 Research Article
Research Article
The Value of a Second Operation in Patients with Glioblastoma Multiforme: is it Medically Justified and Ethically Appropriate?
Javad Hekmatpanah1, David Slotwiner2, Mehrnoush Gorjian3 and Robert Wollman4
1Department of Neurosurgery, University of Chicago, Medicine, USA
2Weill Cornell or Columbia Faculty, New York- Presbyterian Queens, USA
3Department of Neurology, University of New Mexico School of Medicine, USA
4Department of Pathology, University of Chicago, USA
Javad Hekmatpanah M.D., Department of Neurosurgery, University of Chicago, Medicine, USA.
Received Date: November 08, 2021; Published Date: November 30, 2021
Abstract
Objective: Glioblastoma multiforme (GBM) is the most common primary malignant brain tumor, having a high incidence of local recurrence.
The question remains if a second operation offers the patient an enough and meaningful longer survival to be medically justified and ethically
appropriate. In this paper we attempt to come up with a reasonable answer through the results in our patients and in similar ones in the literature
Materials and Methods: The records of 49 patients with gross total resection of GBM were reviewed for two groups, comparing 20 patients
with symptomatic recurrence, with that of 29 patients having a second operation. Effects of age, inter-operative duration, and adjuvant therapies on
survival were studied, using univariate and multivariate analysis. The results were compared with similar studies in the literature.
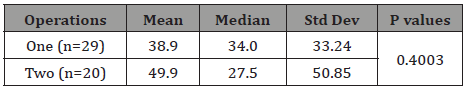
Results: The results indicate median survival of 34 months (mean 38.9) for one operation, and 27.5 months (mean 49.9) for two operations
(p=0.4). Adjuvant therapy, including radiation and chemotherapy with BCNU and Podophyllotoxins, had no significant survival benefit in either
group. Patients younger than 60 years had significantly longer survival (median 32 months) compared with older patients (median 19 months,
p=0.03). Interoperative interval of more than 2 years had significantly longer survivals. (p=0.02).
Conclusions: This study as with those similar in the literature indicates that patients with histological diagnoses of “glioblastoma” significantly
have varied duration of survival; likely caused by difference in the genetic makeup of the tumors, their location, and comorbidity. That creates
uncertainty in recommending a second operation. Yet, a second operation can extend survival in months for younger patients, especially for those
younger than 40 years. Thus, it should be reasonable both ethically and clinically, if selected based on high ethical standard, patient’s informed
consent, and realistic clinical expectation that the extending survival would somehow improve the patient’s medical condition.
Introduction
Glioblastoma multiforme (GBM) is one of the most malignant and rapidly progressing primary brain tumors in adults [1,2]. The standard treatment has traditionally been a single operation, followed by radiation and in some cases chemotherapy. At times a second operation is offered, hoping to extend the survival. Questions often arise if a second operation does offer the patient a meaningful longer survival that is both medically justified and ethically appropriate. This study is about the results in our patients together with results reported in the literature.
Materials and Methods
Eighty-three patients with a histological diagnosis of glioblastoma were operated between 1964 and 1995. In 49 patients there was sufficient data for analysis: 29 patients had one operation, and 20 patients had two operations. The purpose of each operation was to remove tumors as extensively as possible without causing neurological abnormalities. A second operation was done because of recurrence of symptoms and radiographic evidence of regrowth of the tumor.
The histological diagnosis of GBM was made by a
neuropathologist (author RW). Except for one patient having the
first operation elsewhere, all patients were operated by the first
author. Other co-authors independently reviewed, accumulated,
and wrote about the data and two independently reviewed
statistical data. All patients were followed until their deaths; the
last patient died in 2007. The records were reviewed to assess
age, duration before recurrence of symptoms, and results after the
second operation.
The Kaplan-Meier method was used to analyze survival for
each group; a log-rank and two-sided test were used to compare
the survivals; p values less than 0.05 were considered to be
statistically significant. The chi-squared test was used to assess
the homogeneity of the groups, and the Cox proportional hazards
model to test the association of age, inter-operative period, and
adjuvant therapy on survival. All statistical analysis was conducted
in SAS 9.4 (SAS Institute, Cary, NC, USA). The adjuvant therapy
consisted of radiation therapy and chemotherapy with BCNU and
Podophyllotoxins.
