 Research Article
Research Article
Senegalese Experience of Acute Polyradiculoneuritis: Cross-Sectional Study Over 7 Years at the Department of Neurology in Fann Teaching Hospital
Adjaratou Dieynabou Sow*, Wassim Aidibe, Anna Modji Basse, Halladain Mpung Mansoj, Ngor Side Diagne, Marième Soda Diop, Ndiaga Matar Gaye, Maouly Fall, Abdou Aziz Fall, Ousmane Cisse, Lala Bouna Seck, Moustapha Ndiaye, Amadou Gallo Diop and Kamadore Touré
Department of Neurology, Fann National Teaching Hospital, Dakar – Senegal
Adjaratou Dieynabou Sow, Department of Neurology, Fann National Teaching Hospital, Dakar, Senegal
Received Date: November 13, 2023; Published Date: November 23, 2023
Abstract
Introduction: Acute PRNs are a group of inflammatory conditions of the peripheral nerves of which GBS is the most typical. The objective of this work is to determine the epidemiological, clinical, paraclinical, etiological and therapeutic aspects of acute PRNs.
Methodology: We conducted a retrospective, descriptive and analytical study from January 1, 2013, to December 31, 2020 based on 34 cases recruited at the I.P Ndiaye neuroscience clinic of Fann teaching hospital in Dakar.
Results: The hospital prevalence was 0.52%. The average age was 39.4 years, there was no gender difference. A prodromal event preceded the onset of neurological signs in 58.8% of patients, it was an infectious event in 44.1% of patients. At the clinical level, the symptomatology was typical, i.e, a bilateral, symmetrical extensive motor deficit with an upward progression in less than 4 weeks, with abolition of ROT. The ENMG confirmed the diagnosis of acute PRN in all our patients, the majority of whom were demyelinating (58.8%). Lumbar puncture showed albuminocytological dissociation in 76.5% of patients. All patients received symptomatic treatment, and specific treatment consisted of immunoglobulins in 8.8% of patients and corticosteroid therapy in 47% of patients. The course was favourable in 64.7% of patients but was fatal in 1 patient (2.9%).
Conclusion: GBS is an uncommon neuropathy whose early diagnosis is based mainly on clinical and EMG conditions the prognosis of patients.
Keywords:Polyradiculoneuritis; Guillain-barre; Fann teaching hospital
Letter to Editor
Acute polyradiculoneuropathy (APRN) is a disease of the peripheral nervous system, of autoimmune origin,primarily affecting the myelin sheath. It is a so-called rare disease that can start at any age. APRNs are a heterogeneous group of which Guillain- Barré syndrome is the most common form. The typical form is characterized by the rapid, progressive, even abrupt onset over a few days of a symmetrical proximo-distal sensory-motor deficit of the 4 limbs of upward evolution with involvement of the bulbar cranial nerves, making the severity of the disease with the need for hospitalization in intensive care. The prognosis of the pathology has been greatly improved by the advent of respiratory mechanical ventilation and the development of immunotherapy and plasma exchange. In Africa, and particularly in Senegal, the unavailability of new therapies restricts management to treatments that are contraindicated but often remain the only local alternative to offer. We report our experience through a series of patients collected over 7 years (2013-2020) to evaluate the epidemio-clinical-paraclinical, therapeutic and evolutionary aspects.
Patients and Methods
We performed a retrospective descriptive and analytical study from January 1, 2013, to December 31, 2020 concerning patients hospitalized at the Ndiaye IP Neuroscience Clinic of the Fann teaching hospital in Dakar for an aspect APRN with peripheral neurogenic syndrome of ascending extension over less than 4 weeks with a ENMG in favor. Data were collected on the basis of a pre-established questionnaire listing socio-demographic, clinical, paraclinical (biology and electrophysiology), therapeutics and evolutionary with analysis using SPSS software version 17.0.
