 Research Article
Research Article
Increased Familial Risk and Evidence of Genetic Factor in Migraine
Ghaydaa Ahmed Shehata*
Department of Neurology and Psychiatry, Assiut University Hospital, Egypt
Ghaydaa Ahmed Shehata, Department of Neurology and Psychiatry, Assiut University Hospital, Assuit, Egypt.
Received Date: May 15, 2020; Published Date: June 10, 2020
Abstract
Background: Migraine is 7th Disabler according to the World Health Organization’s recent Global Burden of Disease report, due to it’s a wide range of complications and comorbidities. In addition, to the fact that it runs in families. So, our study was designed to determine genetic evidence of migraine through family history method, detect depression in migraine through HDRS, assess any cognitive changes in migraine through CASI and Event-Related Potential, detect any abnormalities in EEG and Neurological Soft Signs. Since these factors are the main causes of disability over time, they should be carefully assessed.
Methods: The sample included 80 study participants,50 cases, and 30 control, identified in hospital-based Study recruited from Neurology Outpatient Clinic, Assiut University Hospital. Migraine was ascertained by direct clinical interview using ICHD-III criteria for Migraine. A structured history of headache was also collected from patients regarding their relatives.
Results: Positive family history of migraine in 58% of migraineurs of which more than half is of 1st-degree relatives. Depression found in 82% of migraineurs and it’s severity increased with an increasing disability experienced by migraine patients and EEG changes in migraine.
Conclusion: There were strong genetic bases of migraine, high comorbidity of depression with migraine. We should take potential steps to prevent this disability through early detection and management.
Keywords: Migraine; Family history; Risk factors; Depression
Introduction
Migraine is a chronic neurological disease characterized by recurrent moderate to severe headaches often in association with a number of autonomic nervous system symptoms [1]. Migraines are believed to be due to a mixture of environmental and genetic factors [2]. About two-thirds of cases run in families [3]. Changing hormone levels may also play a role, as migraines affect slightly more boys than girls before puberty, but about two to three times more women than men after puberty [4,5]. The risk of migraines usually decreases during pregnancy [4]. The exact mechanisms of migraine are not known. It is, however, believed to be a neurovascular disorder [3]. The primary theory is related to increased excitability of the cerebral cortex and abnormal control of pain neurons in the trigeminal nucleus of the brainstem [5].
Environmental influences play a role, but family studies suggest that defective genes may be primarily involved in disease causation. The mode of transmission of typical migraine in families is still unclear, but it is widely believed to be multifactorial [6], although a role for a major susceptibility gene cannot be excluded [7]. Aim of study: we aim to study the familial risk for developed migraine and its relation to EEG changes
Patients and Methods
Administrative and ethical design
The protocol of the study performed in accordance with the Declaration of Helsinki . Our institutional review boards (ethical committee of Assiut University) approved this study. Written informed consent was given to each subject. This study is Hospital based study. We recruited 80 adult persons, 50 migraine cases (43 females and 7 males, their age ranged from 15 to 45 years with mean value ± SD[31.78 ± 8.89] ) , 30 control ( 24 females and 6 males, their age ranged from 15 to 45 years with mean value ± SD [28.10 ± 7.73] ). The patients and control groups were recruited from Neurology Outpatient clinic at Assiut University Hospital within 6 months duration. All subjects included in this study aged 18 years and more, fulfils migraine criteria of International Headache Society with normal or corrected-to-normal vision, and normal hearing capability. We excluded from our study patients had general neurological disease as epilepsy, stroke, Psychiatric illness as schizophrenia, mixed headache types &brain injuries.
Methods
All subjects applied to thorough history; personal data included name, age, sex, residence, phone, occupation, marital status and children. The socio- economic scoring in this study covered education of the father, work and education of the mother, crowding index and income. The socioeconomic level of the families was determined based on the scoring system of Fahmy and El-Sherbini (1983) after modification [8]. Total socioeconomic score is = 23, scores 19+ are considered of high socioeconomic. Scores 15-<19 are considered of middle socioeconomic standard. Scores <15 are considered of low socioeconomic standard.
