 Review Article
Review Article
Source of Free Radicals and Consequences of Oxidative Stress Following Secondary Brain Injury after Intracerebral Haemorrhage
Leta Melaku*
Department of Biomedical Sciences, College of Health Sciences, Arsi University, Asella, Oromia, Ethiopia
Leta Melaku, Department of Biomedical Sciences, College of Health Sciences, Arsi University, Asella, Oromia, Ethiopia.
Received Date: April 05, 2023; Published Date: April 13, 2023
Abstract
Free radicals are reactive chemical species having a single unpaired electron in an outer orbit. Intracranial haemorrhage (ICH) is an acute and spontaneous extravasation of blood into the cranial vault. The most important sites of ICH are basal ganglia followed by cerebral hemispheres. The pathological mechanisms of hematoma after ICH within brain parenchyma trigger a series of adverse events causing secondary brain injury and severe neurological deficits. The global incidence of ICH is increasing year by year, with a trend towards growing incidence at a younger age. A variety of pathways can induce the generation of free radicals in secondary brain injury after ICH. During ICH, mitochondria dysfunction occurs, and substantial ROS production follows. Iron overload is also involved in secondary brain injury, leading to neuronal death, brain edema, and neurodeficits after ICH. Neuroinflammation is recognized as a vital factor in the pathophysiology of ICH-induced brain injury. After ICH, both oxidative and ER stress levels are upregulated and NADPH oxidase is thought to play an important contact role during the oxidative and ER stress process. Excessive free radicals can cause the peroxidation of lipid, protein, and nucleic acid through direct and indirect pathways, leading to apoptosis. Autophagy may also play different roles in pathogenesis at different stages of cerebral hemorrhage. Electronic search was carried out through the period up to 2020.
Keywords:Free radicals; Oxidative stress; Intracerebral haemorrhage; Secondary brain injury after intracerebral haemorrhage
Introduction
As the key life-supporting element, oxygen was independently discovered by Priestly, in 1775 [1], and Scheele, in 1777 [2]. Within a few years of these seminal findings, oxygen toxic side effects that did not support life were also discovered. This revelation was made by Lavoisier in 1785 by a simple experiment in which guinea pigs exposed to oxygen in a container showed congestion of the right heart as well as lungs and died before the oxygen was fully utilized [3]. The good and bad facets of oxygen are played out by its unique molecular structure. Free radicals are reactive chemical species having a single unpaired electron in an outer orbit [4]. This unstable configuration creates energy that can initiate autocatalytic reactions so that molecules to which they react are themselves converted into free radicals [5]. The term oxidative stress is used to describe the condition of oxidative damage to a wide range of cellular structures as a result of an imbalance between free radical production and antioxidant defenses [6]. Short-term oxidative stress may occur in tissues injured by trauma, infection, heat injury, hypertoxia, toxins, and excessive exercise [7]. And harmful effects are balanced by the action of antioxidants, some of which are enzymes present in the body [8]. However, long-term oxidative stress despite the presence of the cell’s antioxidant defense system, ROS have been implicated in the induction and complications of various cardiovascular diseases [9]. The reactive species generated in cells include hydrogen peroxide (H2O2), hypochlorous acid (HClO), the hydroxyl radical (·OH), the superoxide anion radical (O2-·), the nitric oxide radical (NO·), and the lipid peroxyl radical (LOO·) [10,11]. Although ROS (reactive oxygen species) are more common in biological systems (5), free radicals also include RNS (reactive nitrogen species) [12]. The endogenous sources of ROS are the mainly by-products formed in the cells of aerobic organisms within mitochondria. Additional sources are certain enzyme, neutrophils, eosinophil’s, macrophages, microsomes and peroxisomes [13,14]. ROS can be also produced by a host of exogenous sources such as xenobiotics, chlorinated compounds, environmental agents, metals (redox and nonredox), ions, and radiation [13,15].
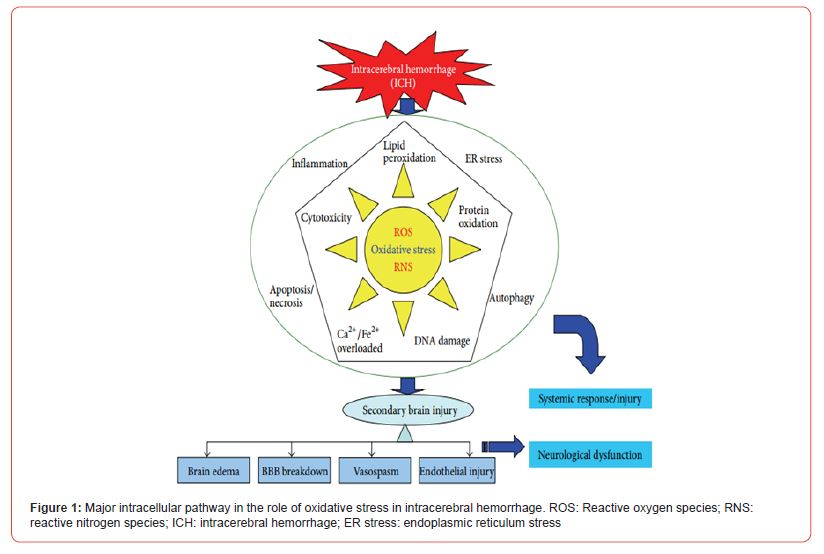
Intracerebral Haemorrhage and Oxidative Stress
Intracranial haemorrhage (ICH) is an acute and spontaneous extravasation of blood into the cranial vault [16]. It comprises intracerebral haemorrhage, subdural hematoma, epidural bleeds, and subarachnoid haemorrhage. The most common sites of ICH are basal ganglia (35 – 50% of cases), cerebral hemispheres (approx. 30%), thalamus (10 – 15%), brainstem (predominantly the pons (5 – 12%), and cerebellum (7%) [17-19]. The gross and microscopic changes in the brain depend on the location of ICH, but the general appearance is similar [20]. Microscopically, in the acute stage, the ICH consists of a liquid or semiliquid mass of blood with well-preserved red blood cells without any inflammation surrounding oedema. Subsequently, the RBC begins to lyse and neutrophils appear. This is followed by infiltration of macrophages whose main role is to phagocytosis blood products and necrotic tissue [21]. After a few days, the haematoma changes its consistency and adopts a brown colour, while oedema begins to recede. The brown discolouration of the slightly older haematomas noted macroscopically is due to the presence of two major haemoglobin-derived pigments, haemosiderin and haematoidin. One of the late events involves proliferation of astrocytes, some containing haemosiderin reflecting their phagocytic activity. The transfer of haemosiderin from macrophages to astrocytes, an event that rarely happens in infants, is common in the adult [22]. After several months or years, depending on its size, the haematoma becomes a cavity. These pathological mechanisms of hematoma after ICH within brain parenchyma trigger a series of adverse events causing secondary brain injury and severe neurological deficits [23]. Numerous preclinical studies show that secondary brain injury after ICH is caused by the interaction of cytotoxicity, excitotoxicity, oxidative stress, and inflammation from the products of red blood cell lysis and plasma components [24,25]. Depending on the underlying cause of bleeding, ICH is classified as either primary or secondary. Primary ICH, which accounts for 78 – 88% of cases, originates from the spontaneous rupture of small vessels damaged by chronic hypertension or amyloid angi opathy. Conversely, secondary ICH occurs in association with trauma, vascular abnormalities, tumours or impaired coagulation [17]. The global incidence of ICH is increasing year by year, with a trend towards growing incidence at a younger age. Despite significant progress in clinical treatment, ICH still remains a significant cause of morbidity and mortality throughout the world, with the 5-year mortality rate remains 52% for males and 56% for females older than 45 years [26]. ICH not only causes serious morbidity and mortality in patients, but also incurs a serious burden on families and society. Even after surgical treatment, 20% of ICH patients experience varying degrees of neurological dysfunction, requiring longterm hospitalization and rehabilitation [27]. Oxidative stress plays a role not only in the pathological process of ICH, but also at various important stages of pathophysiological response during ICH and secondary brain injury (SBI) after ICH (Figure 1) [24,28].
