 Research Article
Research Article
Lumbar Microdiscectomy with Preservation of Ligamentum Flavum as A Means of Preventing Epidural Fibrosis
Vladimer Baziashvili1,3* and Mirza Khinikadze2,3
1Batumi Republic hospital, Batumi, Georgia/ head of neurosurgery, Batumi Clinic named after Khozrevanidze, Batumi, Georgia/head of neurosurgery, Medcenter, Batumi, Georgia/neurosurgeon
2Caucasus Medical Centre, Georgia
3New-Vision University, Georgia
Vladimer Baziashvili, New Vision University, Georgia
Received Date: July 03, 2023; Published Date: July 20, 2023
Abstract
Background: Postoperative scarring stands as the prevailing cause of failure after lumbar microdiscectomies. To avoid the formation of epidural fibrosis, several preventive measures have been recommended, including the application of fat, hemostatic sponges, amniotic membrane, and various anti-scarring barrier materials on the resection window and epidural space, as well as the use of drainage. In terms of surgical tactics, the preservation of the Ligamentum Flavum (LF) during microdiscectomy is recommended to prevent the development of fibrosis.
Objective: The aim of the research was to determine:
a) Would preserving the LF reduce the development of postoperative epidural fibrosis?
b) and whether the results of lumbar microdiscectomies with preserved LF would be improved compared to classic microdiscectomy.
Methods: Overall, 108 patients diagnosed with lumbar discogenic radiculopathy were selected from 2020 to 2022. They were randomly divided into two equal groups. The patients in Group A underwent classic microdiscectomy with preservation of the LF, while the patients in Group B underwent classic microdiscectomy. Patients were assessed with the Visual Analogue Pain Scale (VAPS) and the Oswestry Disability Index (ODI) before the surgery and 12 months after the surgery. The degree of postoperative epidural fibrosis was assessed 12 months after surgery by instrumental studies (MRI).
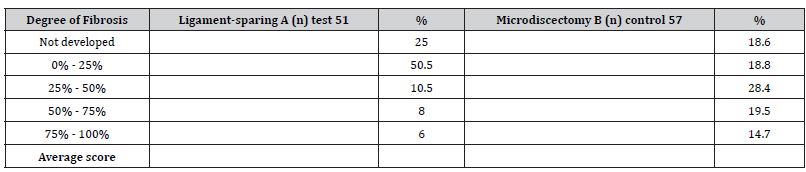
Results: In both groups, clinical data improved significantly 12 months after the surgery. According to ODI, pre-operative scores in Group A were 87.4 and postoperative scores were 13.2 (P 0.05); VAPS scores before the surgery were 8.4 and 1.7 after the surgery (P 0.05). In Group B, the ODI scores before the surgery were 89.1 and after the surgery were 23.1 (P 0.05); the VAPS scores before the surgery were 8.9 and after the surgery were reduced to 3.4 (P 0.05). The degree of scarring was less in Group A than in Group B.
Conclusion: The clinical results demonstrated in both groups were satisfactory. However, the group where LF was preserved revealed significantly fewer local scarring processes 12 months after surgery. The provided surgical method can be considered tissue sparing, reducing the complications caused by postoperative fibrosis and ultimately leading to a better clinical result after microdiscectomy.
Keywords:Epidural fibrosis; Ligamentum flavum; Microdiscectomy; Tissue-sparing surgery
Introduction
Epidural fibrosis is one of the most common reasons for failed back surgeries (48%), followed by recurrent intervertebral disc herniation (27%), and spinal stenosis (20%). The failure rate varies from 20% to 50%, depending on the evaluation criteria used during assessment. There are many methods to prevent postoperative epidural fibrosis; our method has several advantages: it is literally an organ-sparing surgery; it allows microdiscectomy to be performed in a minimal window; and it prevents the development of epidural fibrosis, which in turn gives us better clinical outcomes than classic microdiscectomy. The frequency of recurrence decreases, and what is more important, in the case of repeated surgery, it is much safer for the surgeon to perform the operation.
Materials and Methods
The study involved 108 patients, who were divided into two groups. They were randomly assigned to groups. Group A included patients who underwent microdiscectomy with LF preservation (51 patients), while in Group B, the classic microdiscectomy was performed (57 patients). Indications for surgical interventions were based on the clinical picture (pain syndrome on the side of the injury lasting from 6 weeks to 3 months); the ineffectiveness of conservative treatment; recurrent symptoms; the presence of clinical signs of radiculopathy; and also, the MRI data, which confirmed intervertebral disc herniation and compression of nerve structures.
The results of surgical interventions were evaluated 12 months after the surgery by means of VAPS and ODI scales and questionnaires, which were compared to the pre-operative data. The MRI scan was also performed 12 months after surgery, and the results were evaluated in a blind fashion by neuroradiologists. The scar formation and its degree were assessed as well. The 12-month postoperative interval was chosen because, as described, scar tissue is very stable 6–12 months after the surgery (5,60).
