 Research Article
Research Article
Simultaneous Monitoring of Intracranial Pressure and Cerebral Blood Flow in Patients with Severe Brain Injury
Andriy Sirko1,2*, Dmytro Romanukha1, Vyacheslav Grishin3,4, Igor Yovenko3,5 and Alexander Skripnik6
1Departmentof Nervous Diseases and Neurosurgery, Dnipropetrovsk Medical Academy, Ukraine
2Department Cerebral Neurosurgery, Mechnikov Dnipropetrovsk Regional Clinical Hospital, Ukraine
3Department of Anesthesiology, Dnipropetrovsk Medical Academy, Ukraine
4Department Anesthesiology and Intensive Therapy, Mechnikov Dnipropetrovsk Regional Clinical Hospital, Ukraine
5Department of Intensive Therapy and Polytrauma, Mechnikov Dnipropetrovsk Regional Clinical Hospital, Ukraine
6Dnipropetrovsk Medical Academy, The Ministry of Healthcare of Ukraine, Ukraine
Andriy Sirko, Dnipropetrovsk Medical Academy, the Ministry of Healthcare of Ukraine, Head of Cerebral Neurosurgery Department No. 2, Mechnikov Dnipropetrovsk Regional Clinical Hospital, Ukraine.
Received Date: September 28, 2018; Published Date: October 22, 2018
Abstract
37 patients with severe brain injury (admission Glasgow Coma Score 8 or below) were simultaneously monitored for intracranial and cerebral perfusion pressure and cerebral blood flow using transcranial Doppler ultrasonography. Pearson correlation coefficients were calculated to analyze relationships between quantitative variables. It was established that the increase of intracranial pressure leads to formation of Doppler ultrasonographic pattern of reduced perfusion, which involves relative decrease of mean linear blood flow velocity (primarily due to decrease of end-diastolic blood flow velocity) and increase of peripheral resistivity indices (pulsatility and resistivity indices).
Keywords: Severe brain injury; Cerebral blood flow; Intracranial pressure; Cerebral perfusion pressure
Abbreviation: CBF: Cerebral Blood Flow; CPP: Cerebral Perfusion Pressure; APmean: Mean Arterial Pressure; ICP: Intracranial Pressure; TCDU: Transcranial Doppler Ultrasonography; BI: Brain Injury; AP: Arterial Pressure; LBFV: Linear Blood Flow Velocity; DC: Decompressive Craniectomy; SaO2: Saturation; APsyst: Systolic Arterial Pressure; APdiast: Diastolic Arterial Pressure; MCA: Middle Cerebral Artery; LBFVmean: Mean Linear Blood Flow Velocity; LBFVsyst : Systolic Linear Blood Flow Velocity; LBFVdiast: Diastolic Linear Blood Flow Velocity; PI: Pulsatility Index; RI: Resistivity Index; М: Mean Value; SD: Standard Deviation; CI: Confidence Interval
Introduction
The main doctrine of cerebrovascular physiology is that the uninterrupted supply of metabolic substrates to the brain is ensured by constant cerebral blood flow (CBF) at the level of cerebral perfusion pressure (CPP) within certain value range. CPP is a difference between mean arterial pressure (APmean) and intracranial pressure (ICP) [1-3]. CBF remains relatively constant in the CPP value range 40 to 150 mmHg. There is a dynamic system of arterial vasocon striction and vasodilation to maintain normal CBF level. In case of increased ICP, CPP as low as 40-60 mmHg leads to decrease of CBF and lower limit of its autoregulation [1,4]. Increase of ICP causes mechanical compression of cerebral veins. Veins compression leads to compensatory dilatation of cerebral arteries to maintain CBF. Therefore, a “vicious circle” is created when the increase of brain blood volume creates additional volume in cranial cavity causing further ICP growth [5,6].
CBF monitoring using transcranial Doppler ultrasonography (TCDU) allows to dynamically assess the condition of the CBF system, diagnose different pathologic conditions and forecast complications related to secondary brain injuries. Complications may occur due to disturbance of CBF regulation and volumetric ratios inside the scull, which are interrelated [7-9]. Notwithstanding the long history of studying CBF in severe brain injury (BI), many issues remain not studied thoroughly and the data have many contradictions [10-12]. Mostly it is caused by different approaches in assessment of nature and severity of brain injury, use of different devices and methods for determining CBF, and heterogeneous changes in CBF even in one hemisphere [13].
In view of the above, we defined the following study objective: study the nature of relationship between pressure parameters (ICP, AP, CPP) and indices of CBF measured with TCDU. The following study tasks were defined to achieve the objective:
a. Study the structure of variables relationships measured using TCDU and ICP monitoring.
b. Identify parameters by which groups of patients with and without intracranial hypertension are distinguished.
c. Identify parameters by which groups of patients with low and high values of mean linear blood flow velocity (LBFV) are distinguished.
