 Research Article
Research Article
Variability Index of Optic Nerve Diameter with Hyperosmolar Therapy in Patients with Acute Neurological Injury
Lleny Bocanegra Flores1* , Felipe de Jesús Montelongo2 , María Magdalena Reyes Pérez1 and Blanca Estela Herrera Morales3
1Attending physician in the Neurointensive and Intensive Care Unit, Hospital General de Ecatepec Las Américas ISEM, Mexico
2Chief of the Neurointensive and Intensive Care Unit, Hospital General de Ecatepec Las Américas ISEM, Mexico
3Health Education and Research Coordinator at HGR 196 IMSS, Mexico
Lleny Bocanegra Flores, Attending physician in the Neurointensive and Intensive Care Unit, Hospital General de Ecatepec Las Americas ISEM, Mexico
Received Date: October 07, 2021; Published Date: October 28, 2021
Abstract
Introduction: Intracranial hypertension (IH) is a serious complication derived from increased pressure of brain content. It has been shown that dilation of the optic nerve sheath is a manifestation prior to the elevation of intracranial pressure (ICP) since it is a central nervous system continuum. Acute neurological injury (ANI) is a serious disease with a high risk of mortality and sequelae with non-specific clinical signs. ICP monitoring requires invasive instrumentation; however, now there is non-invasive monitoring by transorbital ocular ultrasonography, which measures the optic nerve sheath diameter (ONSD). Therefore, we propose a new variability index of the optic nerve sheath diameter (VIONSD) that allows determining the response to hyperosmolar therapy treatment as it is an alternative neuromonitoring method.
Objective: To determine the VIONSD for the treatment with hyperosmolar therapy in patients with ANI in the Neurointensive Care Service of the Hospital General de Ecatepec “Las Américas” in the State of Mexico, Mexico, by carrying out a prospective longitudinal observational analytical study.
Results: The trial included 29 patients with a median age of 42 years with a range of 30 to 58 years. A significant difference was obtained between the Glasgow score and the variability of the optic nerve diameter of < 0.05 in pre- and post-treatment. A ROC curve for the VIONSD was made by finding a cut-off point between the responder (6 %) and a cut-off point for the VIONSD with the score of the Glasgow coma scale (4.72 %).
Conclusions: The VIONSD measurement was determined as a tool for monitoring patients with ANI, which is a dynamic process in the changes of intracerebral pressure that has a change in the ONSD in a 30-minutes average time.
Keywords:Optic nerve sheath diameter; Intracranial hypertension; Neurological injury; Variability index of the optic nerve sheath diameter; Optic transcranial ultrasound
Introduction
According to the State Population Council (COESPO), the State of Mexico reports diseases with neurological affection as a health problem due to high incidence, where head trauma is the main cause that affects the population of productive working age between the ages of 35 to 44 years [1]. Therefore, the functional and economic impact of these patients is highly worrisome because those who survive present significant sequelae and often require assistance or medical care for many years. Elevated intracranial pressure (ICP) is a serious complication often leading to adverse outcomes [2,3]. This is both a medical and a surgical emergency, which represents an indication for its close monitoring and aggressive treatment [4- 7]. It is identified as a syndrome with multiple etiologies, whose diagnosis and treatment must be carried out urgently to save the patient’s life and prevent the development of significant disabilities.
The dilation or increase of the Optic Nerve Sheath Diameter (ONSD) by the pressure increase of the cerebrospinal fluid has been shown to be the manifestation prior to the ICP elevation because it is a continuum of the brain and the structures that wrap it. The ONSD measurement is quite easy to visualize and perform with ultrasonography through the insonation of the eye through the orbit, where intracranial hypertension (IH) can be evaluated and diagnosed in traumatic brain injury, as well as in intracranial hemorrhage, and extensive cerebral infarcts [2,4,5]. It is important to determine comprehensive neurological monitoring to help the clinician in the patient’s diagnosis, decision-making, and followup [6]. The use of ultrasound in daily clinical practice and at the bedside of the patient has been implemented as an integral part of the clinical approach in units receiving seriously ill patients [7-10].