A variety of pathways can induce the generation of free radicals in secondary brain injury after ICH, of which there are two major pathways. First, blood cell decomposition products such as iron ions, heme, and thrombin can induce the production of free radicals [29,30]. Second, inflammatory cells, such as microglia and neutrophils, can generate free radicals [31]. Damage to nerve cells caused by free radicals ranges from cell membrane damage to DNA interruption or even apoptosis. The lipid-rich brain tissue is particularly sensitive to ROS that can enhance lipid peroxidation, cause membrane damage, and increase cell membrane permeability and calcium ion influx [32]. In the meantime, crosslinking and polymerization of membrane lipids will occur due to lipid peroxidation, which will indirectly inhibit the activities of membrane proteins such as calcium pumps, sodium pumps, and Na+/Ca2+ exchangers [33]. This leads to a further increase in intracellular calcium concentration which subsequently stimulates mitochondrial calcium pumps to take in calcium [34]. Calcium and phosphate in the mitochondria combine and form insoluble calcium phosphate, which causes interference in mitochondrial oxidative phosphorylation and leads to a decrease in ATP production [35]. Meanwhile, increased intracellular calcium ion concentration can activate phospholipase, promoting membrane phospholipid decomposition and causing damage to the structure of cell and organelle membranes [36,37] (Figure 1).
Sources of Free Radical in Secondary Brain Injury After Intracerebral Haemorrhage
Mitochondria Dysfunction
Physiologically, 1 – 3% of all electrons in the electron transport chain in mitochondria leak, generating superoxide radicals that can be neutralized by normal antioxidant systems [38]. During ICH, mitochondria dysfunction occurs, and substantial ROS production follows. Kim-Han et al, detected an obvious reduction in the oxygen consumption rates of mitochondria in ICH patients, indicating that mitochondria dysfunction, and not ischemia, is responsible for the decreased oxygen metabolites after ICH [39]. Direct evidence of ROS from malfunctioning mitochondria was reported in a recent study, which found that a mitochondrial ROS-specific scavenger can significantly alleviate the increased ROS following ICH [40]. The mechanism of excessive ROS formation by mitochondria after ICH remains unclear but may be partially attributable to mitochondrial permeability transition pore (MPTP) because the inhibition of MPTP can attenuate ROS production.
A. Hb-Heme-Iron
As the most abundant erythrocyte protein, hemoglobin (Hb) is released into the extracellular space via complement-mediated cell lysis in the hours after ICH and is a potent mediator of OS-induced injury. Both in vitro and in vivo investigations have shown that ROS is highly produced after exposing Hb to cell culture or injecting Hb into mouse striatum [41-43]. It is commonly believed that iron released from its degradation is responsible for oxidative damage because an iron chelator may block Hb-induced neurotoxicity [44]. In fact, Hb itself can release a large amount of superoxide during spontaneous, nonenzymatic oxidation to oxyhemoglobin and methemoglobin [45,46]. Heme, released from methemoglobin, quickly oxidizes to form hemin, which also triggers oxidative damage in brain tissue around the hematoma [47]. In vitro experiment demonstrated that hemin exposure leads to cell death, preceded by a significant, iron-dependent increase in ROS [48]. Another in vitro study showed that hemin could stimulate lipid peroxidation, irrespective of iron mediation, because the reaction could not be inhibited by deferoxamine or transferrin [49]. Hence, the mechanism of hemin-related oxidative damage partly involves its breakdown to iron by HO, similar to that of Hb [50]. Indeed, hemin is redoxactive and can react with peroxides to produce cytotoxic free radicals. Moreover, hemin can intercalate into the cell plasma membrane, facilitating lipid peroxidation [51]. Given the effect of hemin in preclinical studies, biphasic functions are observed. Hemin-induced brain injury is evidenced by increased brain water content at 24 hours after intracerebral hemin infusion [46]. In contrast, systemic hemin treatment is neuroprotective after ICH [52]. Although the mechanisms underlying the protection provided by systemic hemin administration are poorly understood, it is clear that most hemin is in circulation rather than in the brain [47].
Iron overload is also involved in secondary brain injury, leading to neuronal death, brain edema, and neurodeficits after ICH [53,54]. Intracerebral iron overload begins within 24 h, peaks at 7 days, and continues for at least a month after hemorrhage [55]. Excessive iron in the extracellular space induces oxidative damage via the Fenton reaction, which yields ROS, especially toxic hydroxyl radicals [56]. Direct evidence of iron-mediated oxidative injury has shown that injecting FeCl2 into rat brain causes oxidative DNA damage [57,58]. The strongest finding supporting the hypothesis of iron-mediated oxidative brain injury is that iron chelators decrease iron accumulation, attenuate ROS generation, exert anti-inflammatory effects, and improve neurological function.
B. Inflammation
Neuroinflammation is recognized as a vital factor in the pathophysiology of ICH-induced brain injury and is characterized by microglia activation, leukocyte infiltration, and cytokine and chemokine production [24,59,60]. In addition to the release of inflammatory factors, the activation of inflammatory cells following ICH, initially to remove oxidative toxins, also participates in ROS production [47]. As one type of innate immune cell with in the brain, microglias are rapidly activated within 1 h after ICH, peaking at 3–7 days and persisting for several weeks [61]. The imbalance of the phenotypic shift between the M1 and M2 phenotypes of microglia contributes to a large release of ROS in addition to proinflammatory factors [62]. Cell experiments have shown that microglia can induce ROS production in vitro [61,63]. Furthermore, the inhibition of microglia was reported to decrease the ROS production and brain damage volume in an ICH animal model [64]. Neutrophils are the earliest leucocytes to enter the brain after ICH. The role of neutrophils in radical production during ischemic brain stroke has been confirmed by reduced radical formation after neutrophil depletion [65]. OS-related brain injury is part of the pathogenesis mechanism of neutrophil infiltration after ICH [66]. The inflammation linked to OS following ICH indicates that neuroinflammation and OS are intercalated in ICH-induced secondary brain injury.