Results
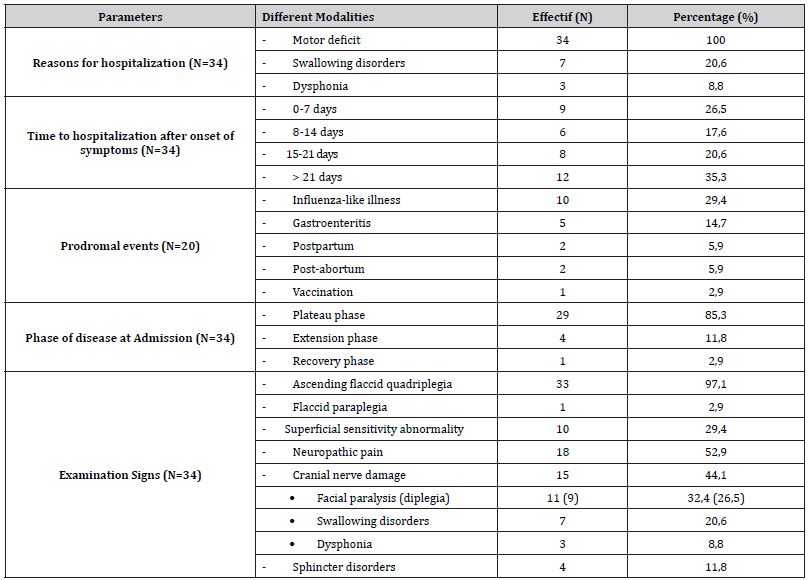
During the study period, 6517 patients were hospitalized in the neurological department, including 43 cases suggestive of APRN but 9 were eliminated for rapid death before assessment (4 cases), incompleteness of the file (2 as), associated comorbidity (2 cases of associated encephalitis) and 1 case of uncertain diagnosis. Finally, 34 patients were included for a hospital prevalence of 0.52% with extremes of 0.17% in 2014 and 1.42% in 2019; Figure 1 shows the epidemiological distribution of cases across the seven years of study. The modal age range of 31-40 years and the extremes of 10 and 79 years. The sex ratio was 1 with age variability by gender; The mean age of patients was 39.4 years, compared with 45.8 years for men and 33.1 years for women. Before the age of 40, women predominated, and after the age of 40, men were in the majority, as shown in Figure 2. Table 1 summarizes all the clinical parameters of our patients; however, there were also haemodynamic abnormalities, including tachycardia (44.1%), hypertension (14.7%) and dyspnea (8.8%) (Figures 1, 2 and Table 1).
Table 1:Summary of clinical parameters of our study population.


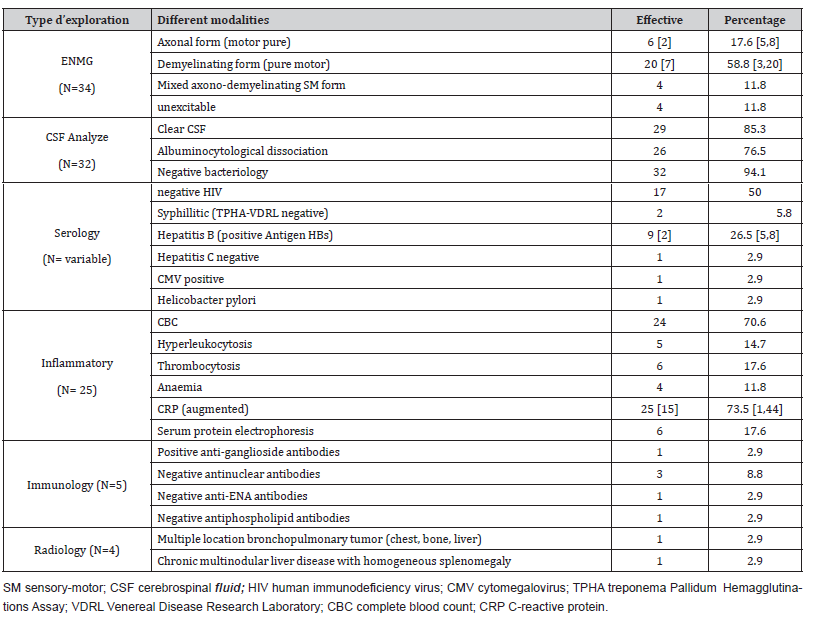
At the paraclinical level, electroneuromyography, lumbar puncture, and inflammatory assessment were performed in almost all patients and the remainder of the etiological assessment on a case-by-case basis, as summarized in Table 2.
Table 2:Summary of paraclinical data from our study population.