Inclusion criteria:
i. Patients with lumbar disc herniation who have an indication for microdiscectomy
ii. Patients aged 16 to 76 years
iii. Patients of both sexes
Exclusion criteria:
i. Patients with recurrent lumbar disc herniation
ii. Polydiscopathy: herniation of more than one intervertebral disc
iii. Extraforaminal hernia
iv. Expressed psychopathology
v. Patients with intervertebral disc herniation status after other surgical interventions on the spine
vi. Patients under 15 and over 76 years old with lumbar intervertebral disc herniation
MRI examination
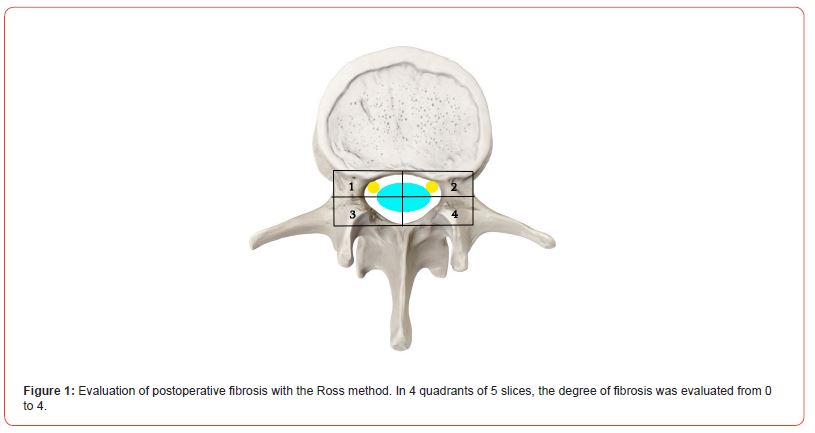
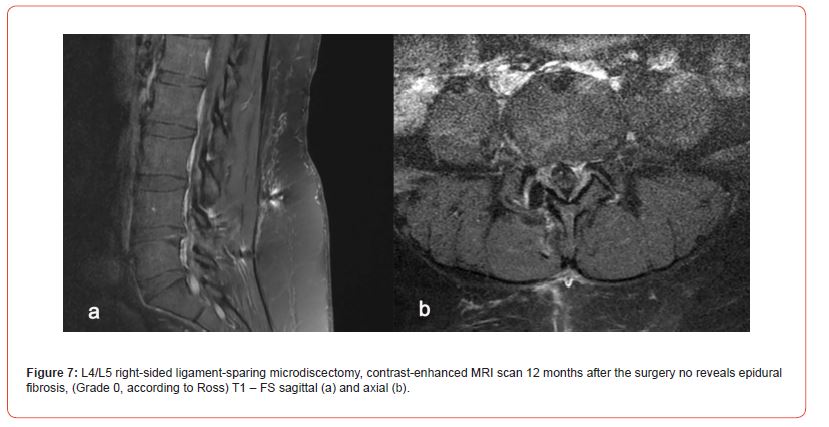
All patients underwent a contrast-enhanced MRI examination of the lumbar spine, and the contrast material was injected intravenously. A standard T1, T2, and T3 scan was performed before the injection of the contrast material. Each examination detailed epidural fibrosis in five slices on the projection of the operated disc. The degree of epidural fibrosis was graded in four quadrants, with scores ranging from 0 to 4 in each quadrant. According to the method of Ross and co-authors (50, 51), grade 0: absence or trace of fibrosis; grade 1: 25% or less of the quadrant is marked by scars; grade 2: more than 25% up to 50%; grade 3: 50–75% or less; grade 4: more than 75% (Figure 1). A total of 108 patients were evaluated (5 slices, 4 quadrants, 20 evaluations for ach).
Anatomy of the Ligamentum Flavum and Stages of Surgery
Histologically, the LF differs from other human ligaments in that it consists of very high-quality and dense elastic fibers. The LF is of fibrous structure, and the fibers run along its length. The superior edge of the ligament is attached to the inferior part of the superior vertebral arch, and the inferior edge is attached to the superior part of the inferior vertebral arch. The anterior surface of the LF runs towards the spinal canal, which is separated from the dural sac and the root by epidural fat and the vascular layer. And the posterior surface of the ligament is covered by the anterior surface of the paravertebral muscles. The medial portion of the ligament meets the medial edge of the contralateral LF in the midline and attaches to the base of the spinous process. Part of the lateral edge (the fibrous part) meets the medial edge of the inferior articular process of the superior vertebra (picture). The LF will then pass along the medial part of the articular process and under the plates into the spinal canal. The lateral edge of the LF runs towards and attaches to the fibrous membrane of the facet joint. This part covers the superior and lateral surfaces of the root.