Continuous neuromonitoring of these patients will make it possible to have a functional impact on the prognosis (considering the score of the Glasgow coma scale) and probably on mortality, as well as to provide treatment for IH with the use of hyperosmolar therapy [6,7]. This is a frequently used treatment measure in which various invasive and non-invasive devices are used to assess the behavior of the IH [8-13]. Thus, transorbital ocular ultrasound monitoring is a very acceptable proposal, but up to now the dynamic changes that the optic nerve sheath could have with hyperosmolar therapy have not been reported. The index of variability of the optic nerve sheath diameter (VIONSD) was proposed by Dr. Montelongo in 2015, after the analysis of 189 cases [14] with acute neurological injury, 92 % of them with severe cranioencephalic trauma. In this index, dynamic changes in the ONSD to hyperosmolar treatment with or without hyperventilation, and changes that were observed in a period of 15 to 60 minutes after administering said therapy are described. Likewise, since the ONSD is influenced by the subarachnoid space thickness, an index of variability (similar to the vena cava since it is a fluid) of the flow of cerebrospinal fluid is proposed. This represents a percentage, objective, and measurable change in the response to hyperosmolar treatment and can be a reference method for targeted treatments, in this case for acute brain injury, mainly with disruption and/or integrity of the blood-brain membrane. Therefore, the purpose of our study is to calculate the variability index to determine the responders and non-responders to hyperosmolar treatment, allowing us to make better clinical and surgical decisions.
Material and Methods
This is a prospective, longitudinal, analytical, and observational study. The target group consisted of hospitalized patients in the Neurointensive Care Unit of the Hospital General de Ecatepec “Las Américas”, Instituto de Salud del Estado de México, Mexico, from May 2018 to August 2019.
Inclusion criteria
• Patients aged 16 years or older with a diagnosis of acute brain injury
Exclusion criteria
• Patients under 16 years
• Patients who did not complete the ultrasonographic measurement of the ONSD
• Patients without criteria for acute brain injury
• Patients who did not require treatment with hyperosmolar therapy (20 % mannitol or 3 % saline solution)
Procedures and technique
• Firstly, the informed consent from the relatives due to the patient’s critical condition was obtained. Siemens Acuson Freestyle and Sonostar ultrasounds were used. The measurements to the optic nerve sheath were performed as follows: a linear transducer (7.5 – 13 MHz) was placed on each patient’s eyelid, after placing a sterile jelly. This was performed without direct pressure on the eyeball to avoid retinal detachment or increased intraocular pressure. The optic nerve was collected in B-mode and then the retina-optic nerve junction was measured by counting 3.0 mm vertically. Subsequently, a perpendicular line was drawn, and the sheath diameter was measured (from outer edge to outer edge of the dura mater). This was performed three times in a cross and longitudinal section to reduce the margin of error in the measurement to obtain an average of the right eye sheath (ARONSD) and the left eye (ALONSD). In addition, a global average of both sheaths (GAONSD) was performed. Following Raffiz et al. [15], intravenous hyperosmolar therapy with mannitol at 1 g/kg/ dose or 3 % saline solution at 2 ml/kg/bolus was administered to patients that presented a diameter greater than 5.20 mm. After 30 minutes, the same measurement was performed and the percentage of variability of the optic nerve sheaths (VIONSD) was determined, thus concluding the procedure for each administered treatment (Figure 1 & 2).
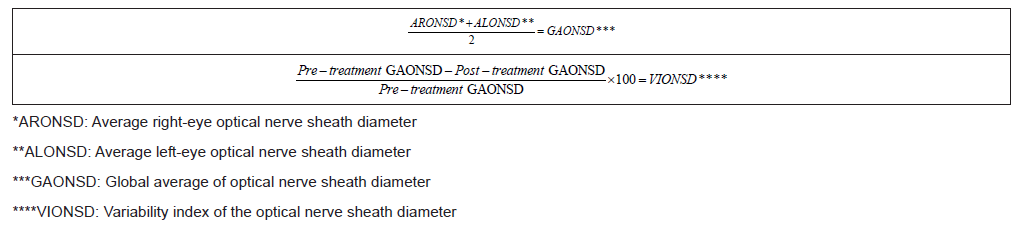
The percentage variation was determined by a variability index with the following formulas: (Table 1)
• The optic nerve sheath measurements were always performed by the same operator and were then validated by another physician expert in ultrasonography with a p > 0.05, interobserver.
Table 1:The percentage variation was determined by a variability index.
• Descriptive and analytical statistical analysis was performed with the IBM SPSS Statistics v.23 software. Differences in the pre- and post-treatment variables of the optic nerve sheath and Glasgow coma scale were obtained with the Wilcoxon test for variables related to free distribution, while Student’s t distribution was used for normal distribution variables.
• The χ2 test was performed to determine the association between the responder and non-responder groups.
• To perform VIONSD cut-off points to determine a score on the Glasgow coma scale, a ROC curve and a 2x2 square are used, where sensitivity, specificity, positive and negative predictive value, and likelihood ratio are obtained.