The etiological classification resulted in GBS in 33 patients (97.1%), including 3 in whom a causative agent was identified: CMV in 1 patient and hepatitis B virus in 2 patients. One patient had secondary acute PRN of paraneoplastic origin (2.9%). The length of hospital stay ranged from 1 to 45 days with an average of 18.5 days. Only one patient was admitted to intensive care for respiratory distress. Management was mainly symptomatic with nursing and bladder catheterization (100%), nasogastric tube (20.6%), preventive anticoagulation with Enoxaparin (38.2%), treatment of neuropathic pain with Pregabalin (8 cases), Clomipramine (6 cases), Carbamazepine (4 cases) and Amitriptyline (2 cases) and physiotherapy in 25 patients (73.5%). Corticosteroid therapy was used as the main specific treatment in 16 patients (47%) with Prednisolone 60mg/day or Solumedrol bolus 120mg/day over 3 days. A course of IV immunoglobulin at 0.4g/kg over 5 days was administered in 3 patients (8.8%). Figure 3 shows the mostly favorable outcome in our patients (Figure 3).

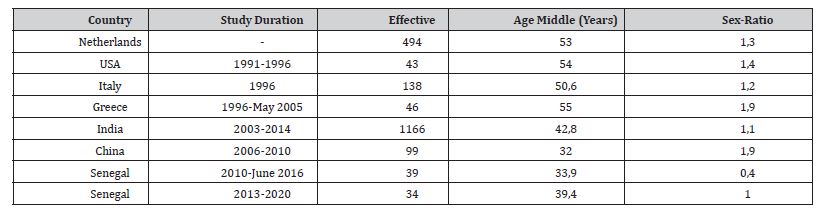
Table 3:Epidemiological aspects of APRNs in the literature.
Discussion
We have identified 34 cases of PRNA out of a total of 6517 hospitalizations in 7 years, making a hospital prevalence of 0.52%, below that of Basse et al from 2010 to 2016 which was 1.44%. The latter is close to the annual prevalence of 2019 which was 1.52%, probably in relation to the Covid 19 pandemic where there was a relative upgrowth, empirically noted, in elderly subjects and children with increasing calls for advice from geriatric and paediatric services, not included in this cohort. Table 3 reviews the socio-demographic particularities of PRNA populations in different countries, showing a lower average age in developing countries such as Senegal, in relation to the youth of the general population, in contrast to the ageing of European populations. Also, we noted a higher frequency in women before the age of 40, unlike men after the age of 40. This fits perfectly with the pathophysiology of autoimmune diseases, which are more common in young women because they are intertwined with hormones. Thus, APRNs increase within 2 weeks postpartum in relation to the overall increase in pro-inflammatory cytokines, as noted in 4 of our patients [35].
Two-thirds of GBS cases are preceded by acute infection within 15 days prior to the onset of neurological signs. In the literature, it is found between 0.3 and 4% of patients with a prodromal event of vaccination or serotherapy [3,21]; as in our study (2.9%). An influenza vaccination campaign in the United States between 1976 and 1977 was followed by an increase in the incidence of GBS [20]. However, no link has been formally established between influenza vaccine and GBS [11]. In our study, we noted a delay in patient consultation: nearly 75% had consulted more than one week after the onset of neurological signs, including 35% after 3 weeks. Hence the vast majority of patients (85.3%) were in the plateau phase at admission. This delay in consultation may be correlated with the mode of progression of the disease but may be attributable to other factors, including:
a) The remoteness of our patients, 85.3% of whom came from a peri-urban or rural area and first consulted in a local health centre before a referral if necessary to other structure.
b) The low socio-economic level of our patients who consult only when they are perceived to be serious.
c) Belief in traditional practices to the detriment of medical treatment.
Clinically, sensory disturbances were found in 70.6% of patients and neuropathic pain, a disabling symptom of acute PRNs, was found in 52.9%; This is close to the figures found by Markoula et al. (52.2%) [13] and Fokke et al. (54%) [7]. Cranial nerve involvement was present in 44.1% of patients. In the literature, extremes of 34.9% and 57% were found [21,23]. The facial nerve remains the most affected nerve: 32.4% in our patients and 34% respectively in the studies by Bogliun et al. [3] and Sudulagunta et al. [23]. In the study by Dhar et al, 71% of patients entered intensive care for acute respiratory failure and only 11% for rapid extension of motor deficit [5], whereas it concerned only one of our patients, probably due to * the late admission of our patients to the plateau phase; * early death of patients with respiratory distress who were not included in our study despite an acute PRN picture; * respiratory failure in the face of neurological symptomatology that leads these patients directly to the intensive care unit, which may indicate a lack of collaboration between neurologists and intensive care specialists.