The patient is lying in the prone position on the operating table, and the skin is cut in the middle line in the interspinales area of the corresponding segments lengthwise. The length of the incision is 2-3 cm. After skeletonizing the long back muscle, the interlaminar section is visualized. Mobilization of soft tissues is managed with a Caspar retractor. As soon as LF is visualized, an operating microscope is positioned. In the microscope, the operating field is represented by the superior vertebral arch on the right, the inferior vertebral arch on the left, the spinous process medially, the articular plate laterally, and the interlaminar section. The central part of the incision is represented by LF. The LF is visible in the section where the ligament attaches the articular capsule; in this area, the ligament covers the inferior articular process of the superior vertebra. We must be careful not to damage the articular capsule here. The LF should be carefully dissected with a surgical dissector at the articular capsule lengthwise, in the craniocaudal direction, in accordance with the anatomic orientation of its fibers. The ligament is easily dissected, and the epidural fat tissue and root can be visualized.
Due to its elasticity, the ligament can be stretched and mobilized, making it easier to access the intraspinal structures.
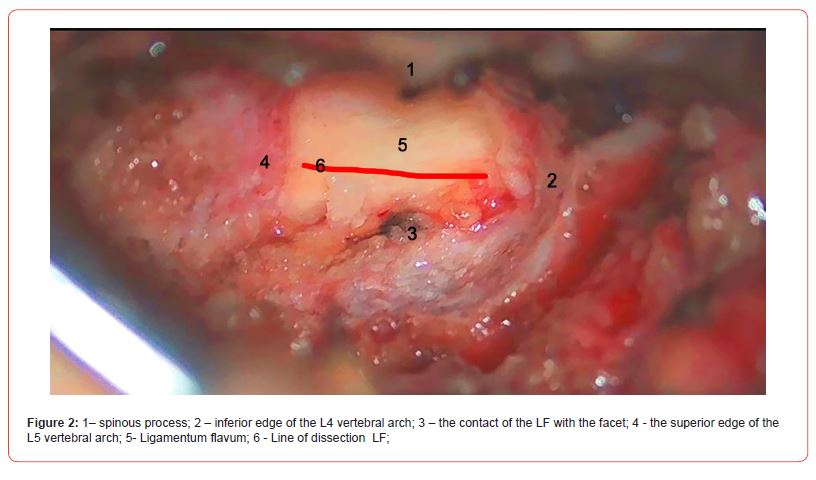
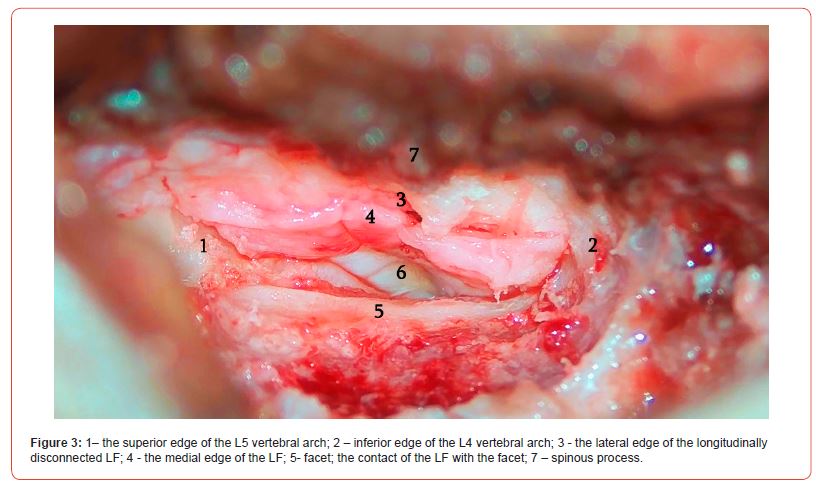
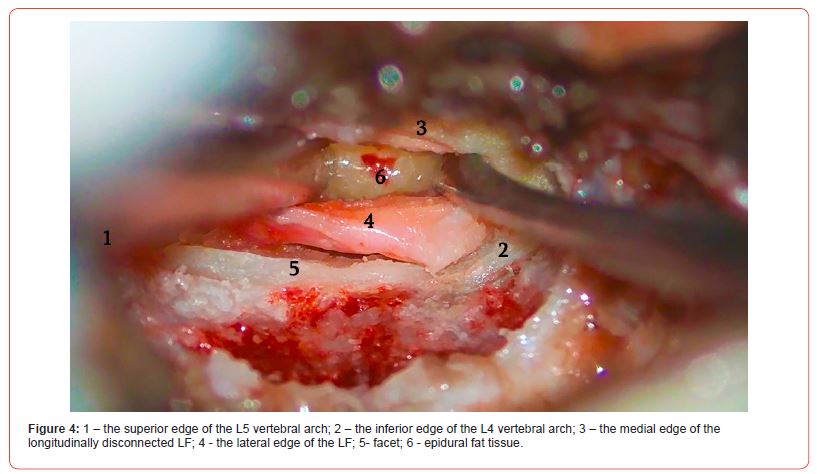
The lateral part of the ligament covers the root, and the medial part covers the dural sac. At this point, it is preferable to perform hemostasis with bandages rather than using bipolar electrocoagulation. It must be noted that if the mobile operating window of the ligament is not enough to safely perform the operation, you can perform the resection of the arch with a drill to create more space. If this approach is also ineffective, it is better to continue the operation with the classic method. After discectomy, the edges of the LF are easily attached to each other, leaving no defect and requiring no additional barrier applicator, including hemostatic sponge or fat grafting (Figures 2-4).

