducted on Pubmed using the search strategy of Chiari and obesity. The search yielded 4 references [2-5]. As well studies dealing with the outcomes of treatment for CM1 were included if they considered obesity as defined using BMI and/or intracranial pressure in the assessment [6-13].
An Instructive Case
The patient is a 53-year-old African American woman who began having severe headaches on a nearly continuous basis for the last year. She had never needed to be treated for headaches in the past. Menopause for her has been very trying and she gained over 60 pounds in this period of time. Her symptoms include severe chronic daily headaches that can be “blinding” in nature made worse by coughing or sneezing. Her MRI reveals a 17 mm descent of the cerebellar tonsils with severe distortion of the tonsils with pointing. She also has a bulge below the obex characteristic of a Chiari 1.5 malformation. She has severe sleep apnea as well.
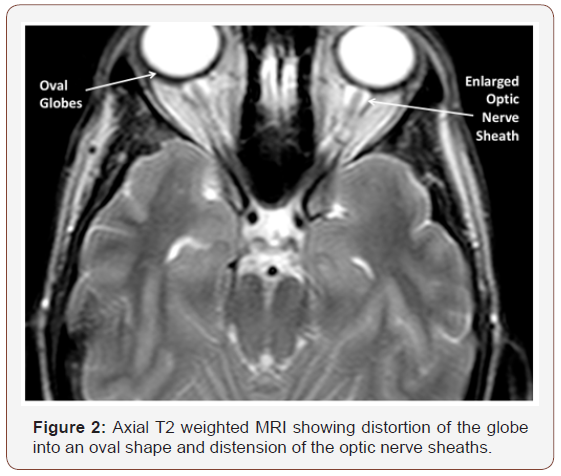
On exam she is obviously severely obese. Her BMI is 39. She had no papilledema seen on funduscopic examination and this has subsequently been confirmed by ophthalmology. Review of the MRI scans shows and empty sella turcica (Figure 1) and mild oval appearance of the globes with distension of the optic nerve sheath (Figure 2). Lumbar puncture was not recommended due to the radiographically severe CM1. These findings strongly suggest the presence of increased intracranial pressure.

She was started on acetazolamide for control of the ICP, referred for possible bariatric surgery and counseled on the need for weight loss. Counseling by nutrition and psychology is part of her workup for bariatric surgery.
Discussion
What is known and what is conjectured about the relationship of CM1 and obesity? There have been no prospective studies of this relationship. As of now no study has suggested that the severity of obesity as assessed using Body mass index (BMI) should play a role in the treatment algorithm for CM1 patients although several have emphasized the increased risk that obesity plays in surgical complications and poor outcomes [3, 4,5,14]. One has recommended attention be paid to the management of obesity as part of the overall management of patients being treated for CM1 [11].
Does obesity cause CM1?
There is only one article found that addresses this issue specifically. In a review of MRI imaging of the brain in 2400 individuals, 1310 had had a measurement of BMI within a year of the imaging. The average position of the cerebellar tonsils was above the foramen magnum in the entire study group. The average BMI was in the overweight category (BMI 26.4 kg/m2). A total of 46 patients 3.5% were found to have tonsillar descent of 5 mm or greater. This study found no correlation between BMI and degree of tonsillar descent. It therefore seems unlikely that the descent of the cerebellar tonsils significant enough to be read as CM1 is directly related to the BMI. The question then becomes does obesity in patients with descent of the cerebellar tonsils lead to symptoms?
Does obesity cause syringomyelia in patients who have tonsillar ectopia and who are obese?
One reference was found that spreads light on this question. Arnautovic and colleagues reviewed 60 adults who had been treated for CM1 [3] of this cohort 26 had associated syringomyelia. The average BMI was in the obese category (30.34±7.6 kg/m2). The degree of tonsillar descent did not correlate with the BMI. While there were no patients who developed syringomyelia related to BMI, patients had lengthening of the syrinx related to significant weight gain of 10 kg/m2. Also in one patient who did not improve following a second decompression surgery with no change in the syrinx, weight loss of 11.8 led to kg/m2 led to complete resolution of the syrinx [15,16]. In this study there is no evidence that obesity is the cause of syringomyelia, but the severity of the obesity does impacts on its severity both clinically and radiographically.