Results
Results from the Kaplan-Meier method are presented in Figure 1. Decimal-rounded results indicate a median survival of 34 months (mean of 39) for one operation, and 28 months (mean of 50) for two operations; the difference is not statistically significant (p=0.4003), Table 1. Survival for one operation was from 2 to 155 months, and for two operations from 10 to 191 months. The interval between the first and second operation had no effect on survival, nor did adjuvant therapy. The second operation was done on an average of 1.6 operations per patient younger than the age of 40, and 1.25 for those older than 40 (Table 1).
Table 1: Survival Outcome in Months.
In general, the mean age for one operation was 52.1 years and for two operations was 34.7, Table 2. Patients younger than the age of 40 had longer mean and median survivals than those older: 53.33 and 34.0 versus 35.89 and 31.5, but the difference was not significant (p=0.18). A similar trend was observed with the cutoff age of 50. However, the mean and the median survival for patients younger than 60 was 47.18 months and 32.0, versus those older: 26.44 and 19. This difference is statistically significant (p=0.03), Table 2,3.
Table 2: Age Distribution in Years.

Table 3: Survival Outcome in Months Based on Age for All Patients.
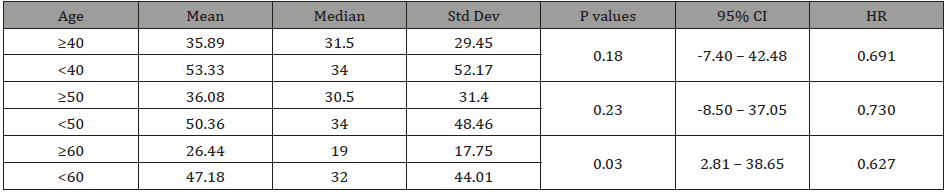
Table 4: Survival Outcome in Months Based on Inter-operative Span and Adjuvant Therapies for Two Operations.
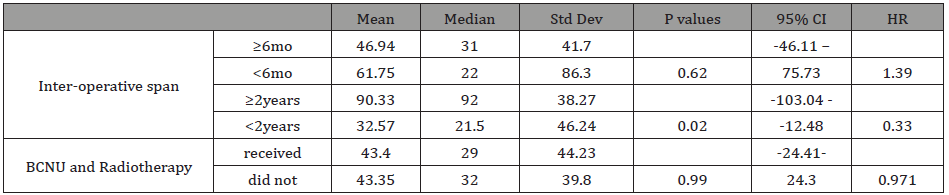
The mean and the median interoperative survival were 46.94
and 31 months for an interoperative span of 6 months and longer,
and 61.75 and 22.5 months for shorter spans; again, this was
statistically not significant (P=0.62). However, they were 90.33 and
92 months for 2 years and longer, and 32.57 and 21.5 months for
shorter spans, constituting a significant difference (P value=0.02),
Table 4.
Additional treatment with BCNU and radiotherapy for a second
operation had no significant effect on survival: there was a mean
and median of 43.40 and 29.0 months as compared with 43.35 and
32 for those who did not receive such treatment (p=0.99), Table 4.
Discussion
This study lacks the statistical significance to support that
a second operation prolongs the duration of survival for GBM
as compared with that of one operation. However, it indicates
an increase in the mean survival by 11 months with decrease of
median survival by 6.5 months (Table 1). In addition, close clinical
observation revealed that a second operation often resulted in a period of relief of neurological symptoms by reducing the tumor
mass and intracranial pressure; the patients were generally content
with the results. We did not perform the Karnofsky test which
would have provided more context to these observations.
The discrepancy between mean and median survival results
from a vast difference in individual length of survival, ranging from
2 to 155 months for one operation and from 10 to 191 months for
two operations. The patients’ ages were also spread across 24 to 76
years for one operation and from 8 to 63 years for two operations.
While the number of patients here is not enough to give a clear
guide, the literature does not contain a clear answer either. Despite
thousands of reoperations, there still is no agreed and concrete
recommendation to give patients.