Consequences of Oxidative Stress Following Secondary Brain Injury after Intracerebral Haemorrhage
i. Inflammation
Inflammation and oxidative stress are closely related. Oxidative stress induces inflammation, while inflammation causes damage through oxidative stress [67]. ROS can induce the expression of acute proinflammatory cytokines directly such as Tumor Necrosis Factor (TNF-𝛼) and Interleukin-10 (IL-10) and also activate nuclear factor-𝜅B (NF-𝜅B) which plays the vital role in inflammation reaction [68,69]. Likewise, proinflammatory cytokines can induce the production of ROS [69]; thus, a positive feedback cycle is formed. Oxidative stress may also initiate the up-regulation of MMP-9 levels in brain damage after ICH [70]. The MMP-9 expression was also increased, accompanied by elevated TNF-𝛼 and IL-1𝛽 levels, and cerebral edema and SBI were aggravated [71]. MMP-9 activity may have dual role and temporal profile in post-ICH [72]. Clinical studies suggest that MMP-9 may be detrimental in the acute phase through destruction of basal lamina, activation of vascular endothelial growth factor, and activation of apoptosis but assist in recovery in the subacute phase through angiogenesis. Additional studies have shown that prostaglandin mediated inflammatory mechanisms are involved in secondary brain damage after ICH. In a collagenase-induced ICH model in mice, prostaglandin E2 receptor 1 (EP1R) was expressed in neurons and axons but not in astrocytes and microglia. EP1R agonists induce brain edema, cell death, neurodegeneration, neuroinflammation, and behavioural defects, while EP1R suppression protects the brain. Research has confirmed that the inhibition effect of EP1R is mainly through the reduction of Scr enzyme phosphorylation levels and MMP-9 activation, thus attenuating oxidative stress and white matter damage [73]. Studies have also shown after ICH the expression of HO-1 and PrxI was induced around the hemorrhagic region. Peroxiredoxin I (PrxI) and heme oxygenase-1 (HO- 1) are considered to be oxidative stress- and heme-related proteins, and heme inhibits PrxI antioxidant activity. PrxI is important for cell protection against oxidative stress, but also works to facilitate production of prostaglandins E2 and D2 (PGE2 and PGD2) through nuclear factor- (erythroidderived 2) like 2 (Nrf2) [74]. In the acute bleeding phase, PrxI and HO-1 are mainly expressed in microglia, while in sub-acute and chronic phases expression is mainly in astrocytes [75]. Acute inflammation is regulated by the time- and cell type-dependent production of cytokines and other signaling molecules including reactive oxygen species and prostaglandins [74]. In SBI after ICH therefore inflammation and oxidative stress may play major roles; however, the relationship between inflammation and oxidative stress is complicated and needs further exploration.
ii. Endoplasmic Reticulum Stress
The pathological conditions may cause an imbalance between ER protein folding load and capacity after ICH, leading to the accumulation of unfolded proteins in the ER lumen, leading to a condition known as ER stress [24]. Moderate activation of unfolded protein response after exposure to oxidation stress may be an adaption mechanism to protect cell function and survival, but ROS accumulation caused by excessive ER stress will further aggravate oxidative stress [76]. Neurons mainly express NOX2 in NADPH oxidase, which comprises gp91 phox catalytic subunit and p47 phox assembly subunit [77]. NMDA receptor is activated after ICH; a large amount of Ca2+ fluxes into the cells, leading to an NADPH oxidase and mitochondrial electron transport chain to produce superoxide [77,78]. Using NADPH oxidase inhibitors and nonspecific ROS scavengers can reduce oxidative stress, improve cerebral vascular function, and reduce cerebral amyloid angiopathy-related microhemorrhages [79]. NADPH oxidase generated by NMDA activation is considered a major superoxide source [80]. After ICH, both oxidative and ER stress levels are upregulated [24,81]. NADPH oxidase is thought to play an important contact role during the oxidative and ER stress process [82]. Studies have shown that NOX-mediated oxidative stress is induced by unfolded protein response/ER stress, whereas ER stress induced apoptosis can be blocked by knockout of NOX2 gene or antioxidant N- acetylcysteine [83,84]. The PERK pathway is considered a molecule pathway which links oxidative and ER stress. Nrf2 induces considerable antioxidant gene expression [29]. After ICH, due to cytotoxicity mediated by heme, hemoglobin, and iron overload, Nrf2 is phosphorylated by PERK and then dissociates from the Nrf2/KEAP1 complex and enters into the nucleus to promote antioxidant gene expression, leading to a resistance to oxidative stress and playing a cell-protective role [29,85]. In Nrf2 knockout (Nrf2(−/−)) mice ICH model, injury volume was significantly larger in 24 h after induction of ICH, which correlated with neurological deficits. This exacerbation of brain injury was also associated with an increase in leukocyte infiltration, production of reactive oxygen species, DNA damage, and cytochrome c release during the critical early phase of the post-ICH period [86]. After subarachnoid hemorrhage (SAH), KEAP1-Nrf2-ARE pathway is activated, and after sulforaphane or tertbutylhydroquinone activates the Nrf2 pathway, NQO1 and GST-𝛼1 levels are increased, thus playing a protective role in the brain [87-89]. Nrf2 and Transcriptional Factor 4 (ATF4) also activate antioxidant response factor (ARE) by up-regulating its expression [90,91], indicating that the ER and oxidative stress signaling pathways have synergistic effects. Endo plasmic reticulum oxidoreductase (ERO1𝛼) forms a disulfide bond, promoting protein refolding and helping reduce ER stress. However, ERO1𝛼 activation transfers the electron to the oxygen molecule and produces ROS [92]. The endoplasmic reticulum stress marker CHOP, a downstream molecule of PERK, induces ERO1𝛼 expression and aggravates ER oxidation; on the contrary, in cells lacking CHOP, the ER stress level induced by ERO1𝛼 is reduced [93]. Other studies have shown that after ER stress inositol 1, 4, 5-trisphosphate receptors (IP3Rs) are activated and calcium release from the endoplasmic reticulum calcium storage is increased, leading to intracellular calcium overload and ROS production [94]. In addition, the elevated ROS level causes the activation of ryanodine receptor (RyRs), another endoplasmic reticulum Ca2+ release channel, and the release of Ca2+ from the ER [95,96]. Thus, Ca2+ activates IP3Rs or RyRs as an input signal, aggravating intracellular calcium overload. After ICH, ER and oxidative stress activate ER Ca2+ release via RyRs and IP3Rs pathways, leading to neuronal toxicity and aggravating SBI.