Results
The surgeries of all 108 patients were performed without complications. The observation period was 12 months. No patient required reoperation due to recurrent intervertebral disc herniations. The VAPS and ODI scores improved significantly in both groups on the second day after the surgery, but 12 months after the surgery, the difference between the groups according to the VAPS (Table 4) and ODI (Table 5) was noticeable; the patients in Group A demonstrated better results (P 0.005). Moreover, 12 months after the surgery, the difference was observed in terms of developing epidural fibrosis as well; the results were better in group A than in group B (Tables 1-5, Figures 5&6).
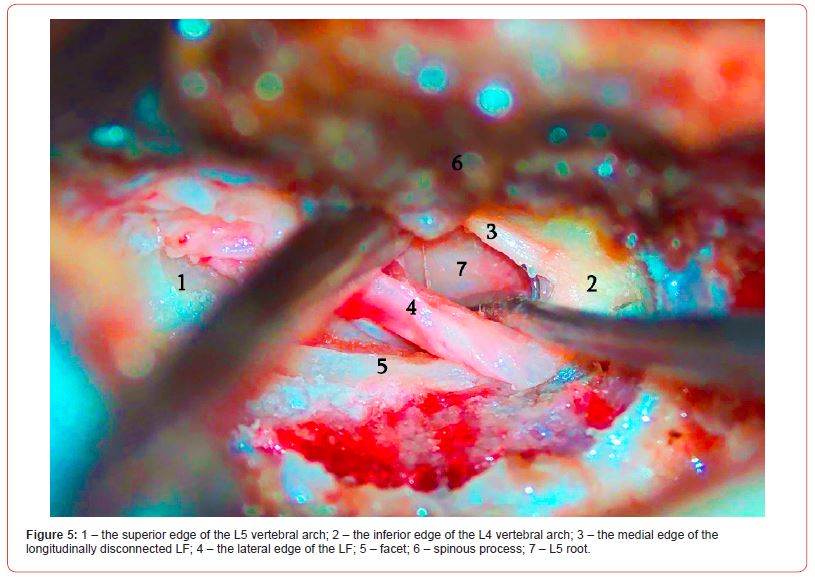

Table 1:Gender Ratio in Groups.

Table 2:Distribution of Operated Patients by Age.
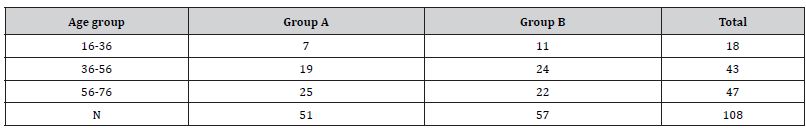
Table 3:Clinical Evaluation of Patients before the Surgery with VAPS and ODI scales.

Table 4:Distribution of Operated Patients after Classic Microdiscectomy according to VAPS and ODI Scales.
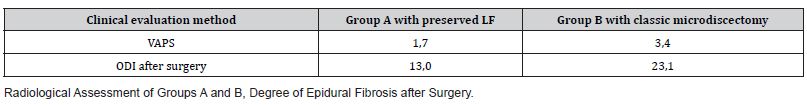
Table 5:Evaluation of MRI results.
Discussion
Based on the obtained data, we can say that both groups, with the ligament-sparing as well as the classic microdiscectomy, showed improvements 24 hours after the surgery, and no significant differences were observed during this time, according to VAS and ODI data. The evaluation conducted 12 months after the operation proved that the VAS and ODI data were better in the group where the LF was preserved, which was also confirmed by the MRI scans evaluating the degree of epidural fibrosis. The failure of back surgery has been studied by many authors and is believed to be a global issue in contemporary spinal neurosurgery [1]. Postoperative complications are quite common after lumbar discectomy, including epidural fibrosis, spinal stenosis, recurrent intervertebral disc herniation, discitis, instability of the operated segment, and others that often require repeated operations [2]. Each of them can be the subject of different studies, but epidural fibrosis remains one of the most common postoperative complications [3]. Since fibrosis is a physiological response to injury, the deeper mechanisms of its development have also been studied. It has been proven that TGFplays a key role in the scar formation process. Histological studies have shown that the elements of the intervertebral disc can trigger the inflammatory process, which leads to the formation of fibrosis [4]. Contemporary studies have also proved that the presence of blood in the spinal canal contributes to the development of epidural fibrosis (H. LaRocca and I. Macnab [5]). The presence of blood in the epidural space causes aseptic inflammation, which eventually leads to fibrosis and scar formation [6].
There are many methods to prevent the development of the Failed Back Surgery Syndrome (FBSS), including: minimizing the hematoma in the epidural space by using different types of drains [7]; using a strong hemostatic agent like a barrier membrane, TachoComb, was recommended in this regard [8]; various artificial [9] and natural anti-scarring barriers have been recommended, including the amniotic membrane [10]. Fat grafting was often used as a method to prevent epidural fibrosis [11]. The preventive methods listed above did not imply revision of surgical nuances until a tissue-sparing method such as microdiscectomy with preservation of the ligamentum flavum was introduced. There are studies conducted by different authors where they describe the dissection of the ligament, formation of a flap from it, microdiscectomy, and reattachment of the flap with or without suturing it; some of them applied the rule of minimal resection. All methods were aimed at preserving this anatomical structure, leaving it in its place, and maintaining its biomechanical, elastic, and barrier functions [12].