What is the effect of significant change in BMI on the signs and symptoms of CM1?
The name Chiari 1 malformation implies that the cause occurred as the skull and central nervous system were developing. It is clear that this is not always the case. Distortion of the cerebellar tonsils due to herniation through the foramen magnum often occurs later in life. It is a mechanical distortion of the tonsils due to mechanical forces. These can be related to the anatomy of the posterior fossa such as a short clivus or inadequate distention of the membranous bone of the occiput. A variant of this mechanism is felt to be the initiating factor in the truly malformative cause of the condition [17]. An acquired pressure differential across the foramen magnum can also lead to herniation of the cerebellar tonsils. This can be due to the use of lumbar drains or lumboperitoneal shunts [6] or due to leaking of cerebrospinal fluid called spontaneous intracranial hypotension [18]. Finally, direct distortion of the foramen magnum from conditions such as basilar invagination can lead to hindbrain herniation [15,16].
The instructive case discussed above is a rather typical scenario. Patients being seen for symptomatic CM1 nearly always ask why have I developed these symptoms now if I have a birth defect or if this has been there most of my life? There are probably several reasons that this occurs. It is not a subject that has been explored widely but hormonal changes in adolescents, neck injuries with hyperextension (whiplash injuries) and weight gain are among the likely culprits. Access to neuroimaging of patients prior to the development of symptoms is a rare event. It is however likely that the tonsilar ectopia has been there for long periods of time prior to the development of symptoms. Batzdorf in a study involving 177 patients including 87 with syringomyelia recognized the importance of obesity in the symptoms of patients with CM! and recommended that management of this obesity was an important part of the overall management of these patients [11].
Are obese patients at greater risk of failed surgery or complications of surgery for CM1 than the non-obese?
In all studies in which the BMI is reported, the rate of failed surgery is always significantly higher than that surgery is in the nonobese. In this discussion, “failed surgery” includes failure to meet the patient’s needs or improve signs and symptoms, complications of surgery, readmission and need for further surgery. In the study by Bhimani for instance, of 672 patients 36 patients were returned to surgery of which 26 were obese. The most common complication was CSF leak. Six patients underwent CSF shunting procedure and the remainder were returned to the operating room for open repair of the CSF leak. They postulated that the complications were due to high intracranial pressure. This had not been diagnosed before surgery [5].
Fagan and colleagues reported the results of CM1 surgery on 192 patients in which 36 (18.8%) showed no improvement in symptoms despite postoperative MRI with flow studies showing the presence of a new cisterna magna and unrestricted flow of CSF. In 15 of these patients a lumbar puncture was done showing elevated ICP and also showed transient improvement in their headaches. Subsequently 14 patients were treated with a shunt and one with acetazolamide and several lumbar punctures. In this study there was no mention of BMI. The authors have given the name Post Chiari pseudotumor cerebri syndrome [9]. It cannot be known whether or not these problems were present and unrecognized preoperatively.
What is the relationship between CM1 and IIH
The most common complication of CM1 surgery after failure to improve relates to CSF leaks including pseudomeningocele and actual external leaking of spinal fluid through the incision. In most of these cases the underlying cause likely relates to CSF dynamics including CSF absorptive issues and increased intracranial pressure.
As stated above, Batzdorf identified obesity failure to improve headaches in CM1 patients is more common in obese patients than in the non-obese patients undergoing that surgery [11]. The relationship between IIH and obesity is well established. There is growing evidence that IIH can be seen as a co-morbidity in patients with CM1. Banik and colleagues reviewed MRI on 68 patients who were diagnosed with IIH. The study showed that 12% (8) of the patients had tonsillar descent of 5 mm or greater implying that they met criteria for CM1. They also measured tonsillar descent that was less than 5mm but 2mm or greater and found a prevalence of 24% [8].