iii. Neural Cell Apoptosis or Necrosis
Apoptosis is a regulated cell death, which is also called programmed cell death (PCD). However, necrosis is characterized by plasma membrane rupture as well as nuclear and cellular swelling, other than regulated cell death. Necrosis was formerly considered to be an accidental, unregulated form of cell death resulting from excessive stress, although it has been suggested that this is an over simplistic view as necrosis may under certain circumstances involve the mobilization of specific transduction mechanisms [97]. The main causes of nerve cell apoptosis in ICH are the release of thrombin during blood coagulation, the toxic effects of hematoma components and its degradation products, and the oxidative stress reaction in perihematomal [24]. Oxidative stress induces apoptosis through pathways, such as the mitochondrial, death receptor, and endoplasmic reticulum stress pathways. These findings suggest that DNA damage cytosolic reactive oxygen species (cROS) generation, and mitochondrial hyperactivation induced necrosis through a PARP1-dependent pathway, while generation of nitric oxide (NO) and mitochondrial ROS (mROS) remained unaffected [98]. It can also induce apoptosis by activating the mitogen-activated protein kinase pathway, activating NF-𝜅B and up-regulating its expression, or activating caspases [99]. Excessive free radicals can cause the peroxidation of lipid, protein, and nucleic acid through direct and indirect pathways, leading to apoptosis [100]. The intrinsic and extrinsic pathways of apoptosis are not necessarily independent of each other; some of the factors in both types of pathway may have a synergistic effect in the regulation of the apoptosis process, initiated by a single stimulator [101]. Hypoxia, nitric oxide (NO), and ROS inducers can all cause the exposure of neuronal membrane phosphatidyl Serine [102]. Hypoxia and nitric oxide (NO) are also inducers, can all cause the exposure of neuronal membrane phosphatidyl Serine [102]. Superoxide production paralleled the increase in iNOS expression, and inhibition of either iNOS (aminoguanidine or iminopiperdine) or superoxide (apocynin) significantly reduced cell death. Furthermore, hydrogen peroxide and NO can lead to nuclear condensation and DNA fragmentation and have a synergistic effect on inducing neuronal apoptosis [103]. Additionally, NO can induce apoptosis of hippocampal and dopamine neurons [104,105], and hydrogen peroxide can induce apoptosis through disrupting mitochondrial function and promoting proapoptosis gene expression [106,107]. Nevertheless, the relationship between necrosis and oxidative stress after ICH is still not fully clear.
Necroptosis was recently discovered as one form of programmed cell death (PCD) that shares characteristics with both necrosis and apoptosis. Necroptosis involves Fas/TNF-𝛼 death domain receptor activation and inhibition of receptor interacting protein I kinase [108]. Recent study identified a novel role for the necroptosis inhibitor, necrostatin-1, in limiting neurovascular injury in tissue culture models of hemorrhagic injury [109]. Another study demonstrated that the specific inhibitor necrostatin-1 suppressed apoptosis and autophagy to exert these neuroprotective effects after ICH and that there existed a cross talk among necroptosis, apoptosis, and autophagy after ICH [110]. Moreover, necrostatin- 1 reduced RIP1-RIP3 interaction and further inhibited microglia activation and TNF-𝛼 and IL-1𝛽 expression after ICH. These findings indicate that RIP1/RIP3-mediated necroptosis is an important mechanism of cell death after ICH [111]. In another study, hemin concentration dependently induced necroptotic cell death in cortical astrocytes within 5 h of treatment. Hemin induced peroxidative injury was associated with a rapid depletion of intracellular glutathione (GSH), culminating in lipid peroxidation and cell death [112]. Together, these studies suggest a novel role for oxidative stress in necroptotic brain injury after ICH.
iv. Autophagy
Autophagy is a lysosomal degradation pathway, which is essential for survival, development, and homeostasis [113]. Autophagy is also involved in the pathological process of cerebral hemorrhage as a degradation process of proteins and organelles within the cells [114-116]. During this process, oxidative stress may contribute to autophagy formation. Likewise, autophagy may reduce oxidative damage by engulfing or degrading stress products [117]. Autophagy plays a dual role in ischemic stroke pathological processes [118]. The intracellular mechanism which regulates autophagy via ROS levels can be summarized as transcriptional and posttranscriptional regulation, including various intracellular signaling pathways such as ROS-FOXO3-LC3/BNIP3 autophagy, ROS-Nrf2-P62 autophagy, ROS-HIF1-BNIP3/NIX autophagy, and ROSTIGAR autophagy [113]. Autophagy can also regulate ROS levels through a chaperone-mediated autophagy pathway, the mitochondrial autophagy pathway, and P62-mediated signaling pathways [119]. Autophagy may play different roles in pathogenesis at different stages of cerebral hemorrhage [120], and further study of the relationship between oxidative stress and autophagy after ICH may provide a theoretical basis for elucidating the pathogenesis of cerebral hemorrhage.
Conclusion
ROS have been implicated in the induction and complications of various cardiovascular diseases. Intracranial haemorrhage (ICH) is an acute and spontaneous extravasation of blood into the cranial vault. The most common sites of ICH are basal ganglia, cerebral hemispheres, thalamus, brainstem and cerebellum. Secondary brain injury after ICH is caused by the interaction of cytotoxicity, excitotoxicity, oxidative stress, and inflammation from the products of red blood cell lysis and plasma components Depending on the underlying cause of bleeding; ICH is classified as either primary or secondary. ICH not only causes serious morbidity and mortality in patients, but also incurs a serious burden on families and society. Oxidative stress plays a role not only in the pathological process of ICH, but also at various important stages of pathophysiological response during ICH and secondary brain injury (SBI) after ICH. The mechanism of excessive ROS formation by mitochondria after ICH remains unclear but may be partially attributable to mitochondrial permeability transition pore (MPTP) because the inhibition of MPTP can attenuate ROS production. The strongest finding supporting the hypothesis of iron-mediated oxidative brain injury is that iron chelators decrease iron accumulation, attenuate ROS generation, exert anti-inflammatory effects, and improve neurological function. In addition to the release of inflammatory factors, the activation of inflammatory cells following ICH, initially to remove oxidative toxins, also participates in ROS production. OS-related brain injury is part of the pathogenesis mechanism of neutrophil infiltration after ICH. In SBI after ICH, inflammation and oxidative stress may play major roles; however, the relationship between inflammation and oxidative stress is complicated and needs further exploration. After ICH, ER and oxidative stress activate ER Ca2+ release via RyRs and IP3Rs pathways, leading to neuronal toxicity and aggravating SBI. The main causes of nerve cell apoptosis in ICH are the release of thrombin during blood coagulation, the toxic effects of hematoma components and its degradation products, and the oxidative stress reaction in perihematoma. Autophagy is also involved in the pathological process of cerebral hemorrhage as a degradation process of proteins and organelles within the cells.