Materials and Methods
The study included 37 patients with severe BI (with admission Glasgow Coma Score of 8 or below), which underwent medical treatment in Public Institution, Mechnikov Dnipropetrovsk Regional Clinical Hospital from 2012 to 2016 inclusive. 32 men and 5 women aged 16 to 65 (average, 34.8±14.3) were examined.
The examined group consisted of 8 patients with diffuse and 29 patients with focal injury (intracranial hematomas, volume 25cm3 and above). Focal injury patients included 18 patients with subdural hematomas, 5 patients with intracerebral hematomas, 4 patients with multiple hematomas, and one patient with epidural hematoma. Intracranial pressure sensors were initially placed in all patients. 31 patients underwent standard decompressive craniectomy (DC) to treat intracranial hypertension (ICH). In 6 cases, the operation was limited to sensor placement with subsequent ICH treatment.
Patients were treated given the indications of multimodal neurophysiological monitoring which included monitoring of ICP, APmean, CPP, saturation (SaO2), and cerebral blood flow using TCDU. ICP was measured with parenchymal sensors. Sensors were placed on the side opposite to the affected area (on the “healthy side”) in an operating room. In all the cases, sensors were placed in Kocher’s point (2 cm anterior to coronal suture and 2 cm lateral to sagittal line) 3-4 cm deep in the frontal lobe. A sensor, placed into the brain parenchyma, was connected with Spiegelberg’s (Hamburg, Germany) Brain Pressure Monitor REF HDM 26.1/FV500, ICP was constantly monitored intra- and postoperatively. ICP monitor was connected to a personal computer (laptop) via 232 interfaces. Spiegelberg collection program, version 7 was used, which allowed to visually study a wave form, save and process the received data. Each value represented a median of 12 ICP values for a past minute, taken once per 5 sec. During TCDU, mean, systolic, diastolic and pulse ICP were automatically recorded. The data were saved as Excel table.
Arterial pressure was measured with an oscillometric method using YuM-300 (ЮМ-300) monitor (Yutas, Ukraine). Mean (APmean), systolic (APsyst), and diastolic (APdiast) arterial pressures were recorded. At the same time, CPP was measured, which provided general indication of cerebral perfusion. CPP was calculated as a difference between APmean and ICP. TCDU was carried out using a portable device, Sonomed 300P produced by SPECTROMED (Russia). In Doppler sonography, М1 and М2 segments of the middle cerebral artery (MCA) were insonated on both sides from anteriofrontal ultrasound window at the depth of 55–70 mm with 2 MHz sensor with determination of mean (LBFVmean), systolic (LBFVsyst) and diastolic (LBFVdiast) linear blood flow velocity. Gosling’s Pulsatility Index (PI) was calculated using the formula [14]:
PI = (LBFVsyst = LBFVdiast)/LBFVmean.
Pourcelot Resistive Index (RI) was calculated using the formula [14]:
RI = (LBFVsyst - LBFVdiast)/LBFVsyst
13 patients died within one month after the surgery; postoperative lethality was 35.1% in the study group.
Table 1:Testing normality of variables distribution using Shapiro-Wilk test.
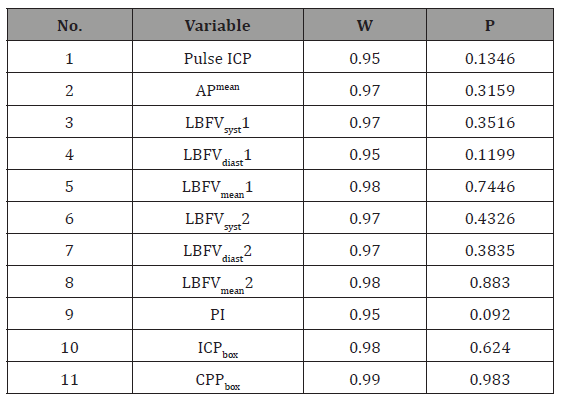
The obtained data were statistically processed using Statistica 6.0 in accordance with key study tasks. The sample included data of one measurement per patient. For each of 37 patients, indices of the first ICP, AP, CPP. and CBF measurement were taken. Normality of variables distribution and equality of dispersion were tested with Shapiro-Wilk test to justify the possibility of using parametric methods of analysis. Pulse ICP, APmean, all LBFVs (LBFVmean, LBFVsyst, LBFVdiast) and PI on healthy and affected sides were distributed normally. It was established that ICP and CPP were not distributed normally. ICP and CPP variables were transformed with Box Cox method. New ICPbox and CPPbox variables were distributed normally. The results of normality of variables distribution testing are specified in Table 1.