Bejjani and colleagues reported the late follow up of 6 patients with failed CM1 surgery. Four of the 6 failed related to pseudomeningocele [7]. It was concluded that this failure resulted from failure to recognize the existence of a CSF absorptive abnormality felt to be compatible with the diagnosis of pseudotumor cerebri. There was no mention of BMI in this study.
In a retrospective review of 8 patients who carried both diagnoses of CM1 and pseudotumor cerebri Alnemari and colleagues identified 5 patients whose symptoms failed to improve after decompressive surgery and finally required shunting. This study is important because it emphasizes the clinical and radiographic findings that could lead to a suspicion of IIH before surgery including flattening of the posterior part of the globe of the eye, CSF within the optic nerve sheath and flattening of the pituitary gland in the sella (“empty sella”). While the authors do not include the presence or absence of obesity in these patients they do discuss the relationship of obesity in IIH patients and all 5 of their patients were women [12].
The question of what the relationship between CM1 and IIH actually is remains a matter of conjecture. There are three possibilities.
a) The IIH resulted from the change in the dynamics of CSF and ICP as a result of the posterior fossa decompression.b) The patient had IIH prior to the decompression but the increased pressure caused a strain on the repair.
c) The patient had IIH prior to the decompression of an incidental otherwise asymptomatic or minimally symptomatic CM1 and the cause of the findings related to the IIH [10,12].
My personal theories on the answer to this question will be discussed below.
What is the proper assessment of CM1 patients who are obese?
As discussed above, three diagnoses are shown to interact including obesity, IIH and CM1. We also know that a high but unknown percentage of individuals with radiographic evidence of CM1 are asymptomatic. There should be a high level of suspicion that the patient will have high intracranial pressure if the patient presents with a BMI of 35 or greater.
The diagnosis of IIH in the context of CM1 can be challenging since there is a significant reluctance to measure ICP via lumbar puncture in patients with Chiari particularly if they are symptomatic. The first thing would be to do a careful funduscopic examination preferably by ophthalmology with mydriatics for good visualization. The MRI scan can be helpful with clues derived from evidence often not noticed by radiology. These include a radiographic “empty sella” which is a concave distortion of the pituitary at the bottom of a prominent sella turcica. The presence of CSF in the optic nerve sheath and an oval appearance of the globe are also suggestive of intracranial hypertension [10].
CM1 without syringomyelia rarely if ever presents as an emergency. It is likely that the weight is contributing to the headaches in these patients and that weight loss may improve or cure the severe headache disorder. Unless the obese patient is allergic to medications containing sulfur, acetazolamide can rapidly lower ICP and lead to amelioration of the headaches. This is probably only a stopgap measure and weight loss is essential for prolonged relief of symptoms. If there is evidence of papilledema or radiographic findings consistent with increased intracranial pressure IIH may be inferred and in the obese the definitive treatment may relate to weight loss [19,20].
Conjecture regarding the interaction of obesity with CM1
While there remains some skepticism, it seems now established that IIH is due to or associated with high pressure in the dural venous sinuses [19]. The most common condition associated with IIH is obesity and the condition is much more common in women than men. In this situation the cause relates to higher pressure in the right atrium attributed to the distribution of fat in the body. In the non-obese the underlying problem relates to restriction of flow out of the dural venous sinuses. This can be measured using interventional radiology techniques and if a pressure gradient is found treatment with the placement of venous stents can be curative [21,22]. Increased intracranial pressure can actually cause distortion of dural venous sinuses leading to reversible pressure gradients. In severely obese individuals there can be a secondary stenosis of the transverse sinus leading to marked increases in already elevated ICP in a positive feedback loop.
IIH in obese patients is not likely to be the primary cause of cerebellar tonsillar herniation of greater than 5 mm as a review of over 1300 mri images in which the BMI of the patient was known showed no relationship between the BMI and the position of the tonsils [2]. It is clear however that the brain volume and ICP are related and dependent on dural venous sinus pressure. At the time of craniotomy or endoventricular endoscopy it is clear that there is small increases in brain volume with each heartbeat. A Valsalva maneuver during open craniotomy will lead to a swelling of the brain, a maneuver that is used to test the adequacy of dural closure during Chiari decompressive surgery. Sustained increase in venous sinus pressure will lead to an increase in the volume of the brain and make it stiffer or more turgid. The work of Oldfield and colleagues demonstrating the pulsations of the tonsils caused by the heartbeat at the foramen magnum in Chiari patients with syrinx shows the effects of this volumetric change [23].
