 Mini Review
Mini Review
Predictors of Chronic Subdural Hematoma Recurrence Following Surgical Intervention: A Review of the Recent Literature?
Cory L Chang* and E Sander Connolly Jr
Department of Neurological Surgery, Columbia University Irving Medical Center, United States of America
Cory L Chang, Columbia University Irving Medical Center, 630 W 168th St., VP&S 5-454, New York, NY 10032, United States of America.
Received Date: December 20,2019; Published Date: January 14, 2020
Abstract
Chronic subdural hematoma (CSDH) is an increasingly common neurosurgical condition worldwide. Surgical evacuation is the mainstay of treatment, but re-operation is necessary in 10-20% of patients due to hematoma recurrence. Though risk factors for CSDH formation are welldescribed, there is less consensus regarding CSDH recurrence, due in part to variations in operative procedure and follow-up protocols. Here we review studies from the past three years to update our understanding of predictors of post-operative CSDH recurrence. Well-evidenced threads include advanced age and loculated hematoma on pre-operative imaging, but other candidate risk factors such as anti-thrombotic therapy are still under active debate. Subsequent prospective, controlled studies are needed to further characterize these findings, which will help refine indications and clinical care guidelines for this growing patient population.
Keywords: Chronic subdural hematoma; Subdural hematoma evacuation; Subdural hematoma recurrence
Abbreviations: SDH: Subdural Hematoma; CSDH: Chronic Subdural Hematoma
Introduction
Chronic subdural hematoma (CSDH) has become one of the most common neurosurgical conditions worldwide, with an estimated incidence of up to 20.6 per 100,000 patients per year [1]. Subdural hematoma (SDH) is characterized by an abnormal accumulation of blood products in the subdural space, classically due to the rupture of bridging veins that drain from the cortical surface to the dural sinuses. Unlike acute SDH, CSDH exhibits an indolent course, clinically manifesting weeks or even months after the initial onset of hemorrhage. The pathophysiology of CSDH formation remains unclear, though proposed mechanisms include direct progression from acute hematoma [2], inflammatory dysfunction [3], or repeated sub-clinical microhemorrhage [4]. Its clinical presentation is also variable and may involve the insidious onset of headaches, nausea, cognitive impairment, alterations in consciousness, or seizures [5].
The mainstay for CSDH treatment is neurosurgical evacuation, via burr hole drainage, craniotomy, or craniectomy. Incidentally diagnosed patients who remain asymptomatic can also be managed medically, though 20% deteriorate clinically and ultimately require surgical intervention [6]. Very few cases of CSDH resolve spontaneously without any intervention [7]. However, even with surgery, the recurrence rates of CSDH are high, with 10-20% of patients requiring repeat operations [1]. The heterogeneity of these outcomes has been inconsistently characterized across individual studies in the literature, due in part to discrepancies in defining CSDH, differences in operative procedure, and variations in followup protocols [8]. Here we aim to review studies from the past three years examining predictors of post-operative CSDH recurrence in order to update our understanding of risk stratification and care guidelines for this growing patient population [9].
Discussion
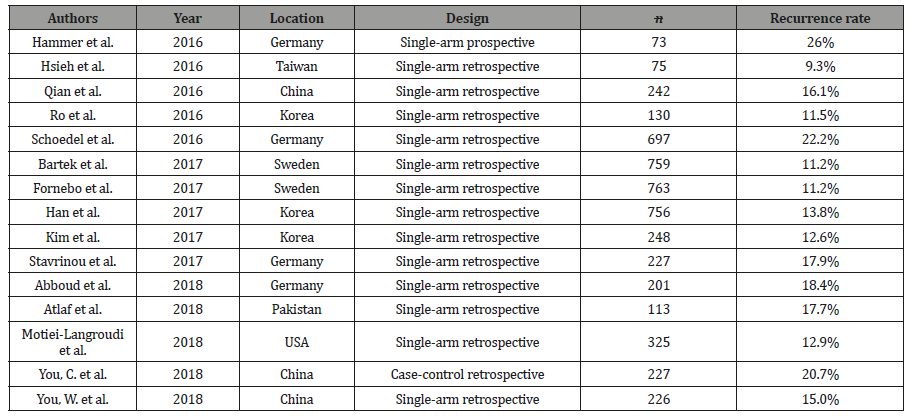
The characteristics of 16 reviewed studies are summarized in Table 1. All but Hammer et al. [10] (single-arm prospective cohort) and You, C. et al. [11] (retrospective case-controlled cohort) were single-arm retrospective cohort studies. Recurrence rates of CSDH ranged from 9.3% to 26% (Table 1).
Table 1:Summary statistics.