Availability of Data and Material
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The author declares that there is no conflict of interests regarding the publication of this paper.
Funding
Nil support in financial or other manner.
Authors’ Contributions
LM had participated in the design of the study, data analyses, and manuscript preparation; and the authors could have read and approved the final manuscript.
Acknowledgements
The Author is grateful to College of Health Sciences Research and Community Office of Arsi University as well as researchers who their documents were used in the preparation of the review.
References
- Priestly J (1894) ‘Experiments and observations on different kinds of air’, Vol II, Sections III-V, 1775:29 203. Reprinted in ‘The discovery of oxygen,’ Part 1, Alembic Club Reprint No. 7. London: Simpkin, Marshall, Hamilton,: Kent.
- Scheele C (1923) Chemische abhandlung von der luft und dem Feuer, Upsala and Leipzig. Reprinted as ‘The discovery of oxygen,’ Part 2. Alembic Club Reprint No. 8. London: Gurney and Jackson.
- Lavoisier A (1785) Alterations qu’eprouve l’air resire. Recueil des memoires de Lavoisier. 1785, Read to the Societe de Medicine. ReprRepinted as part of ‘Memoires sur la respiration et al transpiration des animaux’ in ‘Les maitres de la pensee scientifique. Paris: Gauthier-Villaus et cie;
- Riley P (1994) Free radicals in biology: oxidative stress and effects of ionizing radiation. Int J Rad Biol 65: 27 33.
- Rahman K (2007) Studies on free radicals, antioxidants, and co-factors. Clinical Interventions in Aging 2: 219 36.
- Rock C, Jacob R, Bowen P (1996) Update of biological characteristics of the antioxidant micronutrients Vitamin C, Vitamin E and the carotenoids. J Am Diet Assoc 96: 693 702.
- Lobo V, Patil A, Phatak A, Chandra N (2010) Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev 4: 118 26.
- Halliwell B (1996) Antioxidants in human health and disease. Ann Rev Nutr 16: 33 50.
- Rao A, Bharani M, Pallavi V (2006) Role of antioxidants and free radicals in health and disease. Adv Pharmacol Toxicol 7: 29 38.
- We dinger A, Kosovo A (2015) Biological activities of reactive oxygen and nitrogen species: Oxidative stress versus signal transduction. Biomolecules 5: 472 84.
- Bagchi K, Puri S (1998) Free radicals and antioxidants in health and disease. Eastern Mediterranean Health Journal 4: 350 60.
- Miller A, Budzyn K, Sobey C (2010) Vascular dysfunction in cerebrovascular disease: mechanisms and therapeutic intervention. Clinical Science 119: 1 17.
- Valko M, Rhodes C, Moncol J, Izakovic M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Mini-review Chem Biol Interact 160: 1 40.
- Glade M (2003) The role of reactive oxygen species in Health and Disease Northeast Regional Environmental Public Health Center University of Massachusetts. Amerst Nutrition 19: 401 3.
- Leonard S, Harris G, Shi X (2004) Metal-induced oxidative stress and signal transduction. Free Rad Biol Med 37: 1921 42.
- Papacocea T, Papacocea R, Bădărău A, Ion A, Buraga I, Gaman L, et al. (2011) Oxidative stress and antiOxidant theraphy in intracerebral haemorrhage. Therapeutics, pharmacology and clinical toxicology XV(4): 270 3.
- Qureshi A, Tuhrim S, Broderick J, Batjer H, Hondo H, Hanley D (2001) Spontaneous intracerebral hemorrhage. N Engl J Med 344(19): 1450 60.
- Aguilar M, Brott T (2011) Update in Intracerebral Hemorrhage. The Neurohospitalist 1(3): 148 59.
- Papacocea A, Papacocea T, Dănăilă L, Papacocea R, Ion D, Bădărău A (2010) Primary intracerebellar hematomas: surgical indications, prognosis. Chirurgia 105(6): 805 7.
- Papacocea T, Papacocea A, Dănăilă L, Papacocea R, Ion D, Bădărău A, et al. (2011) Posterior fossa epidural hematomas. Chirurgia 106(3): 309 13.
- Kase C, Caplan L (1994) Intracerebral hemorrhage. Boston: Butterworth-Heinemann.
- McCarron M, Cohen N, Nicoll J (2005) Parenchymal brain hemorrhage. In: In-Pathology & Genetics Cerebrovascular Diseases. Basel, Switzerland: ISN p. 294 300.
- Belur P, Chang J, He S, Emanuel B, Mack W (2013) Emerging experimental therapies for intracerebral hemorrhage: targeting mechanisms of secondary brain injury. Neurosurgical Focus 34(5): E9.
- Aronowski J, Zhao X (2011) Molecular pathophysiology of cerebral hemorrhage: secondary brain injury. Stroke: A journal of cerebral circulation 42(6): 1781 6.
- Chaudhary N, Gemmete J, Thompson B, Xi G, Pandey A (2013) Iron—potential therapeutic target in hemorrhagic stroke. World Neurosurgery 79(1): 7 9.
- Meschia J, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, et al. (2014) American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; Council on Hypertension. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 45(12): 3754 832.
- Gustavsson A, Svensson M, Jacobi F, Allgulander C, Alonso J, Beghi E, et al. (2011) Cost of disorders of the brain in Europe 2010,”European Neuropsychopharmacology. Eur Neuropsychopharmacol 21(10): 718 79.
- Robbins N, Swanson R (2014) Opposing effects of glucose on stroke and reperfusion injury: acidosis, oxidative stress, and energy metabolism. Stroke 45(6): 1881 6.
- Zhao X, Aronowski J (2013) Nrf2 to pre-condition the brain against injury caused by products of hemolysis after ICH. Translational Stroke Research 4(1):71 5.
- Valko M, Morris H, Cronin M (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12: 1161 208.
- Yu Y, Chi X, Liu L (2014) A hypothesis: hydrogen sulphide might be neuroprotective against subarachnoid haemorrhage induced brain injury. The Scientific World Journal 2014: 1 9.
- Toda N, Ayajiki K, Okamura T (2009) Cerebral blood flow regulation by nitric oxide: recent advances. Pharmacological Reviews 61(1): 62 97.