Notes: LBFVmean1 = LBFVmean on the sensor’s side; LBFVmean2 = LBFVmean on the side opposite to the sensor; W = test statistics (specifically formed sums ratio); p = class 1 error probability. At р>0.05, a hypothesis of normality is rejected, a variable is considered normally distributed.
To solve the first task, a correlation matrix was calculated and statistically significant relationships between variables were determined. Pearson correlation coefficients were calculated to analyze relationships between quantitative variables. To solve the second and third tasks, specific hypotheses of samples differences were developed and tested. 20 mmHg was taken as the ICP limit and 60 cm/sec as the LBFV limit. 29 patients had ICP below 20 mmHg and 8 had ICP above 20 mmHg. 22 patients had LBFV below 60 cm/sec and 15 had LBFV above 60 cm/sec. Independent samples Student’s t-test was used for normally distributed variables. Variables values are specified as a mean value (M) and a standard deviation (SD) and in the form of 95% confidence interval [95% CI].
Results and Discussion
Statistically significant (р<0.05) relationships were revealed between mean ICP and PI both on the sensor’s (r=0.37) and the opposite side (r=0.4) (Table 2).
Table 2:Pearson correlation matrix for basic quantitative variables
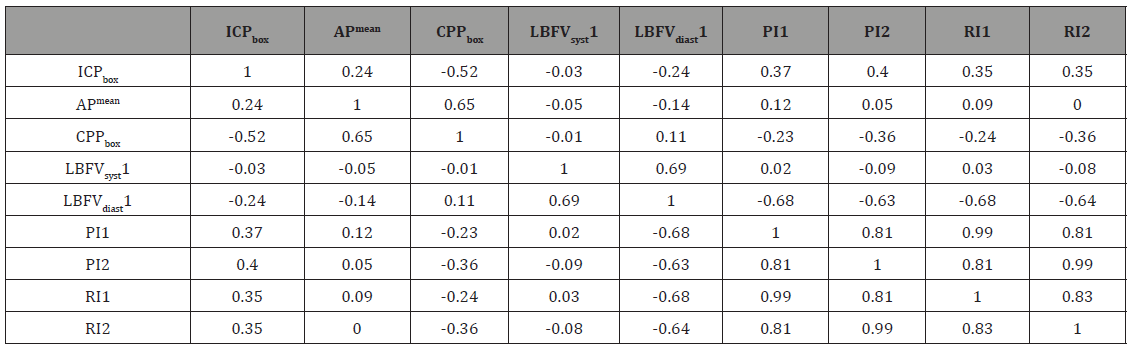
We have confirmed the existence of significant negative correlation between mean ICP and CPP (r=-0.52). LBFVsyst on both sides was not statistically significantly associated with ICP. Statistically significant correlation coefficients were also determined for the following couples of variables: CPP and PI on the side opposite to a sensor (r=-0.36), CPP and RI on the side opposite to a sensor (r=-0.36). It was established that LBFVdiast on the side opposite to a sensor is significantly associated with CPP (r=0.41). LBFV on the sensor’s side was not related with CPP. Strong direct correlation relationship between LBFVsyst and LBFVdiast was found both on the sensor’s (r=0.69) and opposite side (r=0.74). Statistically significant relationships between the two sides were revealed for PI (r =0.47), LBFVsyst (r =0.37), and LBFVdiast (r=0.67). Statistically significant relationships between LBFV and APmean, ICP and APmean were not detected.
Samples of patients with and without intracranial hypertension (ICP above 20 mmHg) were statistically significantly different in terms of the following variables: CPPbox, PI1 and PI2, RI1 and RI2. In ICH patients, PI on the sensor’s side was 092±0.27 (95%CI, 0.69:1.14) and in no-ICH patients, 0.64±0.3 (CI 95%, 0.53:0.76) (р=0.0239). PI on the side opposite to a sensor in ICH patients was 1.07±0.37 (95%CI, 0.77:1.38); in no-ICH patients, 0.66±0.27 (CI 95%, 0.56:0.76) (р=0.0012).
Low and high LBFV (above 60 cm/sec) patient samples had statistically significant difference in APmean and APdiast, PI, and RI. In a group of patients with LBFV below 60 cm/sec, APmean was 94.72±9.31; in a group of patients with LBFV above 60 mmHg, 87.23±8.79 (р=0.026). In a group of patients with LBFV below 60 cm/sec, PI was 0.65±0.33; in a group of patients with LBFV above 60 mmHg, PI was 0.9±0.29 (р=0.026). It can be assumed that the lack of statistically significant relationship between APmean and LBFV based on Pearson correlation coefficient calculation was due to non-linear nature of such relationship.