One of the most comprehensive studies was conducted by Yunus Aydn [13] and the authors. 1500 patients participated in the study, which lasted for several years. Unlike our method, the study involved the formation of a tricuspid flap, which was reattached to its original place after the discectomy. The study by Jigang Li [14] should also be noted in this regard. It also involved the ligament- sparing method, where the researcher performed the resection of the lateral part of the ligament, forming a small resection window sufficient for microdiscectomy. Zahid Askar [15], similar to Aydin, focused on the formation of a flap from the ligament, but with different modifications: the ligament was removed and dissected from the lateral edge, and the flap was moved medially. After the discectomy, the flap was reattached to the ligament. If the ligament could not completely cover the dural sac, fat grafting was applied.
Overall, both studies had similarities and differences, including in terms of the surgery duration, the incision sizes, the volume of blood loss, the degree of arch resection, the tactics of preserving the lateral pocket, as well as the methods of ligament separation and resection, its dissection, and flap formation. Some authors sutured the ligament, while others reattached it without suturing. They also used different materials to cover the defect; some used fat grafting, while others used hemostatic sponge. Different methods were used to evaluate the clinical picture, both pre-operatively and post-operatively [16]. There were differences in clinical assessment scales and neuroimaging methods [17]. The main difference in our study was that no flap was formed from the ligament during the surgery; it was dissected lengthwise along the longitudinal arrangement of its fibers, and after that, the discectomy was performed. The edges of the ligament reattached to each other due to its elasticity, leaving no defect and no need to apply additional artificial barriers or biological material.
Our method allows microdiscectomy to be performed in a minimal operating window to avoid the formation of a flap and resection of the LF. It is possible to get access to the root without exposing the dural sac, which protects it from damage and liquefaction in the postoperative period. After discectomy, contact is restored as soon as its edges are reattached. This method of ligament sparing makes it relatively safe to perform repeated surgery in cases of recurrent herniation. The results of the study showed that the opinion that preserving the ligament reduces epidural fibrosis and improves the clinical picture proved to be true. The limitation of the method involves sequestered and migrated hernias, as they require a larger operating visualization window, due to which the ligament needs a larger dissection or resection for a safe discectomy. Also, unlike classic microdiscectomy, this method is more time-consuming.
Conclusion
Studies have proved that recurrent postoperative pain is mainly associated with epidural fibrosis, and the ligament-sparing microdiscectomy reduces the development of epidural fibrosis, which proves the advantage of this method in the prevention of fibrosis.
Acknowledgement
None.
Conflict of interest
None.
References
- Abdel-Meguid EM (2008) An anatomical study of the human lumbar ligamentum flavum. Neurosciences (Riyadh) 13(1): 11-6.
- Askar Z, Wardlaw D, Choudhary S, Rege A (2003) A ligamentum flavum-preserving approach to the lumbar spinal canal. Spine (Phila Pa 1976) 28(19): E385-E390.
- Aydin Y, Ziyal IM, Duman H, Türkmen CS, Başak M, et al., (2002) Clinical and radiological results of lumbar microdiskectomy technique with preserving of ligamentum flavum comparing to the standard microdiskectomy technique. Surg Neurol 57(1): 5-13.
- Beanes SR, Dang C, Soo C, Ting K (2003) Skin repair and scar formation: the central role of TGF-ß. Expert Rev Mol Med 5(8): 1-22.
- Bosscher HA, Heavner JE (2010) Incidence and severity of epidural fibrosis after back surgery: an endoscopic study. Pain Pract 10(1): 18-24.
- Caspar W, Campbell B, Barbier DD, Kretschmmer R, Gotfried Y, et al. (1991) The Caspar microsurgical discectomy and comparison with a conventional standard lumbar disc procedure. Neurosurgery 28(1): 78-86.
- Cervellini P, Curri D, Bernardi L, Volpin L, Benedetti A, et al. (1988) Computed tomography of epidural fibrosis after discectomy: a comparison between symptomatic and asymptomatic patients. Neurosurgery 23(6): 710-713.
- Dobran M, Brancorsini D, Costanza MD, Liverotti V, Mancini F, et al. (2017) Epidural scarring after lumbar disc surgery: Equivalent scarring with/without free autologous fat grafts. Surg Neurol Int 8: 169.
- Dullerud R, Graver V, Haakonsen M, Haaland AK, Loeb M, el al. (1998) Influence of fibrinolytic factors on scar formation after lumbar discectomy. A magnetic resonance imaging follow-up study with clinical correlation performed 7 years after surgery. Spine 23(13): 1464-1469.