Strong support for this assessment comes from the report of Chung and colleagues in a case report of a patient with symptomatic CM1 and syrinx. Manometric venography revealed a significant pressure gradient in the transverse sinuses. Treatment using venous stenting lead to symptomatic relief, and improvement in both the length of the syrinx and the degree of tonsillar descent [24].
It is clear that decompression of CM1 in severely obese patients has a higher complication rate and higher likelihood of lack of improvement in symptoms. It is also technically more difficult with higher anesthetic risks partly due to difficulty in positioning and the depth of the distance from the skin of the neck to the dura. There is growing data that weight loss in patients with chronic daily headaches and who are obese are likely to respond to bariatric surgery with improvement in headaches. Presumably these patients have abnormal CSF dynamics [25-27].
The report from Alnemari asks the question “are we operating on the wrong population?” [12] I would ask a different question. I would ask are we doing the wrong operation or treatment? Surgery for CM1 is rarely if ever an emergency. In severely obese patients with a BMI >35 or definitely those over a BMI of 40 thought should be given to the potential effect of increased intracranial pressure and dural venous sinus pressure before considering surgical intervention. There have been no prospective studies on this but it has been my practice for at least 20 years to emphasize weight loss and treatment of ICP in such patients prior to a posterior fossa decompression. The patients are told of the severe risks and failure rates for decompression in the face of obesity, are counseled on weight loss and conditioning and told that if they still are symptomatic after significant weight loss it would be time to reassess for appropriate surgical intervention.
Physicians and obesity
Over many years I have cared for patients with severe problems with obesity that are being evaluated for the treatment of CM1. I cannot remember one of these patients who had been told by any previous consultant that there could be a relationship between the headache disorder or CM1 to the obesity. Some had been told that they were excellent candidates for surgery and several had already failed previous decompression. It was rare for any of them to have been counseled about weight management. In a recent edition of Highline a journalist reviewed what is known about the epidemiology of obesity and a brief foray into the science of obesity and weight loss. The article then reported on interviews with individuals with significant medical, social and psychologic difficulties with obesity. Physicians in general and the healthcare system do not come out well in this piece. Highline is published on line by Huffington post publishers and reviews what would be front-page stories [28].
The issues here are extremely complicated. Obese patients are often afraid to see physicians. They are often told that they must lose weight but are given no suggestions about how this could be accomplished.
More frequently however the subject of their weight is ignored. As a physician just mentioning weight issues to a severely obese patient is likely to create an emotionally charged environment. Usually that patient has heard this before and is deeply understanding that this is a problem but does not want to be harassed about it. Once opened this box is difficult to close. Patients tend to feel that they are being judged. Generally, physicians are loath to open the discussion as it will take a long time to a have successful positive conversation.
Even though the conversation is difficult, it is essential in such cases when the presence of obesity plays such a pivotal role in decision-making and outcome. It is essential that in such circumstances the patient is assured that this discussion relates to a medical problem (obesity) that is critical to address in order to make the correct treatment decisions. In the case of CM1 it is important to emphasize thet the decisions should not be made quickly. The first step is to explain that obesity is a central part of the problem that the patient faces. It is likely that the symptoms are related to higher than normal pressure in the head. A high percentage of patients are already aware that the headaches have something to do with pressure.
Weight loss is the most important treatment and may be the end of the struggle [23]. In obesity related IIH the first step would be the use of acetazolamide but to make it clear that this is not a permanent answer. In obesity related IIH acetazolamide can be effective in lowering ICP within a day. If they have not been on the medication before the patient should see some effect very quickly.