Diagnosis of migraine depended of presence of HIS criteria of headache. In which, migraine is a group consisting of one headache type (migraine) and the subtypes of migraine such as 1.2 Migraine with aura constitute the next level (second digit). Migraine with aura is again divided into sub forms, for example 1.2.1 typical aura with migraine headache. Patients must fulfill all criteria listed as A, B, C, D, etc. For each criterion there are specific requirements such as “two of the following four characteristics” [9]. Then patients and control group subjected to full neurological assessment is the assessment of sensor neuron and motor responses, especially reflexes, to determine whether the nervous system is impaired [10]. In general, a neurological examination is focused on finding out whether there are lesions in the central and peripheral nervous systems or there is another diffuse process that is troubling the patient [11], but in our study we used it as a screening tool for exclusion of any neurological disorder whether among cases or controls. In this study we used Neurologic Examination Checklist (2012-2013) which include [12], mental status, cranial nerves, motor, gait and coordination.
Family Pedigree Chart applied to each subject. A pedigree chart is a diagram that shows the occurrence and appearance or phenotypes of a particular gene from one generation to the next. [13-15]. A Pedigree results in the presentation of family information in the form of an easily readable chart. Pedigrees use a standardized set of symbols; squares represent males and circles represent females. Relationships in a pedigree are shown as a series of lines. Parents are connected by a horizontal line and a vertical line leads to their offspring. The offspring are connected by a horizontal line and listed in birth order from left to right. If the offspring are twins, then they will be connected by a triangle. If an offspring dies, then its symbol will be crossed by a line. If the offspring is still born or aborted, it is represented by a small triangle. Each generation is identified by a Roman numeral (I, II, III, and so on), and each individual within the same generation is identified by an Arabic number (1, 2, 3, and so on). Analysis of the pedigree using the principles of Mendelian inheritance can determine whether a trait has a dominant or recessive pattern of inheritance. Pedigrees are often constructed after a family member afflicted with a genetic disorder has been identified. This individual, known as the proband, is indicated on the pedigree by an arrow [16].
We used Headache Disability Index it in our study to detect severity of headache attacks and how much it is disabling to the patient [17]. Also, it is used to periodically evaluate a patient with headache and also to determine the effectiveness of a management strategy over time. It consisted of 2 domains: emotional: 13 items and functional: 12 items. The purpose of this scale is to identify difficulties that may be experiencing because of migraine. Its score ranges from 0-100. The higher the score the greater the disability caused by the headache. A decrease in the total HDI of > 29 points therefore to a management strategy is considered a significant improvement. Electroencephalography (EEG) is the recording electrical activity along the scalp EEG measures voltage fluctuations resulting from ionic current flows within the neurons of the brain [18]. In clinical contexts, EEG refers to the recording of the brain’s spontaneous electrical activity over a short period of time, usually 20–40 minutes, as recorded from multiple electrodes placed on the scalp. Diagnostic applications generally focus on the spectral content of EEG, that is, the type of neural oscillations that can be observed in EEG signals.
In conventional scalp EEG, the recording is obtained by placing electrodes on the scalp with a conductive gel or paste, usually after preparing the scalp area by light abrasion to reduce impedance due to dead skin cells. Electrode locations and names are specified by the International 10–20 system [19] . This system ensures that the naming of electrodes is consistent across laboratories, 19 recording electrodes (plus ground and system reference) are used [20]. In our study we commented on each EEG by filling a standard EEG Reportin order to detect any changes- which include (appendix 10):
Statistical analysis
The data were coded, entered and analyzed using SPSS (version 16) software program. Mean and standard deviation, sample t-test, Pearson chi-squared test, ANOVA, Post Hoc (LSD) Tests, Multiple Linear Regression Test were applied in this study. P value was considered significant >0.05
Results
controls, 50 cases with mean age 31.78 ± 8.89 and 30 control with mean age 28.10 ± 7.73, with male ratio 7:6 (14% : 20% ) ,female ratio 43:24 (86% : 80%). Social scale of cases was 12.74 ± 5.12 and control was 14.93 ± 4.49, with no significant difference between them also no affection of neurological examination among both cases and controls, there was significant difference between them according to depression 41:12 (82%: 40%). As regard family history and consanguinity were present only in cases (Figure 1). This figure of family history showed 60 % +ve family history of migraine of which 32% of 1st degree relative.