- Farrokhi MR, Vasei M, Fareghbal S, Farrokhi N (2011) The effect of methylene blue on peridural fibrosis formation after laminectomy in rats: an experimental novel study. Spine J Off J North Am Spine Soc 11(2): 147-152.
- Gasinski P, Radek M, Jozwiak J, Lyczak P (2000) Peridural fibrosis in lumbar disc surgery - pathogenesis, clinical problems and prophylactic attempts. Neurol Neurochir Pol 34(5): 983-993.
- Grane P, Tullberg T, Rydberg J, Lindgren L (1996) Postoperative lumbar MR imaging with contrast enhancement. Comparison between symptomatic and asymptomatic patients. Acta Radiol 37 (3-1): 366-372.
- Greenwel P, Tanaka S, Penkov D, Zhang W, Olive M, et al., (2000) Tumor necrosis factor alpha inhibits type i collagen synthesis through Repressive CCAAT/Enhancer-Binding Proteins. Mol Cell Biol 20(3): 912-918.
- Hemmo A Bosscher, James E Heavner (2010) Incidence and Severity of Epidural Fibrosis after Back Surgery: An Endoscopic Study. Pain Pract 10(1): 18-24.
- Ivanic GM, Pink PT, Schneider F, Stuecker M, Homann NC, et al., (2006) Prevention of epidural scarring after microdiscectomy: a randomized clinicaltrial comparing gel and expanded polytetrafluoroethylene membrane. Eur Spine J 15(9): 1360-1366.
- Iwanaga J, Ishak B, Saga T, Singla A, Impastato D, et al., (2019) The Lumbar Ligamentum Flavum Does Not Have Two Layers and Is Confluent with the Interspinous Ligament: Anatomical Study with Application to Surgical and Interventional Pain Procedures. Clin Anat 33(1): 34-40.
- Karasu H, Güzel I (2016) Comparison Tachocomb with Surgiwrap, surgical and lyodura in epidural fibrosis: an experimental rat model. Ideggyogy Sz 69(5-6): 195-200.
- Kim CH, Chung CK, Park CS, Choi B, Kim MJ, et al., (2013) Reoperation rate after surgery for lumbar herniated intervertebral disc disease: nationwide cohort study. Spine (Phila Pa 1976) 38(7): 581-590.
- Kotil K (2016) Closed Drainage versus Non-Drainage for Single-Level Lumbar Disc Surgery: Relationship between Epidural Hematoma and Fibrosis. Asian Spine J 10(6): 1072-1078.
- Larionov SN, Sorokovikov VA, Erdyneyev KC, Lepekhova SA, Goldberg OA, et al., (2017) Intervertebral disk mediated postoperative epidural fibrosis: experimental model and methods of prevention. Clin Surg 2.
- LaRocca Н, Macnab I (1974) The laminectomy membrane. Studies in its evolution, characteristics, effects and prophylaxis in dogs. J Bone Joint Surg Br 56(3): 545-550.
- Li J, Ma Q, Wu J, Zhao P, Li T, et al. (2020) Dose preservation of ligament flavum really help prevent postoperative epidural fibrosis and improve outcome in microdiscectomy? J Clin Neurosci 80: 331-335.
- Masopust V, Hackel M, Netuka D, Bradac O, Rokyta R, et al. (2009) Postoperative epidural fibrosis. Clinic J Pain 25(7): 600-606.
- Mastronardi L, Pappagallo M, Tatta C, Roperto R, Elsawaf A, et al. (2008) Prevention of postoperative pain and of epidural fibrosis after lumbar microdiscectomy: pilot study in a series of forty cases treated with epidural vaseline-sterile-oil-morphine compound. Spine 33: 1562-1566.
- McCarron RF, Wimpee MW, Hudkins PG, Laros GS (1987) The inflammatory effects of nucleus pulposus: A possible element in the pathogenesis of low back pain. Spine 12(8): 760-764.
- McLoughlin GS, Fourney DR (2008) The learning curve of minimally-invasive lumbar microdiscectomy. The Canadian journal of neurological sciences 35(1): 75-78.
- Mirzai H, Eminoglu M, Orguc S (2006) Are drains useful for lumbar disc surgery? A prospective, randomized clinical study. J Spinal Disord Tech 19(3): 171-177.
- Mohi Eldin MM, Abdel Razek NM (2015) Epidural fibrosis after lumbar disc surgery: prevention and outcome evaluation. Asian Spine J 9(3): 370-385.
- Olszewski AD, Yaszemski MJ, White AA 3rd (1996) The anatomy of the human lumbar ligamentum flavum. New observations and their surgical importance. Spine (Phila Pa 1976) 21(20): 2307-2312.
- Özay R, Ogur T, Durmaz HA, Turkoglu E, Caglar YS, et al., (2018) Revisiting Ligament-Sparing Lumbar Microdiscectomy: When to Preserve Ligamentum Flavum and How to Evaluate Radiological Results for Epidural Fibrosis. World Neurosurg 114: e378-e387.