While a great many diets have been shown to have effect in such situations weight loss using diet and exercise may help in this situation. Unfortunately, weight loss by diet is rarely sustainable and the Highline article emphasizes that post diet weight gain often leaves a higher weight than the beginning weight. Bariatric surgery is the most effective treatment for IIH in the obese and has the highest likelihood of long-term control of weight [28].
Conclusion
While obesity is not the cause of the anatomic distortion of the cerebellar tonsils we know as CM1, there is growing evidence that obesity leads to symptomatic deterioration in these patients. Symptomatic improvement can be expected with significant weight loss. All overweight and obese patients should have a discussion of the importance of their weight in the pathogenesis and symptoms of CM1. For patients with substantial recent weight gain referral for dietary counseling and conditioning should be emphasized and should be prioritized prior to or instead of CM1 decompression. For severely overweight patients and in particular those with long histories of attempts at weight loss the definitive answer lies with bariatric surgery. This is particularly true when the BMI is greater than 40 kg/m2. It should be recognized by physicians and insurers that CM1 should be considered a co-morbidity of obesity and should lead to a priority given to obese patients with obesity related CM1.
Acknowledgemnet
None.
Conflict of Interest
No conflict of interest.
References
- Barkovich AJ, Wippold FJ, Sherman JL, Citrin CM (1986) Significance of cerebellar tonsillar position on MR. AJNR Am J Neuroradiol 7(5): 795- 799.
- Smith BW, Strahle J, Kazarian E, Muraszko KM, Garton HJ, et al. (2015) Impact of body mass index on cerebellar tonsil position in healthy subjects and patients with Chiari malformation. J Neurosurg 123(1): 226-231.
- Arnautovic KI, Muzevic D, Splavski B, Boop FA (2013) Association of increased body mass index with Chiari malformation Type I and syrinx formation in adults. J Neurosurg 119(4): 1058-1067.
- Lam S, Auffinger B, Tormenti M, Bonfield C, Greene S (2015) The relationship between obesity and symptomatic Chiari I malformation in the pediatric population. J Pediatr Neurosci 10(4): 321-325.
- Bhimani AD, Esfahani DR, Denyer S, Chiu RG1, Rosenberg D, et al. (2018) Adult Chiari I Malformations: An Analysis of Surgical Risk Factors and Complications Using an International Database. World Neurosurg 115: e490-e500.
- Sullivan LP, Stears JC, Ringel SP (1988) Resolution of syringomyelia and Chiari I malformation by ventriculoatrial shunting in a patient with pseudotumor cerebri and a lumboperitoneal shunt. Neurosurgery 22(4): 744-747.
- Bejjani GK, Cockerham KP, Rothfus WE, Maroon JC, Maddock M (2003) Treatment of failed Adult Chiari Malformation decompression with CSF drainage: observations in six patients. Acta Neurochir (Wien) 145(2): 107-16.
- Banik R, Lin D, Miller NR (2006) Prevalence of Chiari I malformation and cerebellar ectopia in patients with pseudotumor cerebri. J Neurol Sci 247(1): 71-75.
- Fagan LH, Ferguson S, Yassari R, Frim DM (2006) The Chiari pseudotumor cerebri syndrome: symptom recurrence after decompressive surgery for Chiari malformation type I. Pediatr Neurosurg 42(1): 14-19.
- Furtado SV, Visvanathan K, Reddy K, Hegde AS (2009) Pseudotumor cerebri: as a cause for early deterioration after Chiari I malformation surgery. Child’s nervous system: ChNS: official. Journal of the International Society for Pediatric Neurosurgery 25: 1007-1012.
- Batzdorf U, McArthur DL, Bentson JR (2013) Surgical treatment of Chiari malformation with and without syringomyelia: experience with 177 adult patients. J Neurosurg 118(2): 232-242.
- Alnemari A, Mansour TR, Gregory S, Miller WK, Buehler M, et al. (2017) Chiari I malformation with underlying pseudotumor cerebri: Poor symptom relief following posterior decompression surgery. Int J Surg Case Rep 38: 136-141.