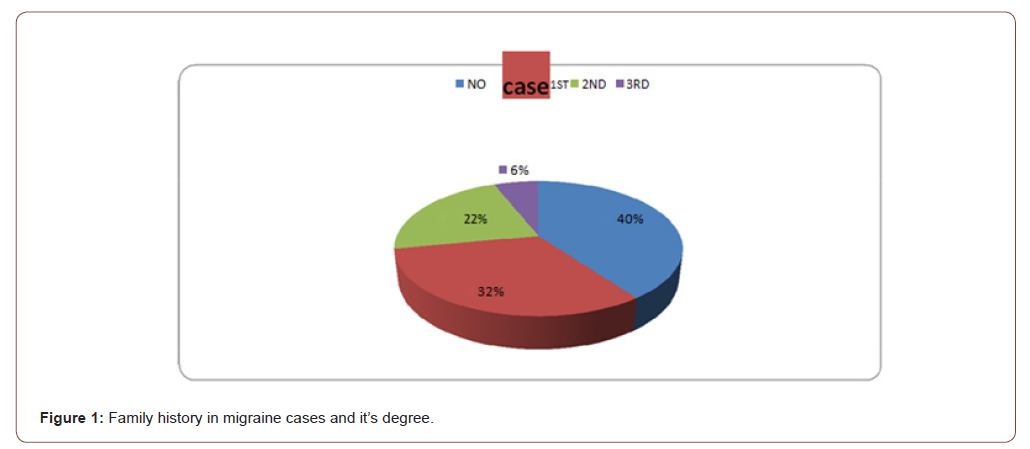
Table 1:Demographic & Clinical data of migraine cases and controls.
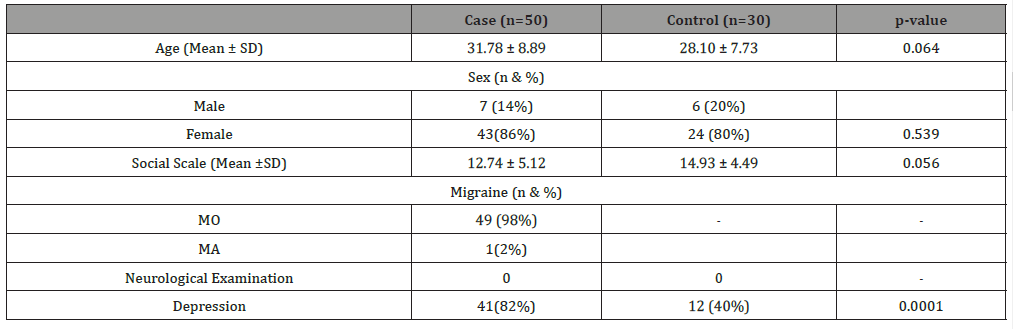
Table 2:Results of multiple linear regression test analysis of Migraine frequency, severity and Headache Disability Index & Related Factors.
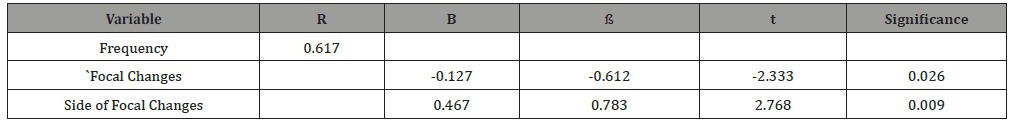
Table 3:Multiple Linear Regression Test Analysis of Hamilton Depression Rating Scale& Related Factors.
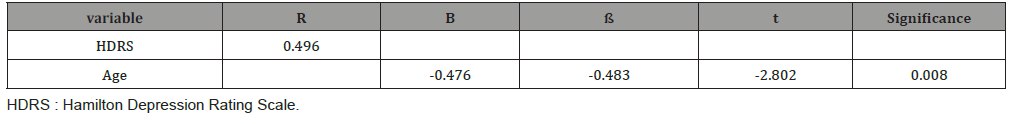
Table 2, showed high frequency of Slow wave and spike slow wave complex in migraine cases than in control. Table 4 showed Results of multiple linear regression test analysis of Migraine frequency, severity and Headache Disability Index & Related Factors, there’s significant difference between frequency and focal changes, side of focal changes only. While, multiple Linear Regression Test Analysis of Hamilton Depression Rating Scale& Related Factors was significant difference between HDRS and age (Table 3).