- Eigel B, Gursahani H, Hadley R (2004) ROS are required for rapid reactivation of Na+/Ca2+ exchanger in hypoxic reoxygenated guinea pig ventricular myocytes. American Journal of Physiology 286(3): H955 63.
- Xiaochun D, Zunjia W, Haitao S, Meifen S, Gang C (2016) Intracerebral Hemorrhage, Oxidative Stress, and Antioxidant Therapy. Oxid Med and Cellu Longevity: 1 17.
- Li Q, Pogwizd S, Prabhu S, Zhou L (2014) Inhibiting Na+/K+ ATPase can impair mitochondrial energetics and induce abnormal Ca2+ cycling and automaticity in guinea pig cardiomyocytes. PLoS ONE 9(4).
- Gu Y, Dee C, Shen J (2011) Interaction of free radicals, matrix metalloproteinases and caveolin-1 impacts blood-brain barrier permeability. Frontiers in Bioscience 3(4): 1216 31.
- Chrissobolis S, Miller A, Drummond G, KempHarper B, Sobey C (2011) Oxidative stress and endothelial dysfunction in cerebrovascular disease. Frontiers in Bioscience 16(5): 1733 45.
- Orrenius S, Gogvadze V, Zhivotovsky B (2007) Mitochondrial oxidative stress: implications for cell death. Annual Review of Pharmacology and Toxicology 47: 143 83.
- Kim-Han J, Kopp S, Dugan L, Diringer M (2006) Perihematomal mitochondrial dysfunction after intracerebral hemorrhage. Stroke 37(10): 2457 62.
- Ma Q, Chen S, Hu Q, Feng H, Zhang J, Tang J (2014) NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Annals of Neurology 75(2): 209 19.
- Regan R, Panter S (1993) Neurotoxicity of hemoglobin in cortical cell culture. Neuroscience Letters 153(2): 219 22.
- Wang X, Mori T, Sumii T, Lo E (2002) Hemoglobin-induced cytotoxicity in rat cerebral cortical neurons: caspase activation and oxidative stress. Stroke 33(7): 1882 8.
- Qu Y, Chen J, Benvenisti-Zarom L, Ma X, Regan R (2005) Effect of targeted deletion of the heme oxygenase-2 gene on hemoglobin toxicity in the striatum. Jour of Cerebral Blood Flow and Metab 25(11): 1466 75.
- Regan R, Rogers B (2003) Delayed treatment of haemoglobin neurotoxicity. Journal of Neurotrauma 20(1): 111 20.
- Misra H, Fridovich I (1972) The generation of superoxide radical during the autoxidation of hemoglobin. The Journal of Biological Chemistry 247(21): 6960 2.
- Huang F, Xi G, Keep R, Hua Y, Nemoianu A, Hoff J (2002) Brain edema after experimental intracerebral hemorrhage: role of hemoglobin degradation products. Journal of Neurosurgery 96(2): 287 93.
- Xin H, Chuanyuan T, Qi G, Jun Z, Hao L, Chao Y (2016) Oxidative Stress in Intracerebral Hemorrhage: Sources, Mechanisms, and Therapeutic Targets. Oxid Medic and Cellular Longe 2016: 1 12.
- Goldstein L, Teng Z, Zeserson E, Patel M, Regan R (2003) Hemin induces an iron-dependent, oxidative injury to human neuron-like cells. Journal of Neuroscience Research 73(1): 113 21.
- Gutteridge J, Smith A (1988) Antioxidant protection by haemopexin of haem-stimulated lipid peroxidation. The Biochemical Journal 256(3): 861 5.
- Kwon K, Kim J, Kim M, Kim S (2013) Neuroprotective effects of valproic acid against hemin toxicity: possible involvement of the down-regulation of heme oxygenase-1 by regulating ubiquitin-proteasomal pathway. Neurochemistry International 62(3): 240 50.
- Chen-Roetling J, Cai Y, Lu X, Regan R (2014) Hemin uptake and release by neurons and glia. Free Radical Research 48(2): 200 5.
- Lu X, Chen-Roetling J, Regan R (2014) Systemic hemin therapy attenuates blood-brain barrier disruption after intracerebral hemorrhage. Neurobiology of Disease 70: 245 51.
- Wu H, Wu T, Xu X, Wang J (2011) Iron toxicity in mice with collagenase-induced intracerebral hemorrhage. Jour of Cere Blood Flow and Metab 31(5): 1243 50.
- Caliaperumal J, Ma Y, Colbourne F (2012) Intra-parenchymal ferrous iron infusion causes neuronal atrophy, cell death and progressive tissue loss: implications for intracerebral hemorrhage. Experimental Neurology 237(2): 363 9.
- Zhao F, Hua Y, He Y, Keep R, Xi G (2011) Minocycline induced attenuation of iron overload and brain injury after experimental intracerebral hemorrhage. Stroke 42(12): 3587 93.
- Ward R, Zucca F, Duyn J, Crichton R, Zecca L (2014) The role of iron in brain ageing and neurodegenerative disorders. The Lancet Neurology 13(10): 1045 60.
- Nakamura T, Keep R, Hua Y, Nagao S, Hoff J, Xi G (2006) Iron-induced oxidative brain injury after experimental intracerebral hemorrhage. Acta Neurochirurgica Supplement 96: 194 8.
- Nakamura T, Keep R, Hua Y, Hoff J, Xi G (2005) Oxidative DNA injury after experimental intracerebral hemorrhage. Brain Research 1039(1 2): 30 6.
- Sinha K, Das J, Pal P, Sil P (2013) Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Archives of Toxicology 87(7): 1157 80.
- Zhou Y, Wang Y, Wang J, AnneStetler R, Yang Q (2014) Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Progress in Neurobiology 115: 25 44.
- Yang Z, Zhong S, Liu Y, Shen H, Yuan B (2015) Scavenger receptor SRA attenuates microglia activation and protects neuroinflammatory injury in intracerebral hemorrhage. Journal of Neuroimmunology 278: 232 8.
- Hu X, Leak R, Shi Y, Suenaga J, Gao Y, Zheng P, Chen J (2015) Microglial and macrophage polarization: new prospects for brain repair. Nature Reviews Neurology 11: 56 64.
- Cui H, He H, Yang A, Zhou H, Wang C, Luo J, et al. (2015) Efficacy of deferoxamine in animal models of intracerebral hemorrhage: a systematic review andstratifiedmeta-analysis. PLoS ONE 10(5).
- Wang J, Tsirka S (2005) Tuftsin fragment 1 3 is beneficial when delivered after the induction of intracerebral hemorrhage. Stroke 36(3): 613 8.
- Matsuo Y, Kihara T, Ikeda M, Ninomiya M, Onodera H, et al. (1995) Role of neutrophils in radical production during ischemia and reperfusion of the rat brain: effect of neutrophil depletion on extracellular ascorbyl radical formation. Jour of Cerebral Blood Flow and Metab 15(6): 941 7.