The uncertainty about second operations stems from the genetic
nature, diagnosis, and management of GBM than from a paucity
of patients: numerous studies [1-15] report favorable outcomes,
others [16-18] report the opposite. This can be explained by the
fact that despite similar histological appearance, GBMs have varied
molecular and genetic attributes and aggressiveness. Other factors
such as location, size, and age contribute. In addition, the presence
of comorbidity, disability, and doctor-patient views have influence
on the length of survival. That is why recommending a second
operation requires a high level of ethical consideration. For there
are patients who welcome any duration of longer survival, while
others forego even longer durations, both views demand respect.
While effective treatment for GBM has been relatively slower
as compared with some other malignant tumor [19], ultimately
better treatment or cure has to come through science. An
important consideration for a second operation is that, at least
for younger patients, the potential longer survival can diminish or
delay disability, and give the time needed for the newer chemo or
radiation therapy to work. Johnson et al. [20], found as our study
shows, that patients surviving longer that 2 years from diagnosis
have a relatively favorable conditional probability of survival into
the future compared to newly diagnosed patients. In addition, the
presence of glioblastoma subtypes [21] is believed to “facilitate the
discovery of therapeutic and diagnostic target candidates.”
While slow, there has been progress. In 1884 Rickman Godlee
operated on a patient with brain tumor, a glioma [22], but the
patient died 28 days later from meningitis [23]. In 1977, the FDA
approved the use of Carmustine (BCNU) for the brain that was used
for decades together with radiotherapy and operation for treatment
of GBM. Others reported a 2.5 fold increase in median survival using
BCNU plus radiation [24]. In 1979 [25], we randomly assigned 21
patients with grade III or IV astrocytoma receiving either BCNU
alone or BCNU with VM-26, a semisynthetic podophillotoxin,
following surgery and radiation therapy. The result indicated that
a single–agent chemotherapy had a median survival of 14 months
while combined chemotherapy had a median survival of 22 months.
However, the difference was not statistically significant, (P> 0.5).
In 1980, Salcman [12] studied data extracted from 17 reports
in the literature comprising 2,532 patients and found the median
survival for operations alone, operations plus radiation, and
operations plus radiation and chemotherapy was 4, 9.25, and 10
months respectively. In 2011, based on recursive partitioning
analysis (RPA) classification, Li et al updated GBM database in
three distinct classes and indicated median survival times of 17.1,
11.2, and 7.5 months for Classes III, IV, and V+VI, respectively [26].
In 2005 temozolomide chemotherapy became a common adjunct
to radiation therapy and operation, after which a large study in
2012 indicated a rise in median survival from 12.0 months to 14.2
months [27].
As for second operation, in 2016 through analysis of 28 studies
of 2,279 patients with a second operation, Montemurro et al. [28]
reported median overall survival from diagnosis and from a second
operation at 18.5 months and 9.7 months, respectively. Despite a
vast difference in numbers our findings essentially mirror theirs.
While operations in our experiment were done several decades
ago, the operative technique has not changed. Except for one, all
patients were operated by one surgeon in one institution; and all
patients were followed until their death. In their follow-ups, the
patients with second operations were essentially happy with their
decision. Now with the advent of more effective chemo-radiation
modalities patients may do better.
Thus, despite the uncertainty and lack of strong statistical
proof, this study and others 4 -16 show that a second operation for
glioblastoma, in patients below the age of 60 and especially 40 can
prolong survival. In addition, because we did the second operations
when the patients’ symptoms recurred, they would have likely not
lived as long as they did, if they did not have the second operation.
We therefor conclude that a second operation on glioblastoma
is reasonable, if it is done based on high ethical consideration;
combined with a realistic clinical expectation that it would extend a
survival that would improve the patient’s medical condition; and is
based on the patient’s informed consent and desire.