Discussion
Migraine is a chronic, neurovascular polygenic disease where genetic and environmental factors are involved in its etiology. Etiology of a migraine attack is only partly understood. Environmental influences play a role, but family studies suggest that defective genes may be primarily involved in disease causation. The mode of transmission of typical migraine in families is still unclear, but it is widely believed to be multifactorial [6], although a role for a major susceptibility gene cannot be excluded [7].
The result of this study proves that family history of migraine is positive in 58% of migraineurs of which 32% 1st degree relative which means that more than half those with positive family history are of 1st degree relatives , So there’re strong genetic bases for migraine. Accuracy of family history method was assessed by Lateef et al (2015) and he proved that the sensitivity and specificity of family history reports of migraine compared with direct interview were 38.6% and 96.8%,respectively,indicatingthat the false positive rate was very low, whereas the false negative rate was substantial .The positive and negative predictive values of migraine diagnosis provided by family member report are 90% and 67.6%,respectively,this means that our results of positive family history most probably in reality is more than 58 % [21].
Our results agreed with Dzoljic et al (2014) who studied characteristics of migraine and some lifestyle habits in migraineurs with and without a positive family history for migraine and found high frequency of positive family history for migraine among migraineurs. They also suggest that subjects with a positive family history have a lower “migraineurs threshold” for the development of migraine [22].
DucrosA talked about Genetics of migraine, stated that family history of migraine is the most potent and consistent risk factor for migraine, with a two- to three fold greater risk of migraine among relatives of people with migraine compared with controls [23]. The relation among the members of the nuclear family (contingency quotient of 0.429) was significantly stronger than the relation to the members of wider family (contingency quotient of 0.338), as he studied Risk of the recurrent headache and migraine appearance within the family, These results go with ours as we also found high frequency of first degree relative affection of migraine [24].
Regarding EEG changes, we found no significant difference between migraineurs and controls, on the other side Kollar (2012), studied the importance of interictal EEG in paroxysmal states found that 5% of migraineurs have epileptiform abnormalities [25].
Also our result doesn’t agree with Bjørk et al (2009) who found that Compared with controls, migraineurs had increased relative theta power in all cortical regions and increased delta activity in the painful fronto-central region, absolute power and asymmetry were similar among groups, in age-adjusted analyses, headache intensity correlated with increased delta activity, also found globally increased relative theta activity in migraineurs and slight interictal brain dysfunction is probably present between attacks [26].
Unlike previous blinded electroencephalography (EEG) studies and controlled quantitative EEG (QEEG) studies showing that EEG abnormality rates are higher in migraineurs compared to headachefree controls [27-30].
Up till now inter_ ictal EEG changes in migraine are still controversial and don’t have clinical significance nor real usefulness as Viticchi (2012) ,confirmed the usefulness of a wide application of IHS guidelines, not recommending EEG for migraine detection [31], recent studies are working on ictal EEG and EEG with photic stimulation.
In our study we found no affection of Neurological Soft Signs in migraine patients, However Tremolizzo et al (2015), studied neurological soft signs in primary headaches using 16-items Heidel-berg scale which, unlike other batteries, excludes primitive reflexes , found that NSS were increased by ∼70 and ∼90% in tension-type headache and migraine headache , respectively, with respect to controls (p<0.001) and the difference remained significant even after controlling for age and education. Headache type and characteristics did not influence NSS presentation, while headache patients with white matter hyperintensities (WMH) at brain MRI had higher NSS scores compared both to normal controls and patients without WMH. NSS identify a subset of primary headache patients sharing the same comorbidities or minimal brain anomalies, suggesting that tailored prophylactic options might apply [32].
The result of our study revealed that depression among migraine cases 82% was detected in more than those without migraine 40% according to total HDRS, this probably associated with significant difference among subunits of HDRS between migraine and control. These results matched with Saunders et al (2014), who worked on Gender differences, clinical correlates, and longitudinal outcome of bipolar disorder with comorbid migraine and concluded that Migraine is a common comorbidity with bipolar disorder and may impact long-term outcome of bipolar disorder, particularly depression [33]. Also, Yong et al (2012) found that depression and anxiety comorbidity in the mainland Chinese migraineurs are also common [34]. In addition, Patel et al (2004), assessed the prevalence of major depression in individuals with migraine and found it 28.1 % among migraine and 10.3% for control [35]. Also, Zwart et al (2003) studied the co-occurrence of headache and depression and found that individuals with migraine headache were more likely to have depression than non- headache control [36]. That was due to the relation between affective disorders and migraine seems to result from bidirectional influences where each disorder increases the risk for first onset of the other [37]. According to Scher et al (2005), such bidirectional relationship between migraine and affective disorders may be underpinned by shared genetics or epigenetic factors able to increase risk of both conditions [38].