- Wang J (2010) Preclinical and clinical research on inflammation after intracerebral hemorrhage. Progress in Neurobiology 92(4): 463 77.
- Mracsko E, Veltkamp R (2014) Neuroinflammation after intracerebral hemorrhage. Frontiers in Cellular Neuroscience 8: 388.
- Hu W, Zhou P, Rao T, Zhang X, Wang W, Zhang L (2015) Adrenomedullin attenuates interleukin-1𝛽-induced inflammation and apoptosis in rat Leydig cells via inhibition of NF-𝜅B signaling pathway. Experimental Cell Research 339(2): 220 30.
- Khaper N, Bryan S, Dhingra S, Singal R, Bajaj A, Pathak C, et al. (2010) Targeting the vicious inflammation-oxidative stress cycle for the management of heart failure. Antioxidants & Redox Signaling 13(7): 1033 49.
- Katsu M, Niizuma K, Yoshioka H, Okami N, Sakata H, Chan P (2010) Hemoglobin-induced oxidative stress contributes to matrix metalloproteinase activation and blood-brain barrier dysfunction in vivo. Jour of Cere Blood Flow and Metab 30(12): 1939 50.
- Han D, Li S, Xiong Q, Zhou L, Luo A (2015) Effect of propofol on the expression of MMP-9 and its relevant inflammatory factors in brain of rat with intracerebral hemorrhage. Cell Biochemistry and Biophysics 72(3): 675 9.
- Chang J, Emanuel B, Mack W, Tsivgoulis G, Alexandrov A (2014) Matrix metalloproteinase-9: dual role and temporal profile in intracerebral hemorrhage. Journal of Stroke and CVD 23(10): 2498 505.
- Zhao X, Wu T, Chang C, Wu H, Han X, Li Q, et al. (2015) Toxicrole of prostaglandin E2 receptor EP1 after intracerebral hemorrhage in mice. Brain, Behavior, and Immunity 46: 293 310.
- Ishii T (2015) Close teamwork between Nrf2 and peroxiredoxins 1 and 6 for the regulation of prostaglandin D2 and E2 production in macrophages in acute inflammation. Free Radical Biology and Medicine B 88: 189 98.
- Nakaso K, Kitayama M, Mizuta E, Fukuda H, Ishii T, Nakashima K, et al. (2000) Co-induction of heme oxygenase-1 and peroxiredoxin I in astrocytes and microglia around hemorrhagic region in the rat brain. Neuroscience Letters 293(1): 49 52.
- Malhotra J, Kaufman R (2007) Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxidants and Redox Signaling 9(12): 2277 93.
- Bedard K, Krause K (2007) The NOX family of ROS generating NADPH oxidases: physiology and pathophysiology. Physiological Reviews 87(1): 245 313.
- Cavallucci V, Bisicchia E, Cencionietal M (2014) Acute focal brain damage alters mitochondrial dynamics and autophagy in axotomized neurons. Cell Death & Disease: 5.
- Han B, Zhou M, Johnson A, Singh I, Liao F, Vellimana A, et al. (2015) Contribution of reactive oxygen species to cerebral amyloid angiopathy, vasomotor dysfunction, and microhemorrhage in aged Tg2576 mice. PNAS 112(8): E881 90.
- Brennan A, Suh S, Won S, Narasimhan P, Kauppinen TM, et al. (2009) NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nature Neuroscience 12(7): 857 63.
- Jeanne M, Labelle-Dumais C, Jorgensen J, Kauffman W, Mancini G, Favor J, et al. (2012) COL4A2 mutations impair COL4A1 and COL4A2 secretion and cause hemorrhagic stroke. The American Journal of Human Genetics 90(1): 91 101.
- Li G, Scull C, Ozcan L, Tabas I (2010) NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. The Journal of Cell Biology 191(6): 1113 25.
- Laurindo F, Araujo T, Abrahao T (2014) Nox NADPH oxidases and the endoplasmic reticulum. Antioxidants & Redox Signaling 20(17): 2755 75.
- Santos C, Nabeebaccus A, Shah A, Camargo L, Filho S, Lopes L (2014) Endoplasmic reticulum stress and nox-mediated reactive oxygen species signaling in the peripheral vasculature: potential role in hypertension. Antioxidants and Redox Signaling 20(1): 121 34.
- Zhao X, Sun G, Ting S, Song S, Zhang J, Edwards N, et al. (2015) Cleaning up after ICH: the role of Nrf2 in modulating microglia function and hematoma clearance. Journal of Neurochemistry 133(1): 144 52.
- Wang J, Fields J, Zhao C, Langer J, Thimmulappa R, Kensler T, et al. (2007) Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radical Biology & Medicine 43(3): 408 14.
- Chen G, Fang Q, Zhang J, Zhou D, Wang Z (2011) Role of the Nrf2-ARE pathway in early brain injury after experimental subarachnoid hemorrhage. Journal of Neuroscience Research 89(4): 515 23.
- Liu Y, Qiu J, Wang Z, You W, Wu L, Ji C, et al. (2015) Dimethylfumarate alleviates early brain injury and secondary cognitive deficits after experimental subarachnoid hemorrhage via activation of Keap1-Nrf2-ARE system. Journal of Neurosurgery 123(4): 915 23.
- Wang Z, Ji C, Wu L, Qiu J, Li Q, Shao Z, et al. (2014) Tert-butylhydroquinone alleviates early brain injury and cognitive dysfunction after experimental subarachnoid hemorrhage: role of Keap1/Nrf2/ARE pathway. PLoS ONE 9(5): e97685.
- Miller D, Singh I, Wang J, Hall E (2015) Nrf2-ARE activator carnosic acid decreases mitochondrial dysfunction, oxidative damage and neuronal cytoskeletal degradation following traumatic brain injury in mice. Experimental Neurology 264: 103 10.
- Afonyushkin T, Oskolkova O, Philippova M, Resink T, Erne P, Binder B, et al. (2010) Oxidized phospholipids regulate expression of ATF4 and VEGF in endothelial cells via NRF2-dependent mechanism: novel point of convergence between electrophilic and unfolded protein stress pathways. Arteriosclerosis, Thrombosis, and Vascular Biology 30(5): 1007 13.
- Dandekar A, Mendez R, Zhang K (2015) Cross talk between ER stress, oxidative stress, and inflammation in health and disease. In: In Stress Responses. New York, USA: Springer p. 205 14.
- Marciniak S, Yun C, Oyadomari S, Novoa I, Zhang Y, Jungreis R, et al. (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes & Development 18(24): 3066 77.
- Pathak N, Mitra S, Khandelwal S (2013) Cadmium induces thymocyte apoptosis via caspase-dependent and caspaseindependent pathways. Jour of Biochem and Molecular Toxico 27(3): 193 203.