Results
Of the 29 patients studied, 69 % were male and the median age was 42 years. The most common initial diagnosis was severe cranioencephalic trauma. 41.3 % of the patients received 3 % saline treatment, while 58.7 % received mannitol treatment. All patients underwent pre- and post-treatment optic nerve sheath measurements, which are described in Table 2.
Table 2:General characteristics of neurological patients.
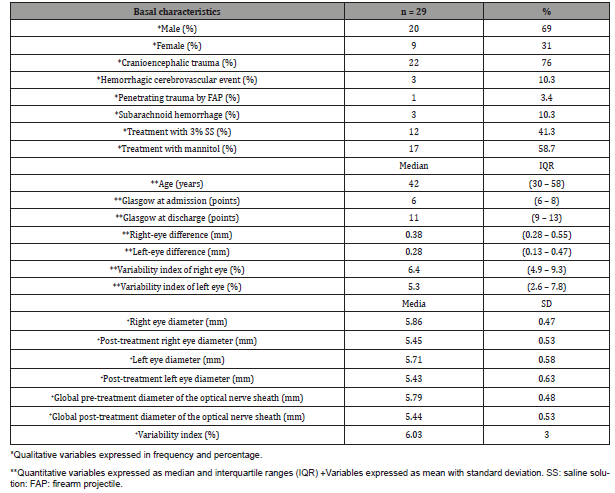
Table 3:Differences in the variables of the optic nerve sheath and the pre- and post-treatment Glasgow coma scale.
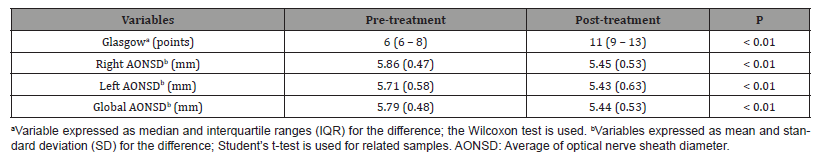
Differences in the averages of the right, left and global optic nerve sheath are shown in Table 3, as well as the pre-treatment and post-treatment Glasgow score (Table 3).
Cut-off points for patients responding to hyperosmolar treatment are identified by a ROC curve; then considering the percentage change of the dynamic change in ONSD, a VIONSD of 6.03 % was obtained. Later, an association using x2 was made to verify those responding patients with a VIONSD > 6 %, and it was considered significant with a p < 0.05. Additionally, a VIONSD cutoff point was identified with the score of the Glasgow coma scale, and a score of 4.72 % was determined with a 76 % sensitivity and a specificity of 50 %. With an area under the curve of 0.58 and a 95 % confidence interval (CI) (0.362, 0.814), a positive predictive value (PPV) of 55 % and a negative predictive value (NPV) of 65 % are obtained. This was obtained considering a 2.1 positive likelihood ratio and a 1.5 negative likelihood ratio (Figure 3).
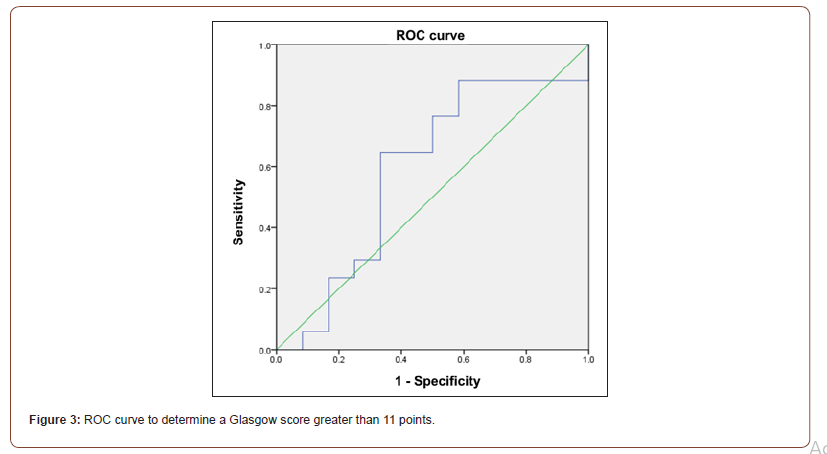
There were 13 patients with a VIONSD > 4.72 % and a Glasgow score greater than 11 points at discharge, which corresponds to 76.5 % of all those who do not have a major neurological deficit (Table 4).
Table 4:Glasgow and VIONSD ratio.