- Pacca P, Altieri R, Zenga F, Garbossa D, Ducati A, (2017) Is Pseudotumor Cerebri An Unusual Expression of Chiari Syndrome? A Case Report and Review of the Literature. Surg Technol Int 30: 486-489.
- Bejjani GK (2003) Association of the Adult Chiari Malformation and Idiopathic Intracranial Hypertension: more than a coincidence. Med Hypotheses 60(6): 859-863.
- Milhorat TH, Chou MW, Trinidad EM, Kula RW, Mandell M, et al. (1999) Chiari I malformation redefined: clinical and radiographic findings for 364 symptomatic patients. Neurosurgery 44(5): 1005-1017.
- Kim LJ, Rekate HL, Klopfenstein JD, Sonntag VK (2004) Treatment of basilar invagination associated with Chiari I malformations in the pediatric population: cervical reduction and posterior occipitocervical fusion. J Neurosurg 101: 189-95.
- Milhorat TH, Nishikawa M, Kula RW, Dlugacz YD (2010) Mechanisms of cerebellar tonsil herniation in patients with Chiari malformations as guide to clinical management. Acta Neurochir (Wien) 152: 1117-1127.
- Schievink WI, Meyer FB, Atkinson JL, Mokri B (1996) Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. J Neurosurg 84(4): 598-605.
- Karahalios DG, Rekate HL, Khayata MH, Apostolides PJ (1996) Elevated intracranial venous pressure as a universal mechanism in pseudotumor cerebri of varying etiologies. Neurology 46(1): 198-202.
- Nadkarni T, Rekate HL, Wallace D (2004) Resolution of pseudotumor cerebri after bariatric surgery for related obesity. Case report. J Neurosurg 101(5): 878-880.
- Owler BK, Parker G, Halmagyi GM, Dunne VG, Grinnell V, et al. (2003) Pseudotumor cerebri syndrome: venous sinus obstruction and its treatment with stent placement. J Neurosurg 98(5): 1045-1055.
- Ahmed RM, Zmudzki F, Parker GD, Owler BK, Halmagyi GM (2014) Transverse sinus stenting for pseudotumor cerebri: a cost comparison with CSF shunting. AJNR Am J Neuroradiol 35(5): 952-958.
- Oldfield EH (2002) Cerebellar tonsils and syringomyelia. J Neurosurg 97(5): 1009-1010.
- Chung CY, John S, Luciano MG, Hui FK (2016) Reduction in Syrinx Size and Severity After Venous Sinus Stenting in a Patient with Pseudotumor Cerebri and Chiari Malformation: Technical Case Report. Oper Neurosurg (Hagerstown)12(2): E197-E201.
- Bond DS, Vithiananthan S, Nash JM, Thomas JG, Wing RR (2011) Improvement of migraine headaches in severely obese patients after bariatric surgery. Neurology 76(13): 1135-1138.
- Novack V, Fuchs L, Lantsberg L, Kama S, Lahoud U, et al. (2011) Changes in headache frequency in premenopausal obese women with migraine after bariatric surgery: a case series. Cephalalgia 31(13): 1336-1342.
- Razeghi Jahromi S, Abolhasani M, Ghorbani Z, et al. (2018) Bariatric Surgery Promising in Migraine Control: a Controlled Trial on Weight Loss and Its Effect on Migraine Headache. Obes Surg 28(1): 87-96.
- Hobbes MMF (2018) Everything you know about obesity is wrong. Highline.
-
Harold L Rekate. What is the Relationship between Chiari I Malformation and Obesity?. Arch Neurol & Neurosci. 1(4): 2018. ANN. MS.ID.000518.
-
Chiari I Malformation, Obesity, Body weight, Diagnosis, Pathophysiology, Syringomyelia, Neuroradiologists, Barkovich, Asymptomatic, Tonsillar descent, Tonsillar ectopia, Cerebellar dystopia, Asymptomatic, Coughing, Sneezing, Cerebellar tonsils, Sleep apnea, Ophthalmology, Pituitary gland, Sella turcic, Optic nerve sheaths, Bariatric surgery, Syringomyelia, Syrinx, Nervous system, Membranous bone
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