Electroneuromyography helps with classification. Acute inflammatory demyelinating polyradiculonevitis (AIDP) is the most common classic form of GBS. This form was found in 58.8% of our patients. In the literature, the proportion of AIDP ranges from 24% to 84.8% [10,13]. In the 1990s, an acute motor axonal form of GBS called AMAN was first described in northern China [10]. The frequency of this form varies from country to country. It is more common in Asia, reaching 56% in Bangladesh [28], 47% in Japan [17] and up to 65% in China [10]. Our study found the axonal shape in 5 patients (17.6%), of whom 2 had pure motor impairment (5.9%). The AMAN form therefore affected 5.9% of patients. Finally, mixed axono-demyelinating and inexcitable forms were found in 11.8% of patients each. Albuminocytological dissociation (ADC) defined by proteinorachy greater than 0.5 g/l and cellularity less than 10 elements/mm³ was found in 81.2% of patients who performed a lumbar puncture. The same is true of the Italian studies with 83% of their cases [3,4], but well below Basse et al. who found CAD in 45.4% of his patients [2].
From a therapeutic point of view, hospitalization was systematic in all our patients, as shown in the literature. The mean length of hospital stay in our study was 18.5 days with extremes of 1 and 45 days, as for Markoula et al. with a mean length of hospital stay of 17.5 days [13]. All of our patients received symptomatic treatment, while 17 patients (50%) received specific treatment with corticosteroid therapy in 47% of patients despite evidence of its ineffectiveness. The largest therapeutic trial randomized 242 patients receiving IV methylprednisolone 500 mg (118 patients) versus placebo (118 patients) for 5 days [9]. No significant differences were shown between the two groups with respect to motor deficit. The study concluded that a brief, high dose of IV corticosteroids at an early stage of the disease was ineffective [9]. In our study, the frequent use of corticosteroid therapy could be related to the inaccessibility of IVIG, due to its high price for our patients or its immediate unavailability. These were used in only 3 patients (8.8%), 2 of which were in a postpartum setting. In our study, the frequent use of corticosteroid therapy could be related to the inaccessibility of IVIG, due to its high price for our patients or its immediate unavailability.
These were used in only 3 patients (8.8%), 2 of which were in a postpartum setting. As plasma exchange (PE) is not available in Senegal outside of the management of sickle cell disease in children, no patient has benefited from it. According to recent data, IVIG and PE have a similar ability to accelerate recovery time in GBS and the combination treatment does not produce any significant difference compared to either therapy alone [6]. In the patients who received IVIG, only one had partial recovery of motor skills while in the other 2, no recovery was noted. This can be explained by the late delay in consultation, who had consulted 21 and 29 days respectively after the onset of neurological signs. However, IVIG treatment is all the more effective the earlier it is started, so it is recommended to start it during the extension phase of the disease [19].
Acknowledgement
None.
Conflict of Interest
No Conflict of interest.
References
- Alam TA, Chaudhry V, Cornblath DR (1998) Electrophysiological studies in the Guillain-Barré syndrome: distinguishing subtypes by published criteria. Muscle Nerve 21(10): 1275-127
- Albers JW, Donofrio PD, McGonagle TK (1985) Sequential electrodiagnostic abnormalities in acute inflammatory demyelinating polyneuropathy. Muscle Nerve 8(6): 528-539.
- Alshekhlee A, Hussain Z, Sultan B, Katirji B (2008) Guillain-Barre syndrome: incidence and mortality rates in US hospitals. Neurology 70 (18): 1608-1613.
- Asbury AK, Cornblath DR (1990) Assessment of current diagnostic criteria for Guillain-Barré Ann Neurol 27(suppl): S21-S24.
- Azulay JP, Verschueren A, Attarian S, Pouget J (2002) Le syndrome de Guillain Barre et ses frontiè Rev neurol (Paris); 158 suppl 123: 21-26.
- Basse AM, Boubacar S, Sow AD, Diagne NS, Diop MS, et al. (2017) Epidemiology of acute polyradiculoneuritis at Fann department of neurology Dakar, Senegal. Clinical Neurology and Neuroscience1(4): 76-79.
- Bersano A, Carpo M, Allaria S, Franciotta D, Citterio A, et al. (2006) Long term disability and social status change after Guillain-Barré J Neurol 253(2): 214-218.
- Bogliun G, Beghi E (2004) Incidence and clinical features of acute inflammatory polyradiculoneuropathy in Lombardy, Italy, 1996. Acta Neurol Scand 110(2): 100-106.
- Brown WF, Feasby TE, Hahn AF (1993) Electrophysiological changes in the acute axonal form of Guillain-Barré Muscle Nerve 16: 200-205.