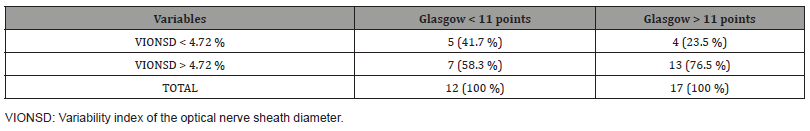
However, it is not significant to associate this cut-off point with Glasgow at discharge since this study has a small number of participants, so it is necessary to expand the target group to determine if there is significance. In addition, it can be adjusted with some other variables that may influence the prognosis.
Discussion
In our study, 69 % of the patients were male, with an average age of 40 years (a productive age), and severe head trauma was the main cause of admission, which was probably related to a poor culture of road and labor safety. The Glasgow average score at hospital admission was 6 points (min. 6 points) and at discharge from the Neurointensive Unit was 11 points (max. 13 points), which represents a likely satisfactory neurological recovery. The use of the hyperosmolar drug with 3 % saline solution and 20 % mannitol was almost homogeneous and was mainly determined by its availability in the Neurointensive Care Unit of 41 % and 58 %, respectively. This study demonstrated that the use of hyperosmolar therapy ostensibly decreased the average of the right, left, and global ONSD per administered hyperosmolar treatment by .34 mm. In addition, we found that the right optic nerve modified its diameter more intensely than the left optic nerve with a median of .38 mm. This was attributed to the least-damaged side of the cerebral hemisphere which kept the blood-brain barrier mostly complete, and the movement of cerebrospinal fluid had a better absorption.
Given the demonstrated dynamic changes in the ONSD, we propose a variability index (VIONSD) equal to or greater than 6 % as a reference point for responders and non-responders to hyperosmolar treatment for all patients of this small-scale study. This is an innovative proposal for the management of neurocritical patients. Likewise, in our study, when comparing the Glasgow at admission and discharge of the responders and considering those who recovered clinically better (a Glasgow of 11 points or greater), a VIONSD > 4.72 % was obtained in 13 patients. This means that a patient with a VIONSD < 4.72 % has a 55 % probability of having a neurological deficit, while those with a VIONSD > 4.72 % have a 65 % probability of not presenting a major neurological deficit, which translates as a Glasgow score at discharge greater than 11 points.
This could have a significant impact on the magnitude of neurological sequelae and a faster recovery.
In addition, in a different sub-analysis, the patients with head trauma responded adequately or better, considering the cause of hospital admission.
Conclusions
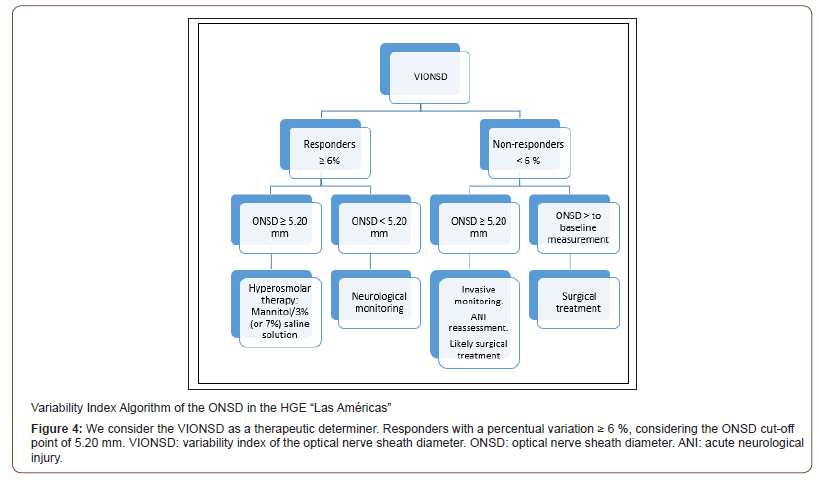
In this study, we determined the variability index of the optic nerve sheath diameter as a reference point that allows the clinician to make decisions quickly, at the bedside, through a simple method of neuromonitoring that can impact treatment, the prognosis, and, probably, the sequelae. The early diagnosis of intracranial hypertension with a non-invasive method favors the neuromonitoring of neurocritical patients. A larger sample of patients is required to be conclusive. Finally, Raffiz M et al in their study determined that an ONSD of 5.205 mm allows the detection of traumatic and non-traumatic neurosurgical patients with an early increase in IH [12]. Therefore, based on our results and taking this ONSD cut-off point, we suggest the following management algorithm (Figure 4):
Participation Consent and Ethics Committee
The study was carried out with prior authorization from the Hospital Ethics and Research Committee, registration number ESMEEC-01-081, as well as with the written consent from the patients and their families.