As these two main Ideas proved worldwide -migraine has genetic bases and co morbidity of depression- recent studies searching the mechanism of linkage and whether this linkage has genetic base as Ligthart et al (2014) who presented a new application of polygenic (genetic risk) score analysis to investigate the mechanisms underlying the genetic overlap migraine and major depression [39].
Our study found no relation between frequency of migraine attacks and depression, unlike Zwart et al (2003) who proved that there was a linear trend associated with headache frequency ,thus for migraine headache occurring on fewer than 7 days per month,7-14 days per month or 15 or more days per month, the associations with depression [40].
Conclusion
We found positive correlation between depression and Headache Disability Index which means that as long as disability increase the more risk for depression, but no relation was found between depression and migraine severity or family history, so these results partially agree with Yong et al (2012) who discovered Possible risk factors for depression in migraineurs including headache intensity of first onset of migraine, migraine with family history and migraine disability, these differences may be due different methods used as he measured headache intensity using 11-point pain scale and migraine disability using Migraine Disability Assessment questionnaire and depression assessed using Hospital Anxiety and Depression Scale [34].
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Anderson Kenneth, Anderson EL, Glanze (1994) p.. WDe. Mosby's Medical, Nursing & Allied Health Dictionary. In: Mosby (Ed.), New York: Karger, USA.
- Piane M, Lulli P, Farinelli I, Simeoni S, De Filippis S, et al. (2007) Genetics of migraine and pharmacogenomics: some considerations. The journal of headache and pain 8(6): 334-339.
- Bartleson JD, Cutrer FM (2010) Migraine update. Diagnosis and treatment. Minnesota medicine 93(5): 36-41.
- Lay CL, Broner SW (2009) Migraine in women. Neurologic clinics 27(2): 503-511.
- Stovner LJ, Zwart JA, Hagen K, Terwindt GM, Pascual J (2006) Epidemiology of headache in Europe. European journal of neurology 13(4): 333-345.
- Russell MB, Olesen J (1993) The genetics of migraine without aura and migraine with aura. Cephalalgia 13: 245-248.
- Mochi M, Sangiorgi S, Cortelli P, Carelli V, Scapoli C, et al. (1993) Testing models for genetic determination in migraine. Cephalalgia 13: 389-394.
- Gag AH, Abd El Ghany GM (2012) Effect of Socio-economic Factors on the Onset of Menarche in Mansoura City Girls. Journal of American Science 8(3): 546.
- http://ihs-classification.org/en/01_einleitung/02_einleitung/Source: 2013 Journal Citation Reports® (Thomson Reuters, 2014)
- Nicholl DJ, Appleton JP (2014) Clinical neurology: why this still matters in the 21st century". J Neurol Neurosurg
- Fuller, Geraint (2004) Neurological Examination Made Easy. Churchill Livingstone. p. 1.
- http://www.med.umich.edu/medstudents/curRes/cca/m2/resources/Neurology_Exam_C hecklist_Website2012_13.pdf
- HELP - Ancestral File - Pedigree Chart
- Pedigree chart Genealogy Glossary - About.com, a part of The New York Times Company.
- Documenting Your Pedigree Chart By Melody Daisson - GeneaSearch.com
- Michael R. Cummings "Human Heredity Principles and issues" pg 59-60.
- Jacobson GP, Ramadan NM, SK Aggarwal, CW Newman (1994) The Henry Ford Hospital Headache Disability Inventory (HDI). Neurology 44: 837-842.
- http://www.psychcongress.com/saundras-corner/scales-screeners/depression/hamilton-depression-rating-scale-ham-d
- Towle Vernon L, Bolaños José, Suarez Diane, Tan Kim, Grzeszczuk Robert, et al. (1993) The spatial location of EEG electrodes: Locating the best-fitting sphere relative to cortical anatomy. Electroencephalography and Clinical Neurophysiology 86 (1): 1-6.