Conclusion
A review of the literature indicates mixed results about the value
of a second operation for glioblastoma. Numerous investigators
report that it prolongs survival, others indicate the opposite. Our
data indicate that when symptoms of GBM recurred, a second
operation extended the mean survival by 11 months; a few months
more in those younger that 60, and 17 months in patients younger
than aged 40 years. But the data lacks statistical significance, likely
caused by vast difference in the length of survival; and by varied
molecular genetics of the tumors, size, location, and patients’ ages.
This creates uncertainty to recommend a second operation.
Yet, there is empirical evidence that a second operation does
prolong survival, especially in younger patients, that can be both
desirable and give more time for other treatments to work. Thus,
despite the uncertainty, it should be reasonable both ethically
and clinically for an individual patient, if selected based on high
ethical standard, patient’s informed consent, and realistic clinical
expectation that the extended survival would somehow improve
the patient’s medical condition.
Contributors
• J.Hekmatpanah, MD, did all the operations at except for
one patient who had the first operation elsewhere.
• D. Slotwiner, MD, did the first chart reviews and retrieved
the data while a senior medical student at the University of
Chicago.
• M. Gorjian, MD, a research assistant at the University
of Chicago, neurosurgery in 2017 reviewed all the records
tabulated, and made the tables.
• All the above three authors were involved In writing and
reviewing the paper
• R. Wollmann, MD. professor of neuropathology made the
histological diagnosis of the tissues
• All authors have read the final manuscript and agree
• Kristen Wroblewski, MS, a senior. Biostatistician. At the
Biostatistics laboratory, the university of Chicago reviewed the
data and advised on statistics.
Competing Interest
There is none.
Patient consent for publication: not required. This was a
retrospective record study, all patients had died, there is nothing in
the manuscript to identify patients.
Ethical Approval
As this was a retrospective data review without identifying photo, name or record number. The senior author is a faculty member in medical ethics.
Funding
No specific funding for this paper.
Acknowledgement
JH, acknowledges the Philanthropic nonrestricted contribution
to his academic activities by:
Mr. Frank Linden, Mr. and Mrs. Samuel Krauss Jr., and Dr. and
Mrs. Donald W. King; none of whom have any financial connection
to this.
References
- Chaichana KL, Zadnik P, Weingart JD, Alessandro Olivi, Gary L Gallia, et al. (2013) Multiple resections for patients with glioblastoma: prolonging survival. J Neurosurg 118(4): 812-820.
- Harsh GR, Levin VA, Gutin PH, M Seager, P Silver, et al. (1987) Reoperation for recurrent glioblastoma and anaplastic astrocytoma. Neurosurgery 21(5): 615-621.
- Azizi A, Black P, Miyamoto C, S E Croul (2001) Treatment of malignant astrocytomas with repetitive resections: a longitudinal study. Isr Med Assoc J 3(4): 254-257.
- Barker FG, Chang SM, Gutin PH, M K Malec, M W McDermott, et al. (1998) Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery 42(4): 709-20.
- Bloch O, Han SJ, Cha S, Matthew Z Sun, Manish K Aghi, et al. (2012) Impact of extent of resection for recurrent glioblastoma on overall survival: clinical article. J Neurosurg 117(6): 1032-1038.
- Chang SM, Parney IF, McDermott M, Fred G Barker, Meic H Schmidt, et al. (2003) Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the Glioma Outcome Project. J Neurosurg 98(6): 1175-1181.
- Dirks P, Bernstein M, Muller PJ, W S Tucker (1993) The value of reoperation for recurrent glioblastoma. Can J Surg 36(3): 271-275.
- Helseth R, Helseth E, Johannesen TB, C W Langberg, K Lote, et al. (2010) Overall survival, prognostic factors, and repeated surgery in a consecutive series of 516 patients with glioblastoma multiforme. Acta Neurol Scand 122(3): 159-67.
- Landy HJ, Feun L, Schwade JG, S Snodgrass, Y Lu, et al. (1994) Retreatment of intracranial gliomas. South Med J 87(2): 211-4.
- Mandl ES, Dirven CM, Buis DR, T J Postma, W P Vandertop (2008) Repeated surgery for glioblastoma multiforme: only in combination with other salvage therapy. Surg Neurol 69(5): 506-509.
