 Case Report
Case Report
Mioclonic Epilepsy with Red Ribbed Fibers (Merrf): Report of a Case and Review in Literature
Gabriel Miranda Nava*
Head of Neurology of the hospital Center of the presidential staff, Mexico
Gabriel Miranda Nava, Military Doctor specialized in Neurology, Chief of Neurology of the Hospital Center of the Presidential General Staff, Mexico.
Received Date: August 24, 2018; Published Date: September 11, 2018
Abstract
MERRF is a multiple system disorder characterized by myoclonia that is often the first symptom followed by generalized epilepsy, ataxia, weakness and dementia. The onset normally manifests in childhood, after normal early development. The clinical diagnosis of MERRF is based on the following four “canonical” traits: myoclonus, generalized epilepsy, ataxia and red-ripped fibers (RRF) in muscle biopsy. A 23-year-old female patient who began her illness at 19 years of age, previously healthy, first with generalized tonicclonic seizures that were difficult to control initially, since she currently had 2 medications; the following year he has generalized weakness, ataxic gait, weight gain, dysarthria and prostration in a wheelchair, with progressive and irreversible deterioration. Muscle biopsy is performed demonstrating red fibers torn through the Gomori trichrome technique. The electrocardiogram is normal.
Keywords: Myoclonic epilepsy; Torn red fibers; Muscle biopsy; Gomori trichrome; Mitochondrial disease
Introduction
Mitochondrial diseases result from the failure of mitochondria, the specialized compartments present in every cell of the body, with the exception of red blood cells. Mitochondria are responsible for creating more than 90% of the energy the body needs to sustain life and support growth. When they fail, less and less energy is generated inside the cell. It can then present cell injury or even cell death. If this process is repeated throughout the body, the entire systems begin to fail and the life of the person who suffers it is at serious risk. This disease affects mainly children, but outbreaks in adults are becoming more and more common. Mitochondria diseases appear to cause the greatest damage to the brain, heart, liver, skeletal muscle, kidney, as well as the endocrine and respiratory systems; Depending on which cells are affected, symptoms may include loss of motor control, muscle weakness and pain; gastrointestinal disorders and difficulties swallowing; poor growth, heart disease, liver disease, diabetes, respiratory complications, seizures, visual and hearing problems, lactic acidosis, developmental delays and susceptibility to infections.
Case Report
Myoclonic epilepsy with torn red fibers (MERRF) is a multiple system disorder characterized by myoclonia which is often the first symptom followed by generalized epilepsy, ataxia, weakness and dementia. The onset normally manifests in childhood, after normal early development. The clinical diagnosis of MERRF is based on the following four “canonical” traits: myoclonus, generalized epilepsy, ataxia, and red-ripped fibers (RRF) in muscle biopsy. The molecular genetic study for mutations with mitochondrial DNA (mt DNA) associated with MERRF are clinically available; The most common mutation, present in more than 80% of patients with typical results, is a U-to-G transition at nucleotide-8344 in the t RNALys gene of mt DNA. Mutations are normally present in all tissues [1-5].
Objectives
The knowledge of mitochondrial diseases, the diagnosis through the clinical picture and the performance of histopathological studies such as muscle biopsy and the knowledge of its form of genetic transmission.
A 23-year-old female patient who began her condition at 19 years of age, previously healthy, first with generalized tonic-clonic seizures of difficult initial control, since it was currently possible to control with 2 medications; the following year he has generalized weakness, ataxic gait, weight gain, dysarthria and prostration in a wheelchair, with progressive and irreversible deterioration. Muscle biopsy is performed demonstrating red fibers torn through the Gomori trichrome technique. The electrocardiogram is normal.