Demographics, comorbidities, and anti-thrombotic therapy
Advanced age is a well-known risk factor for the initial formation of CSDH, [1] and this association for CSDH recurrence also appears to be significant, with Schoedel et al., [12] Han et al., [13] and Qian et al. [14] reporting higher rates of recurrence in patients aged ≥75. You, W. et al., [15] Bartek et al., [16] and Stavrinou et al.’s [17] analyses of age as a continuous variable did not yield significance, suggesting that age only becomes predictive of recurrence at the older end of the spectrum. Sex was not found to be an independent predictor of recurrence in any study, though male sex trended towards significance in two studies [16,17].
The great majority of studies reported no associations between CSDH recurrence and medical comorbidities, including hypertension, diabetes mellitus, heart disease, cerebrovascular disease, liver disease, renal failure, malignancy, substance use, and dementia [13,15,17]. The only exception was Kim et al. [18] who found diabetes mellitus to be an independent risk factor for recurrence.
Though anti-thrombotic medication use is a risk factor for CSDH formation, its effect on CSDH recurrence rates remains controversial [1]. Six of eight studies found no association between anti-thrombotic (including anti-platelet and anti-coagulation) therapy at time of presentation and recurrence [13,15,16,17,19,20]. However, Kim et al. [18] found anti-coagulation therapy to be predictive, and Motiei-Langroudi et al. [21] identified warfarin and clopidogrel therapy specifically as independent predictors of CSDH recurrence.
Clinical presentation and pre-operative imaging
Patients whose CSDHs were precipitated by known head trauma were not more likely to experience post-operative recurrence than other patients [15,17]. Headache as a presenting symptom was found to be predictive of recurrence by Hammer et al. [10] but not Bartek et al. [16] No studies reported any significant associations between CSDH recurrence and paresis, speech disturbances, Glasgow Coma Scale, or seizures on presentation. Interestingly, Hammer et al. found presenting aphasia as an independent predictor of recurrence, but none of the other studies included aphasia as a variable in their analyses [10].
With the exception of Stavrinou et al., all other reporting studies found larger hematoma size and loculated-type hematomas to be independently predictive of higher recurrence rates [10,14,21]. Pre-operative homogenous hyperdense hematoma [15] and mixed-density hematoma [16] have also been identified as risk factors for recurrence, while homogeneous isodense hematoma has been identified as a protective factor [10]. The utility of other imaging metrics is not as clear, with a minority of studies finding a midline shift of ≥10mm [14,18] and bilateral hematoma13,16 to be predictors for recurrence. Side of hematoma was not predictive in any study.
Procedure types and post-operative metrics
The majority of studies only included cases of burr hole drainage in their analyses. The remaining four studies found no difference in CSDH recurrence rates among cases of single burr hole drainage, double burr hole drainage, and craniotomy [12,13,17,21]. Type of peri-procedural anesthesia was also not predictive [13]. The use of a subdural drain was found to be protective of CSDH recurrence in two of four studies [10,22] and You, W. et al. also identified longer duration of drainage as a protective factor [15].
Post-operative pneumocephalus was predictive of CSDH recurrence (along with longer hospital stay and poor neurological outcome) in the only case-control study included in this review [11], which is consistent with results of meta-analyses in the past literature [23,24]. Other post-operative metrics were less consistently investigated in the recent literature, with only individual studies finding degree of hematoma drainage [17], residual hematoma density [17], and degree of brain expansion [25] as predictors of CSDH recurrence.
Conclusion
Recurrence rates of CSDH following surgical intervention reported in the past three years remain comparable to those of prior literature despite advancements in care and an increasing incidence of CSDH. Independent predictors of recurrence have been found to encompass a host of pre- and post-operative factors, such as advanced age and hematoma loculation on imaging. Nevertheless, current evidence is insufficient to validate the significance of several other potential predictors, such as antithrombotic therapy at presentation and the use of subdural drains. This review is limited by the retrospective, single-arm nature of the great majority of included studies as well as variations in follow-up protocols (and thus operational definitions of CSDH recurrence), making inter-study comparisons difficult to interpret. Further controlled prospective studies are necessary to broaden our understanding of this heterogenous clinical entity, which will refine surgical indications and clinical care guidelines for this patient population.
Acknowledgement
None.
Conflict of Interest
The authors have no conflicts of interest to report.
References
- Yang W, Huang J (2017) Chronic Subdural Hematoma: Epidemiology and Natural History. Neurosurg Clin N Am 28(2): 205-210.
- Lee KS (2004) Natural history of chronic subdural haematoma. Brain Inj 18(4): 351-358.
- Chen JC, Levy ML (2000) Causes, epidemiology, and risk factors of chronic subdural hematoma. Neurosurg Clin N Am 11(3): 399-406.
- Lee KS (2016) Chronic subdural hematoma in the aged, trauma or degeneration? J Korean Neurosurg Soc 59(1): 1-5.
- Mori K, Maeda M (2001) Surgical treatment of chronic subdural hematoma in 500 consecutive cases: Clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med-Chir 41(8): 371-381.
- Bender MB, Christoff N (1974) Nonsurgical Treatment of Subdural Hematomas. Arch Neurol 31(2): 73-79.