- Ozer AF, Oktenoglu T, Sasani M, Bozkus H, Canbulat N, et al. (2006) Preserving the ligamentum flavum in lumbar discectomy: a new technique that prevents scar tissue formation in the first 6 months postsurgery. Neurosurgery 59(1 Suppl 1): ONS126-133.
- Pappas CT, Harrington T, Sonntag VK (1992) Outcome analysis in 654 surgically treated lumbar disc herniations. Neurosurgery 30(6): 862-866.
- Petty PG, Hudson P, Hare WS (2000) Symptomatic lumbar spinal arachnoiditis: fact or fallacy? Clin Neurosci 7(5): 395-399.
- Ran B, Song YM, Liu H, Liu LM, Gong Q, et al., (2011) Novel biodegradable alpha- TCP/poly (amino acid) composite artificial lamina following spinal surgery for prevention of intraspinal scar adhesion. Eur Spine J 20(12): 2240-2246.
- Ross JS, Robertson JT, Frederickson RC, Petrie JL, Obuchowski N, et al., (1996) Association between peridural scar and recurrent radicular pain after lumbar discectomy: magnetic resonance evaluation. Neurosurgery 38(4): 855-861.
- Ross JS, Robertson JT, Frederickson RC, Petrie JL, Obuchowski N, et al. (1996) Association between peridural scar and recurrent radicular pain after lumbar discectomy: magnetic resonance evaluation ADCON-L European Study Group. Neurosurgery 38(4): 855-861.
- Schaller B (2004) Failed back surgery syndrome: the role of symptomatic segmental single-level instability after lumbar microdiscectomy. European spine journal 13(3): 193-198.
- Schofferman J, Reynolds J, Herzog R, Covington E, Dreyfuss P, et al., (2003) Failed back surgery: etiology and diagnostic evaluation. Spine J 3(5): 400-403.
- Sen O, Kizilkilic O, Aydin MV, Yalcin O, Erdogan B, et al. (2005) The role of closed-suction drainage in preventing epidural fibrosis and its correlation with a new grading system of epidural fibrosis on the basis of MRI. Eur Spine J 14(4): 409-414.
- Smith C, Berry M, Clarke W, Logan A (2001) Differential expression of fibroblast growth factor-2 (FGF-2) and FGF receptor 1 (FGFR1) in a scarring and non-scarring model of CNS injury. Eur J Neurosci 13(3): 443-456.
- Smith S (2003) The adhesive arachnoiditis syndrome.
- Giovanni F Nicoletti , Nunzio Platania, Vincenzo Albanese (2005) Smooth dissection of ligamentum flavum for lumbar microdiscectomy. Preliminary report of this personal technique. Surgical Neurology 64(3): 232-235.
- Song J, Park Y (2000) Ligament-sparing lumbar microdiscectomy: technical note. Surg Neurol 53(6): 592-596.
- Tatsui CE, Martinez G, Li X, Pattany P, Levi AD et al. (2006) Evaluation of DuraGen in preventing peridural fibrosis in rabbits. J Neurosurg Spine 4(1): 51-59.
- Vakis A, Koutentakis D, Karabetsos D, Kalostos G (2006) Use of polytetrafluoroethylene dural substitute as adhesion preventive material during craniectomies. Clin Neurol Neurosurg 108(8): 798-802.
- Van Goethem JW, Van de Kelft E, Biltjes IG, Van Hasselt BH, Van den Hauwe L, et al. (1996) MRI after successful lumbar discectomy. Neuroradiology 38: 90-96.
- Waguespack A, Schofferman J, Slosar P, Reynolds J (2002) Etiology of long-term failures of lumbar spine surgery. Pain Med 3(1): 18-22.
- Xu H, Li P, Liu M, Liu C, Sun Z, et al., (2015) CCN2 and CCN5 exerts opposing effect on fibroblast proliferation and transdifferentiation induced by TGF-ß. Clin Exp Pharmacol Physiol 42(11): 1207-1219.
- Yang J, Ni B, Liu J, Zhu L, Zhou W (2011) Application of liposome-encapsulated hydroxycamptothecin in the prevention of epidural scar formation in New Zealand white rabbits. Spine J 11(3): 218-223.
- Liu ZC, Li Y, Zang Y, Cui G, Sang HX, et al., (2013) Clinical assessment of a CMC/PEO gel to inhibit postoperative epidural adhesion formation after lumbar discectomy: a randomized, control study. Arch Orthop Trauma Surg 133(3): 295-301.
- Wu CY, Huang YH, Lee JS, Tai TW, Wu PT, et al. (2015) Efficacy of topical cross-linked hyaluronic acid hydrogel in preventing post laminectomy/laminotomy fibrosis in a rat model. J Orthop Res 34(2): 299-306.
- Häckel M, Masopust V, Bojar M, Ghaly Y, Horínek D (2009) The epidural steroids in the prevention of epidural fibrosis: MRI and clinical findings. Neuro Endocrinol Lett 30(1): 51-55.
- Lane Moore M, Deckey DG, Pollock JR, Smith JH, Tokish JM, et al., (2022) The Effect of Amniotic Tissue on Spinal Interventions: A Systematic Review. Int J Spine Surg 17(1): 32-42.