Conclusion
The increase of intracranial pressure leads to formation of Doppler ultrasonographic pattern of reduced perfusion, featuring relative decrease of mean linear blood flow velocity (primarily due to decrease of end-diastolic blood flow velocity) and increase of peripheral resistivity indices (pulsatility and resistivity indices). Differences in correlation coefficients between ICP, CPP and values of cerebral blood flow on the sensor’s side and the opposite side indicate multidirectional changes of CBF autoregulation. ICH forecast is possible based on PI analysis in two brain hemispheres.
Acknowledgemnet
None.
Conflict of Interest
No conflict of interest.
References
- Abraham P, Rennert RC, Gabel BC, Sack JA, Karanjia N, et al. (2017) ICP Management in patients suffering from traumatic brain injury: a systematic review of randomized controlled trials. Acta Neurochir 159(12): 2279-2287.
- Tang A, Pandit V, Fennell V, Jones T, Joseph B, et al. (2015) Intracranial pressure monitor in patients with traumatic brain injury. J Surg Res 194(2): 565-570.
- Tsai TH, Huang TY, Kung SS, Su YF, Hwang SL, et al. (2013) Intraoperative intracranial pressure and cerebral perfusion pressure for predicting surgical outcome in severe traumatic brain injury. Kaohsiung J Med Sci 29(10): 540-546.
- Sauvigny T, Göttsche J, Czorlich P, Vettorazzi E, Westphal M, et al. (2018) Intracranial pressure in patients undergoing decompressive craniectomy: new perspective on thresholds. Journal of Neurosurgery 128(3): 819-827.
- Lang EW, Kasprowicz M, Smielewski P, Santos E, Pickard J, et al. (2015) Short pressure reactivity index versus long pressure reactivity index in the management of traumatic brain injury. J Neurosurg 122(3): 588-594.
- Han J, Yang S, Zhang C, Zhao M, Li A (2016) Impact of Intracranial Pressure Monitoring on Prognosis of Patients with Severe Traumatic Brain Injury: A PRISMA Systematic Review and Meta-Analysis. Medicine (Baltimore) 95(7): e2827.
- Biersteker HA, Andriessen TM, Horn J, Franschman G, van der Naalt J, et al. (2012) Factors influencing intracranial pressure monitoring guideline compliance and outcome after severe traumatic brain injury. Crit Care Med 40(6): 1914-1922.
- Xie Q, Wu H, Yan Y, Liu M, Wang ES (2017) Mortality and Outcome Comparison Between Brain Tissue Oxygen Combined with Intracranial Pressure/Cerebral Perfusion Pressure-Guided Therapy and Intracranial Pressure/Cerebral Perfusion Pressure-Guided Therapy in Traumatic Brain Injury: A Meta-Analysis. World Neurosurg 100: 118-127.
- Sánchez-Porras R, Santos E, Czosnyka M, Zheng Z, Unterberg AW, et al. (2012) “Long” pressure reactivity index (L-PRx) as a measure of autoregulation correlates with outcome in traumatic brain injury patients. Acta Neurochir (Wien) 154(9):1575-1581.
- Zhang D, Xue Q, Chen J, Dong Y, Hou L, Jiang Y, et al. (2017) Decompressive craniectomy in the management of intracranial hypertension after traumatic brain injury: a systematic review and meta-analysis. Scientific Reports 7(1): 8800.
- Shen L, Wang Z, Su Z, Qiu S, Xu J, et al. (2016) Effects of Intracranial Pressure Monitoring on Mortality in Patients with Severe Traumatic Brain Injury: A Meta-Analysis. PLoS One 11(12): e0168901.
- Bor-Seng-Shu E, Figueiredo EG, Amorim RL, Teixeira MJ, Valbuza JS, et al. (2012) Decompressive craniectomy: a meta-analysis of influences on intracranial pressure and cerebral perfusion pressure in the treatment of traumatic brain injury. J Neurosurg 117(3): 589-596.
- Gosling RG, King DH (1974) Continuous wave ultrasound as an alternative and complement to X-rays in vascular examination. In: Reneman RS (edn), Cardiovascular applications of ultrasound, pp. 262-282.
- Pourcelot L (1975) Indications of Doppler’s ultrasonography in the study of peripheral vessels. Rev Prat 21(12): 4671-4680.
-
Sirko A, Romanukha D, Grishin V, Yovenko I, et al. Simultaneous Monitoring of Intracranial Pressure and Cerebral Blood Flow in Patients with Severe Brain Injury. Arch Neurol & Neurosci. 1(3): 2018. ANN.MS.ID.000515.
-
Severe brain injury, Cerebral blood flow, Intracranial pressure, Cerebral perfusion pressure, Doppler ultrasonography, Physiology, Cerebrovascular, Vicious circle, Pathologic conditions, Pressure sensors, Cerebral blood flow, Parenchymal sensors, Brain pressure monitor, Intracranial hypertension, Pulsatility, Resistivity indices
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