- Parlato C, Guarracino A, Moraci A (2000) Spontaneous resolution of chronic subdural hematoma. Surg Neurol 53(4): 312-317.
- Xu CS, Lu M, Liu LY, Yao MY, Cheng GL, et al. (2017) Chronic subdural hematoma management: clarifying the definitions of outcome measures to better understand treatment efficacy – a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci 21(4): 809-818.
- Kolias AG, Aswin C, Santarius T, Hutchinson PJ (2014) Chronic subdural haematoma: Modern management and emerging therapies. Nat Rev Neurol 10: 570-578.
- Hammer A, Tregubow A, Kerry G, Schrey M, Hammer C, et al. (2017) Predictors for Recurrence of Chronic Subdural Hematoma. Turk Neurosurg 27(5): 756-762.
- You CG, Zheng XS (2018) Postoperative pneumocephalus increases the recurrence rate of chronic subdural hematoma. Clin Neurol Neurosurg 166: 56-60.
- Schoedel P, Bruendl E, Hochreiter A, Scheitzach J, Bele S, et al. (2016) Restoration of Functional Integrity After Evacuation of Chronic Subdural Hematoma—An Age-Adjusted Analysis of 697 Patients. World Neurosurg 94: 465-470.
- Han MH, Ryu JI, Kim CH, Kim JM, Cheong JH, et al. (2017) Predictive factors for recurrence and clinical outcomes in patients with chronic subdural hematoma. J Neurosurg 127(5): 1117-1125.
- Qian Z, Yang D, Sun F, Sun Z (2017) Risk factors for recurrence of chronic subdural hematoma after burr hole surgery: potential protective role of dexamethasone. Br J Neurosurg 31(1): 84-88.
- You W, Zhu Y, Wang Y, Liu W, Wang H, et al. (2018) Prevalence of and risk factors for recurrence of chronic subdural hematoma. Acta Neurochir 160(5): 893-899.
- Bartek J, Sjåvik K, Kristiansson H, Ståhl F, Fornebo I, et al. (2017) Predictors of Recurrence and Complications After Chronic Subdural Hematoma Surgery: A Population-Based Study. World Neurosurg 106: 609-614.
- Stavrinou P, Katsigiannis S, Lee JH, Hamisch C, Krischek B, et al. (2017) Risk Factors for Chronic Subdural Hematoma Recurrence Identified Using Quantitative Computed Tomography Analysis of Hematoma Volume and Density. World Neurosurg 99: 465-470.
- Kim SU, Lee DH, Kim YI, Yang SH, Sung JH, et al. (2017) Predictive Factors for Recurrence after Burr-Hole Craniostomy of Chronic Subdural Hematoma. J Korean Neurosurg Soc 60(6): 701-709.
- Fornebo I, Sjåvik K, Alibeck M, Kristiansson H, Ståhl F, et al. (2017) Role of antithrombotic therapy in the risk of hematoma recurrence and thromboembolism after chronic subdural hematoma evacuation: a population-based consecutive cohort study. Acta Neurochir 159(11): 2045-2052.
- Abboud T, Dührsen L, Gibbert C, Westphal M, Martens T (2018) Influence of antithrombotic agents on recurrence rate and clinical outcome in patients operated for chronic subdural hematoma. Neurocirugia 29(2): 86-92.
- Motiei-Langroudi R, Stippler M, Shi S, Adeeb N, Gupta R, et al. (2018) Factors predicting reoperation of chronic subdural hematoma following primary surgical evacuation. J Neurosurg 129(5): 1143-1150.
- Altaf I, Shams S, Vohra AH (2018) Radiolological predictors of recurrence of chronic subdural hematoma. Pak J Med Sci 34(1): 194-197.
- Liu W, Bakker NA, Groen RJ (2014) Chronic subdural hematoma: A systematic review and meta-analysis of surgical procedures. J Neurosurg 121(3): 665-673.
- Almenawer SA, Farrokhyar F, Hong C, Alhazzani W, Manoranjan B, et al. (2014) Chronic subdural hematoma management: A systematic review and meta-analysis of 34,829 patients. Ann Surg 259(3): 449-457.
- Ro HW, Park SK, Jang DK, Yoon WS, Jang KS, et al. (2016) Preoperative predictive factors for surgical and functional outcomes in chronic subdural hematoma. Acta Neurochir 158(1): 135-139.
- Hsieh CT, Siu IC, Hsu SK, Huang CT, Lian FJ, et al. (2016) Chronic subdural hematoma: Differences between unilateral and bilateral occurrence. J Clin Neurosci 34: 252-258.
-
Cory L Chang, E Sander Connolly Jr. Predictors of Chronic Subdural Hematoma Recurrence Following Surgical Intervention: A Review of the Recent Literature Arch Neurol & Neurosci. 6(3): 2020. ANN.MS.ID.000637.
-
BChronic Subdural Hematoma, Subdural hematoma evacuation; Subdural hematoma recurrence
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