- Chiò A, Cocito D, Leone M, Giordana M T, Mora G et al. (2003) Guillain-Barré syndrome: a prospective, population-based incidence and outcome survey. Neurology 60(7): 1146-1150.
- Chroni E, Thomopoulos C, Papapetropoulos S, Paschalis C, Karatza CL (2003) A Case of Relapsing Guillain-Barré Syndrome associated with exacerbation of chronic Hepatitis B Virus J Neurovirol 9(3): 408-410.
- Cosi V, Versino M (2006) Guillain-Barré Neurol Sci 27: S47-S51.
- Dhar R, Stitt L, Hahn AF (2008) The morbidity and outcome of patients with Guillain-Barré syndrome admitted to the intensive care unit. J Neurol Sci 264(1-2): 121-128.
- Van Doorn PA, Ruts L, Jacobs BC (2008) Clinical features, pathogenesis, and treatment of Guillain-Barré Lancet Neurol 7(10): 939-950.
- Fokke C, Van Den Berg B, Drenthen J, Walgaard C, Antoon van Doorn P (2014) Diagnosis of Guillain-Barré syndrome and validation of Brighton criteria. Brain 137(Pt 1): 33-43.
- Forsberg A, Press R, Einarsson U, De Pedro-Cuesta J, Widén Holmqvist L (2004) Impairment in Guillain- Barré syndrome during the first 2 years after onset : a prospective study. J Neurol Sci 227(1): 131-138.
- Fournier E (2013) Polyradiculonévrites et polyneuropathies démyé Syndrome d’atteinte des nerfs et des muscles. Lavoisier 15: 149-153.
- Fross RD, Daube JR (1987) Neuropathy in the Miller-Fisher syndrome: clinical and electrophysiologic findings. Neurology 37(9): 1493-1498.
- Gigli GL, Bax F, Marini A, Pellitteri G, Scalise A, et al. (2021) Guillain Barré syndrome in the COVID19 era: just an occasional cluster? J Neurol 268(4): 1195-1197.
- Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B (2004) Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 75(8): 1135-1140.
- (1993) Double-blind trial of intraveinous methylprednisolone in Guillain-Barré Guillain-Barré Syndrome Steroid Trial Group. Lancet 341(8845): 586-590.
- Hadden RD, Cornblath RD, Hughes RA, Zielasek J, Hartung HP (1998) Electrophysiological classification of Guillain Barre syndrome. Electrophysiological classification of Guillain-Barré syndrome: clinical associations and outcome. Ann Neurol 44(5): 780-788.
- Hahn AF (1998) Guillain-Barre Lancet 352 (9128): 635-641.
- Ho TW, Mishu B, Li CY, Gao CY, Cornblath DR (1995) Guillain-Barré syndrome in northern China. Relationship to campylobacter jejuni infection and anti-glycolipid antibodies. Brain 118 (Pt 3): 597-605.
- Ho TW, Willison HJ, Nachamkin I, Li CY, Veitch J (1999) AntiGD1a antibody is associated with axonal but not demyelinating forms of Guillain-Barré Ann Neurol 45(2): 168-173.
- Hughes RA, Cornblath DR (2005) Guillain-Barré Lancet 366: 1653-1666.
- Iqbal S, Li R, Gargiullo P (2015) Relationship between Guillain-Barré syndrome, influenza-related hospitalizations, and influenza vaccine coverage. Vaccine 33 (17): 2045-2049.
- Islam Z, Jacobs BC, Van Belkum A, Kevin Klaij, Herbrink P, et al. (2010) Axonal variant of Guillain-Barré syndrome associated with Campylobacter infection in Bangladesh. Neurology 74: 580-587.
- Jacobs BC, Rothbarth PH, van der Meché FG, Herbrink P, et al. (1998) The spectrum of antecedent infections in Guillain- Barré syndrome: a case-control study. Neurology 51(4):1110-1105.
- Kelly JJ, Karcher DS (2005) Lymphoma and peripheral neuropathy : a clinical review. Muscle Nerve 31: 301-313.
- Koga M, Yuki N, Hirata K (2001) Antecedent symptoms in Guillain-Barre syndrome: an important indicator for clinical and serological subgroups. Acta Neurol Scand 103 (5): 278-287.
- Markoula S, Giannopoulos S, Sarmas I (2007) Guillain-Barré syndrome in northwest Greece. Acta Neurol Scand 115(3): 167-173.