Publication Consent
All authors authorize this paper’s publication.
Conflict of Interests
We declare that we have no conflict of interest.
Funding
The study was funded by the authors and the hospital.
Authors’ Contributions
LIBF, FJM, and MMRP contributed to the concept, design, procedures, and patient selection. BEHM and FJM contributed to the study’s methodological design and statistical process.
Acknowledgments
We thank the hospital and our patients without whom it would have been impossible to carry out this study.
Material and Data Availability
The database and additional material are available, previous request to the authors.
References
- Consejo Estatal de Población [base de datos en Internet]. Toluca Estado de México: Gobierno del Estado de México. 2015- [acceso 15 de febrero de 2020]. COESPO con base INEGI.
- Carrillo E Rojo O, Cruz J Romero J (2016) Diámetro de la vaina del nervio óptico. Una herramienta para el monitoreo dinámico de la hipertensión intracraneana. Rev Asoc Mex Med Crit Ter Int 30(4): 249-252.
- Robba C, Santori G, Czosnyka M, Corradi F, Bragazzi N, et al. (2018) Optic nerve sheath diameter measured sonographically as non-invasive estimator of intracranial pressure: a systematic review and meta-analysis. Intensive Care Med 44(8): 1284-1294.
- Lochner P, Czosnyka M, Naldi A, Lyros E, Pelosi P, et al. (2019) Optic nerve sheath diameter: present and future perspectives for neurologists and critical care physicians. Neurol Sci 40(12): 2447-2457.
- Shirodkar CG, Rao SM, Mutkule DP, Harde YR, Venkategowda PM, et al. (2014) Optic nerve sheath diameter as a marker for evaluation and prognostication of intracranial pressure in Indian patients: An observational study. Indian J Crit Care Med 18(11): 728-734.
- Launey Y, Nessier N, Le Maguet P, Mlledan Y, Seguin P (2014) Effect of Osmotherapy on Optic Nerve Sheath Diameter in Patients with Increased Intracranial Pressure. J Neurotrauma 31(10): 984-988.
- Bhardwaj A, I Harukuni, S J Murphy, N J Alkayed, B J Crain, et al. (2000) Hypertonic saline worsens infarct volume after transient focal ischemia in rats. Stroke 31: 1694-1701.
- Hansen HC, Helmke K (1997) Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: Ultrasound findings during intrathecal infusion tests. J Neurosurg 87: 34-40.
- Bratton SL, Chestnut RM, Ghajar J, McConnell-Hammond FF, Harris OA, et al. (2007) Guidelines for the management of severe traumatic brain injury. VII. intracranial pressure monitoring technology. J Neurotrauma 24: 45-54.
- Geeraerts T, Merceron S, Benhamou D, Vigué B, Duranteau J (2008) Noninvasive assessment of intracranial pressure using ocular sonography in neurocritical care patients. Intensive Care Med 34: 2062-2067.
- Carrillo R, Flores OI, Peña CA, Carrillo LD, Carrillo JR, et al. (2014) Evaluación ultrasonográfica del diámetro de la vaina del nervio óptico (DVNO) para la medición de la presión intracraneana (PIC): a propósito de un caso. Gac Med Mex 150: 165-170.
- Shirodkar CG, Rao SM, Mutkule DP, Harde YR, Venkategowda PM, et al. (2014) Optic nerve sheath diameter as a marker for evaluation and prognostication of intracranial pressure in Indian patients: An observational study. Indian J Crit Care Med 18(11): 728-734.
- Stocchetti N, Mass A (2014) Traumatic intracranial hypertension. N Engl J Med 370: 2121-2130.
- Sistema de Información en Salud (SIES) Sistema automático de egresos hospitalarios y estadística del Hospital General de Ecatepec “Las Américas” 2012-2016.
- Raffiz M, Abdullah JM (2017) Optic nerve sheath diameter measurement: a means of detecting raised ICP in adult traumatic and non-traumatic neurosurgical patients. Am J Emerg Med 35(1): 150-153.
-
Lleny Bocanegra Flores, Felipe de Jesús Montelongo, María Magdalena Reyes Pérez, Blanca Estela Herrera Morales. Variability Index of Optic Nerve Diameter with Hyperosmolar Therapy in Patients with Acute Neurological Injury. Arch Neurol & Neurosci. 11(4): 2021. ANN. MS.ID.000766.
-
Dermoid Cyst, Posterior Fossa, Neurosurgery, Bacterial Meningitis, Abscess Formation, Tumors.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