- Ivanic GM, Pink PT, Schneider F, Stuecker M, Homann NC, et al., (2006) Prevention of epidural scarring after microdiscectomy: a randomized clinical trial comparing gel and expanded polytetrafluoroethylene membrane. Eur Spine J 15(9): 1360-1366.
- Mastronardi L, Pappagallo M, Tatta C (2005) The Oxiplex/SP gel-morphine compound after lumbar microdiscectomy in the management of postoperative pain. Report of 20 cases. Surg Neurol 64(1): 75-78.
- Gelalis ID, Papanastasiou EI, Theodorou DJ, Theodorou SJ, Pakos EE, et al., (2019) Postoperative MRI findings 5 years after lumbar microdiscectomy. Eur J Orthop Surg Traumatol 29(2): 313-320.
- Sze GK (1993) Clinical experience with gadolinium contrast agents in spinal MR imaging. J Comput Assist Tomogr 17 Suppl 1: S8-13.
- Sarrazin JL (2003) Imagerie du rachis lombaire opéré [Imaging of postoperative lumbar spine]. J Radiol 84(2 Pt 2): 241-250.
- Papanastasiou EI, Theodorou DJ, Theodorou SJ, Pakos EE, Ploumis A, et al., (2020) Association between MRI findings and clinical outcomes in a period of 5 years after lumbar spine microdiscectomy. Eur J Orthop Surg Traumatol 30(3): 441-446.
- Noh SH, Ndraha E, Shin DA, Cho PG, Kim KN, et al., (2022) Immediate postoperative lumbar spine magnetic resonance imaging: Correlation with postoperative pain in lumbar microdiscectomy. Medicine (Baltimore) 101(43): e31287.
- Rahme R, Moussa R, Bou-Nassif R, Maarrawi J, Rizk T,et al. (2011) Lumbar microdiscectomy: a clinicoradiological analysis of outcome. Can J Neurol Sci 38(3): 439-445.
- Nygaard OP, Jacobsen EA, Solberg T, Kloster R, Dullerud R, et al. (1999) Nerve root signs on postoperative lumbar MR imaging. A prospective cohort study with contrast enhanced MRI in symptomatic and asymptomatic patients one year after microdiscectomy. Acta Neurochir (Wien) 141(6): 619-622.
- Vogelsang JP, Finkenstaedt M, Vogelsang M, Markakis E (1999) Recurrent pain after lumbar discectomy: the diagnostic value of peridural scar on MRI. Eur Spine J 8(6): 475-479.
- Vanni D, Sirabella FS, Guelfi M, Pantalone A, Galzio R, et al. (2015) Microdiskectomy and translaminar approach: minimal invasiveness and flavum ligament preservation. Global Spine J 5(2): 84-92.
- Önen MR, Naderi S (2021) Bone-to-bone ligament preserving laminoplasty technique for reconstruction of laminae. J Craniovertebr Junction Spine 12(1): 61-64.
- Yüce İ, Kahyaoğlu O, Çavuşoğlu H, Aydın Y (2019) Surgical outcome and efficacy of lumbar microdiscectomy technique with preserving of ligamentum flavum for recurrent lumbar disc herniations. J Clin Neurosci 63: 43-47.
- Park YK, Kim JH, Chung HS (2002) Outcome analysis of patients after ligament-sparing microdiscectomy for lumbar disc herniation. Neurosurg Focus 13(2): E4.
- Baranowska A, Baranowska J, Baranowski P (2016) Analysis of Reasons for Failure of Surgery for Degenerative Disease of Lumbar Spine. Ortop Traumatol Rehabil 18(2): 117-129.
- Dobran M, Brancorsini D, Costanza MD, Liverotti V, Mancini F, et al., (2017) Epidural scarring after lumbar disc surgery: Equivalent scarring with/without free autologous fat grafts. Surg Neurol Int 8: 169.
- Rajpal K, Singh J, Bahadur R, Bansal K, Shyam R, et al., (2022) Postoperative Epidural Fibrosis Prevention: Which Is Better-Autologous Fat versus Gelfoam. Asian Spine J 16(3): 343-351.
-
Vladimer Baziashvili* and Mirza Khinikadze. Lumbar Microdiscectomy with Preservation of Ligamentum Flavum as A Means of Preventing Epidural Fibrosis. Arch Neurol & Neurosci. 15(4): 2023. ANN.MS.ID.000866.
-
Freezing of gait (FoG) is a highly disabling and challenging symptom of dyskinesia. At present, the pathophysiological mechanism of frozen gait is not completely clear, and the specific mechanism of action observation and motor relearning rehabilitation training based on mirror visual feedback of embodied cognition is not clear. Cognitive, emotional and sensory signals are involved in each node of gait control motion loop, and multiple interactions among sensory, edge and cognitive systems regulate gait and posture control. This paper makes a brief review from the perspective of embodied cognition in order to provide new ideas for further improving the cognition of FoG, evaluating and developing more effective non-invasive intervention FoG nerve rehabilitation technology.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