Discussion
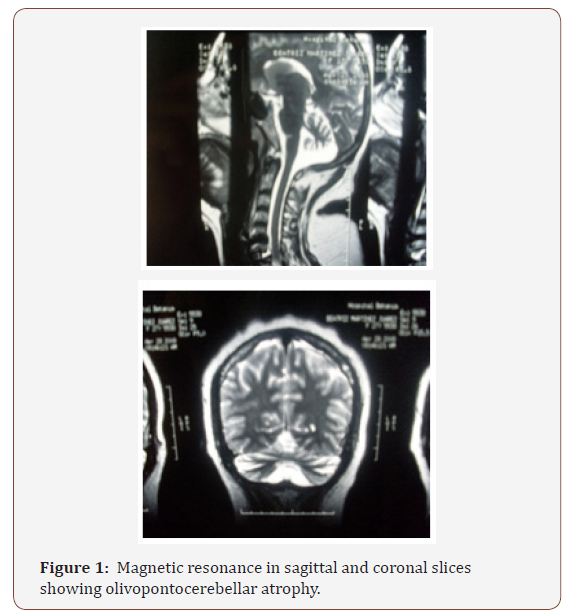
In patients with MERRF and in general the various mitochondrial diseases, the various symptoms and signs appear, among which we can list situations such as lactate and pyruvate are normally elevated at rest and increase excessively after moderate activity; the concentration of the CSF protein may be increased but rarely exceeds 100mg/dl; the electroencephalogram usually shows generalized crests and wave discharges with a delayed background, but also discharges of focal epileptic forms can be seen; the electrocardiogram often shows preexcitation, but no obstruction of the heart has been described; electromyography and nerve conduction studies are consistent with myopathy, but neuropathy can coexist, brain magnetic resonance imaging often shows atrophy of the brain and calcification of the basal ganglia. Muscle biopsy typically shows torn red fibers in the muscle biopsy with the trichrome technique of Gomori and stains on the hyperactive fibers of dehydrogenase succinate (SDH). Some red fibers do not stain the histochemical reaction for cytochrome c oxidase (COX). The biochemical studies of respiratory chain enzymes in muscle extracts usually show a decrease in the complex activities of respiratory chains that contain mtDNA subunits coded, especially COX deficiency. However, biochemical studies may also be normal.
Conclusion
Genetic counseling: MERRF is caused by mutations in the mtDNA and is transmitted by maternal inheritance. The father of a studied individual is not at risk of having the mtDNA mutation that causes the disease. The mother of an individual studied normally has the mtDNA mutation and may or may not have symptoms. A male with a mtDNA mutation can not transmit the mutation to his offspring. A woman with the mutation (both affected and not) transmits the mutation to all her offspring. Prenatal diagnosis for MERRF is available if a mtDNA mutation has been discovered in the mother. However, because the mutation is loaded in the tissues of the mother and in fetal tissue samples (ie, amniocentesis and chorionic villi) it can not correspond to that of other fetal tissues and because the burden of mutation in the Prenatal tissue samples can change in the uterus or after birth due to random mitotic segregation, prediction of the phenotype of prenatal studies is not possible (Figure 1 & 2).

References
- Boulet L, Karpati G, Shoubridge EA (1992) Distribution and threshold expression of the tRNA(Lys) mutation in skeletal muscle of patients with myoclonic epilepsy and ragged-red fibers (MERRF). Am J Hum Genet 51(6): 1187-1200.
- Chinnery PF, Johnson MA, Wardell TM, Singh-Kler R, Hayes C, et al. (2000) The epidemiology of pathogenic mitochondrial DNA mutations. Ann Neurol 48(2): 188-193.
- Chomyn A, Meola G, Bresolin N, Lai ST, Scarlato G, et al. (1991) In vitro genetic transfer of protein synthesis and respiration defects to mitochondrial DNA-less cells with myopathy-patient mitochondria. Mol Cell Biol11(4): 2236-2244.
- Crest C, Dupont S, Leguern E, Adam C, Baulac M (2004) Levetiracetam in progressive myoclonic epilepsy: an exploratory study in 9 patients. Neurology 62(4): 640–643.
- Crimi M, Galbiati S, Moroni I, Bordoni A, Perini MP, et al. A missense mutation in the mitochondrial ND5 gene associated with a Leigh-MELAS overlap syndrome. Neurology 60(11): 1857-1861.
-
Gabriel M N. Mioclonic Epilepsy with Red Ribbed Fibers (Merrf): Report of a Case and Review in Literature. Arch Neurol & Neurosci . 1(1): 2018. ANN.MS.ID.000503.
-
Myoclonic epilepsy, Torn red fibers, Muscle biopsy, Gomori trichrome, Mitochondrial disease, Red ribbed fibers, Mitochondrial, cell death; endocrine, seizures, Ataxia, Dementia, Canonical, electromyography, Myopathy, Neuropathy, Brain, Muscle, Tissue , Amniocentesis, Olivopontocerebellar atrophy, Sagittal, Coronal slices
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