- McKhann GM, Cornblath DR, Ho TW et (1991) Clinical and electrophysiological aspects of acute paralyticdisease of children and young adults in northern China. Lancet 338(8767): 593-597.
- Van der Meche FG, Schmitz PI (1992) A randomized trial comparing intravenous immuneglobulin and plasma exchange in Guillain-Barre syndrome. Dutch Guillain-Barre Study Group. N Engl J Med 326 (17): 1123-1129.
- Meenakshi-Sundaram S, Swaminathan K, Karthik SN, Bharathi S, et al. (2014) Relapsing Guillain-Barre syndrome in pregnancy and postpartum. Ann Indian Acad Neurol 17(3): 352-354.
- Nishimoto Y, Odaka M, Hirata K, Yuki N (2004) Usefulness of anti-GQ1b IgG antibody testing in Fisher syndrome compared with cerebrospinal fluid J Neuroimmunol 148(1-2): 200-205.
- Novy J, Kuntzer T (2007) Prise en charge des polyradiculoneuropathies aiguës et chroniques. Rev Med Suisse 3: 32273.
- Ogawara K, Kuwabara S, Mori M, Hattori T, Koga M, et al. (2000) Axonal Guillain Barré syndrome: relation to antiganglioside antibodies and Campylobacter jejuni infection in Japan. Ann Neurol 48(4): 624-631.
- Pan C-L, Tseng T-J, Lin Y-H, Chiang M-C, Lin W-M, et al. (2003) Cutaneous innervation in Guillain-Barré syndrome: pathology and clinical correlations. Brain J Neurol 126 (Pt 2): 386-397.
- Radziwill AJ, Kuntzer T, Steck AJ (2002) Immunopathology and treatments of Guillain-Barré syndrome and of chronic inflammatory demyelinating polyneuropathy. Rev Neurol (Paris) 158(3): 301-310.
- Rajabally YA, Durand MC, Mitchell J, Orlikowski D, Nicolas G (2015) Electrophysiological diagnosis of Guillain-Barré syndrome subtype: could a single study suffice? J Neurol Neurosurg Psychiatry 86 (1): 115-119.
- Ropper AH (1992) The Guillain-Barre N Engl J Med 326(17): 1130-1136.
- Ruts L, Drenthen J, Jongen JL, Hop WCJ, Visser GH, et (2010) Pain in Guillain-Barre syndrome: a long-term follow-up study. Neurology 75(16): 1439-1447.
- Said G, Goulon-Goeau C (2002) Syndrome de Guillain Barré. Encyclopédie médico-chirurgicale, éditions scientifiques et médicales. Elsevier SAS, Paris, Neurologie 17-095-A-10: 1-6.
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, Keenlyside RA, Ziegler DW, et al. (1979) Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976-1977. Am J Epidemiol 110(2): 105-1
- Sudulagunta SR, Sodalagunta MB, Sepehrar M, Khorram H, Shiva Kumar BR et (2015) Guillain-Barré syndrome: clinical profile and management. Ger Med Sci 13: Doc16.
- Vigliani MC, Magistrello M, Polo P, Mutani R, Chiò A (2004) Risk of cancer in patients with Guillain-Barre syndrome (GBS). A population-based study. J Neurol 251(3): 321-326.
- Winer JB, Hughes RA, Osmond C (1988) A prospective study of acute idiopathic I. Clinical features and their prognostic value. J Neurol Neurosurg Psychiatry 51(5): 605-612.
- Ye Y, Wang K, Deng F, Xing Y (2013) Electrophysiological subtypes and prognosis of Guillain-Barré syndrome in Northeastern China. Muscle Nerve 47(1): 68-71.
- Zochodne DW (1994) Autonomic involvement in Guillain-Barré syndrome: a review. Muscle Nerve 17(10): 1145-1155.
-
Adjaratou Dieynabou Sow*, Wassim Aidibe, Anna Modji Basse, Halladain Mpung Mansoj and Ngor Side Diagne. Senegalese Experience of Acute Polyradiculoneuritis: Cross-Sectional Study Over 7 Years at the Department of Neurology in Fann Teaching Hospital. Arch Neurol & Neurosci. 16(2): 2023. ANN.MS.ID.000881.
-
Acute Polyradiculoneuritis, Neurology, Guillain-barre; Fann teaching hospital, neuropathy, cranial nerves, immunotherapy, Neuroscience, neurogenic syndrome, epidemiological, Dysphonia.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