- Bhandary B, Marahatta A, Kim H, Chae H (2013) An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Inter Jour of Molecular Sciences 14(1): 434 56.
- Cooper L, Li W, Lu Y, Centracchio J, Terentyeva R, Koren G, et al. (2013) Redox modification of ryanodine receptors by mitochondria-derived reactive oxygen species contributes to aberrant Ca2+ handling in ageing rabbit hearts. The Journal of Physiology 591(23): 5895 911.
- Smith C, Yellon D (2011) Necroptosis, necrostatins and tissue injury. Journal of Cellular and Molecular Medicine 15(9): 1797 806.
- Shin H, Kwon H, Lee J, Gui X, Achek A, Kim J, et al. (2015) Doxorubicin induced necrosis is mediated by poly-(ADP-ribose) polymerase 1 (PARP1) but is independent of p53. Scientific Reports 5.
- Maiese K, Chong Z, Hou J, Shang Y (2010) Oxidative stress: biomarkers and novel therapeutic pathways. Experimental Gerontology 45(3): 217 34.
- Crack P, Taylor T (2005) Reactive oxygen species and the modulation of stroke. Free Radical Biology & Medicine 38(11): 1433 44.
- Fujikawa D (2015) The role of excitotoxic programmed necrosis in acute brain injury. Computa and Struct Biotechn Journal 13: 212 21.
- Chong Z, Lin S, Kang J, Maiese K (2003) The tyrosine phosphatase SHP2 modulates MAP kinase p38 and caspase 1 and 3 to foster neuronal survival. Cellular and Molecular Neurobiology 23(4 5): 561 78.
- Wang J, Shum A, Ho Y, Wang J (2003) Oxidative neurotoxicity in rat cerebral cortex neurons: synergistic effects of H2O2 and NO on apoptosis involving activation of p38 mitogen-activated protein kinase and caspase-3. Journal of Neuroscience Research 72(4): 508 19.
- Sharma S, Ebadi M (2003) Metallothionein attenuates 3-morpholinosydnonimine (SIN-1)-induced oxidative stress in dopaminergic neurons. Antioxidants and Redox Signaling 5(3): 251 64.
- Calcerrada P, Peluffo G, Radi R (2011) Nitric oxide-derived oxidants with a focus on peroxynitrite: molecular targets, cellular responses and therapeutic implications. Current Pharmaceutical Design 17(35): 3905 32.
- Higgins G, Beart P, Nagley P (2009) Oxidative stress triggers neuronal caspase-independent death: endonuclease G involvement in programmed cell death-type III. Cellular and Molecular Life Sciences 66(16): 2773 87.
- Higgins G, Devenish R, Beart P, Nagley P (2012) Transitory phases of autophagic death and programmed necrosis during superoxide-induced neuronal cell death. Free Radical Biology and Medicine 53(10): 1960 7.
- Wu W, Liu P, Li J (2012) Necroptosis: an emerging form of programmed cell death. Critical Reviews in Oncology/Hematology 82(3): 249 58.
- King M, Whitaker-Lea W, Campbell J, Alleyne C, Dhandapani K (2014) Necrostatin-1 reduces neurovascular injury after intracerebral hemorrhage. Interna Journal of Cell Biology 2014: 1 10.
- Chang P, Dong W, Zhang M, Wang Z, Wang Y, Wang T, et al. (2014) Anti-necroptosis chemical necrostatin-1 can also suppress apoptotic and autophagic pathway to exert neuroprotective effect in mice intracerebral hemorrhage model. Journal of Molecular Neuroscience 52(2): 242 9.
- Su X, Wang H, Kang D, Zhu J, Sun Q, Li T, Ding K (2015) Necrostatin-1ameliorates intracerebral hemorrhage-induced brain injury in mice through inhibiting RIP1/RIP3 pathway. Neurochemical Research 40(4): 643 50.
- Laird M, Wakade C, Alleyne C, Dhandapani K (2008) Hemin-induced necroptosis involves glutathione depletion in mouse astrocytes. Free Radical Biology & Medicine 45(8): 1103 14.
- Lee J, He Y, Sagher O, Keep R, Hua Y, Xi G (2009) Activated autophagy pathway in experimental subarachnoid hemorrhage. Brain Research 1287: 126 35.
- Wang Z, Shi X, Yin J, Zuo G, Zhang J, Chen G (2012) Role of autophagy in early brain injury after experimental subarachnoid hemorrhage. Journal of Molecular Neuroscience 46(1): 192 202.
- Hu S, Xi G, Jin H, He Y, Keep R, Hua Y (2011) Thrombin induced autophagy: a potential role in intracerebral hemorrhage. Brain Research 1424: 60 6.
- Filomeni G, De Zio D, Cecconi F (2015) Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death and Differentiation 22(3): 377 88.
- Rubio N, Verrax J, Dewaele M, Verfaillie T, Johansen T, Piette J, et al. (2014) P38MAPK-regulated induction of p62 and NBR1 after photodynamic therapy promotes autophagic clearance of ubiquitin aggregates and reduces reactive oxygen species levels by supporting Nrf2-antioxidant signaling. Free Radical Biology and Medicine 67: 292 303.
- Li L, Tan J, Miao Y, Lei P, Zhang Q (2015) ROS and autophagy: interactions and molecular regulatory mechanisms. Cellular and Molecular Neurobiology 35(5): 615 21.
- Shao A, Wu H, Chen S, Ammar A, Zhang J, Hong Y (2014) Resveratrol attenuates early brain injury after subarachnoid hemorrhage through inhibition of NF-𝜅B-dependent inflammatory/MMP-9 pathway. CNS Neuroscience & Therapeutics 20(2): 182 5.
- Jiang T, Harder B, Rojo de la Vega M, Wong P, Chapman E, Zhang D (2015) P62 links autophagy and Nrf2 signaling. Free Radical Biology and Medicine B 88: 199-204.
-
Leta Melaku*. Source of Free Radicals and Consequences of Oxidative Stress Following Secondary Brain Injury after Intracerebral Haemorrhage. Arch Neurol & Neurosci. 15(1): 2023. ANN.MS.ID.000854.
-
Decompression Surgery, Neurosurgery, Anesthesiology, Medical Science, Bupivacaine, Pain relief, Lumbar decompression surgery, Neural complications, Nerve tissue.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
- Abstract
- Introduction
- Intracerebral Haemorrhage and Oxidative Stress
- Sources of Free Radical in Secondary Brain Injury After Intracerebral Haemorrhage
- Consequences of Oxidative Stress Following Secondary Brain Injury after Intracerebral Haemorrhage
- Conclusion
- Availability of Data and Material
- Competing Interests
- Funding
- Authors’ Contributions
- Acknowledgements
- References