- Pinsker M, Lumenta C (2001) Experiences with reoperation on recurrent glioblastoma multiforme. Zentralbl Neurochir 62(2): 43-47.
- Salcman M (1980) Survival in glioblastoma: historical perspective. Neurosurgery 7(5): 435-439.
- Terasaki M, Ogo E, Fukushima S, Kiyohiko Sakata, Naohisa Miyagi, et al. (2007) Impact of combination therapy with repeat surgery and temozolomide for recurrent or progressive glioblastoma multiforme: a prospective trial. Surg Neurol 68(3): 250-254.
- Vick NA, Ciric IS, Eller TW, J W Cozzens, A Walsh, et al. (1989) Reoperation for malignant astrocytoma. Neurology 39(3): 430-432.
- Young B, Oldfield EH, Markesbery WR, D Haack, P A Tibbs, et al. (1981) Reoperation for glioblastoma. J Neurosurg 55(6): 917-921.
- Franceschi E, Bartolotti M, Tosoni A, Stefania Bartolini, Carmelo Sturiale, et al. (2015) The effect of re-operation on survival in patients with recurrent glioblastoma. Anticancer Res 35(3): 1743-1748.
- Skeie BS, Enger PO, Brogger J, Jeremy Christopher Ganz, Frits Thorsen, et al. (2012) gamma knife surgery versus reoperation for recurrent glioblastoma multiforme. World Neurosurg 78(6): 658-669.
- Stromblad LG, Anderson H, Malmstrom P, L G Salford (1993) Reoperation for malignant astrocytomas: personal experience and a review of the literature. Br J Neurosurg 7(6): 623-633.
- Dizon DS, Krilov L, Cohen E, Tara Gangadhar, Patricia A Ganz, et al. (2016) Clinical Cancer Advances 2016: Annual Report on Progress Against Cancer From the American Society of Clinical Oncology. J Clin Oncol 34(9): 987-1011.
- Johnson DR, Ma DJ, Buckner JC, Julie E Hammack (2012) Conditional probability of long-term survival in glioblastoma. Cancer 118(22): 5608-5613.
- Brennan CW, Verhaak RG, McKenna A, Benito Campos, Houtan Noushmehr, et al. (2013) The somatic genomic landscape of glioblastoma. Cell 155(2): 462-477.
- Bennett HMD, Godlee R (1884) Excision of a Tumor from the Brain. The Lancet: 1090-1091.
- Kirkpatrick DB (1984) The first primary brain-tumor operation. J Neurosurg 61(5): 809-13.
- Weiss RB, Issell BF (1982) The nitrosoureas: carmustine (BCNU) and lomustine (CCNU). Cancer Treat Rev 9(4): 313-330.
- Sweet DL, Hendler FJ, Hanlon K, J Hekmatpanah, M L Griem, et al. (1979) Treatment of grade III and IV astrocytomas with BCNU alone and in combination with VM-26 following surgery and radiation therapy. Cancer treatment reports 63(11-12): 1707-11.
- Li J, Wang M, Won M, Edward G Shaw, Christopher Coughlin, et al. (2011) Validation and simplification of the Radiation Therapy Oncology Group recursive partitioning analysis classification for glioblastoma. Int J Radiat Oncol Biol Phys 81(3): 623-630.
- Johnson DR, O'Neill BP (2012) Glioblastoma survival in the United States before and during the temozolomide era. J Neurooncol 107(2): 359-364.
- Montemurro N, Perrini P, Blanco MO, Riccardo Vannozzi (2016) Second surgery for recurrent glioblastoma: A concise overview of the current literature. Clin Neurol Neurosurg 142: 60-64.
-
Javad Hekmatpanah, David Slotwiner, Mehrnoush Gorjian, Robert Wollman. The Value of a Second Operation in Patients with Glioblastoma Multiforme: is it Medically Justified and Ethically Appropriate?. Arch Neurol & Neurosci. 11(5): 2021. ANN.MS.ID.000774.
-
Neural Conductional, Brainstem Auditory, Neonatology, Immature Brain, Neurological Impairment, Asphyxia, Hyperbilirubinemia, Asphyxia, Hypoglycaemia, Neurons.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