- Na (1994) Guideline Journal of Clinical Neurophysiology 11(1): 30-36.
- Lateef TM, Cui L, Nakamura E, Dozier J, Merikangas K (2015) Accuracy of family history reports of migraine in a community-based family study of migraine. Headache 55(3): 407-412.
- Dzoljic E, Vlajinac H, Sipetic S, Marinkovic J, Grbatinic I, et al. (2014) A survey of female students with migraine: what is the influence of family history and lifestyle? Int J Neurosci 124(2): 82-87.
- Ducros A (2013) Genetics of migraine. Rev Neurol (paris) 169(5): 360-371.
- Knezevic-Pogancev M, Filipović D, Ivetić V, Mikov A, Katanić D (2011) Risk of the recurrent headache and migraine appearance within the family. Med Glas (Zenica) 8(2): 216-223.
- Kollar B, Carnicka Z, Siarnik P, Krizova L, Sutovsky S, Traubner P (2014) The importance of interictal electroencephalography in paroxysmal states. Bratislavskelekarskelisty 115(3): 168-170.
- Bjork MH, Stovner LJ, Engstrom M, Stjern M, HagenK, et al. (2009) Interictal quantitative EEG in migraine: a blinded controlled study.
- Bille BS (1962) Migraine in school children. A study of the incidence and short-term prognosis, and a clinical, psychological and electroencephalographic comparison between children with migraine and matched controls. Acta Paediatr 51(Suppl 136): 1-151.
- Giel R, de Vlieger M, van Vliet AG (1966) Headache and the EEG. Electroencephalogr Clin Neurophysiol 21(5): 492-495.
- Whitehouse D, Pappas JA, Escala PH, Livingston S (1967) Electroencephalographic changes in children with migraine. N Engl J Med 276(1): 23-27.
- Rowan AJ (1974) The electroencephalographic characteristics of migraine. Arch Neurobiol (Madr) 37(Suppl): 95-113.
- Viticchi G, Falsetti L, Silvestrini M, Luzzi S, Provinciali L, et al. (2012) The real usefulness and indication for migraine diagnosis of neurophysiologic evaluation. Neurol Sci 33 Suppl1: S161-16W3.
- Tremolizzo L, Ferrario S, Pellegrini A, Fumagalli L, Ferrarese C, et al. (2015) Neurological soft signs in primary headache patients. Neurosci Lett 595: 41-44.
- Saunders EF, Nazir R, Kamali M, Ryan KA, Evans S, et al. (2014) Gender differences, clinical correlates, and longitudinal outcome of bipolar disorder with comorbid migraine. J Clin Psychiatry 75(5): 512-519.
- Yong N, Hu H, Fan X, Li X, Ran L, et al. (2012) Prevalence and risk factors for depression and anxiety among outpatient migraineurs in mainland China. J Headache Pain 13(4): 303-310.
- Patel NV, Bigal ME, Kolodner KB, C Leotta, J E Lafata, et al. (2004) Prevalence and impact of migraine and probable migraine in a health plan. Neurology 63: 1432-1438.
- Zwart JA, Dyb G, Hagen K, K J Ødegård, A A Dahl, et al. (2003) Depression and anxiety disorders associated with headache frequency. The Nord-Trondelag Health Study. Eur J Neurol 10: 147-152.
- Rota E, Mongini F (2014) Muscle tenderness and psychiatric comorbidity: a vicious cycle in migraine chronicization. Front Neurol 5: 148.
- Scher AI, Bigal ME, Lipton RB ((2005)) Comorbidity of migraine. Curr Opin Neurol 18(3): 305-310.
- Ligthart L, Jouke-Jan Hottenga, Cathryn M Lewis, Anne E Farmer, Ian W Craig, et al. (2014) Genetic risk score analysis indicates migraine with and without comorbid depression are genetically different disorders. Hum Genet 133(2): 173-186.
- Mathur VA, Khan SA, Keaser ML, Hubbard CS, Goyal M, et al. (2015) Altered cognition-related brain activity and interactions with acute pain in migraine. Neuroimage Clin 7: 347-358.
-
Ghaydaa Ahmed Shehata. Increased Familial Risk and Evidence of Genetic Factor in Migraine. Arch Neurol & Neurosci. 8(1): 2020. ANN.MS.ID.000677.
-
Migraine, Family history, Risk factors, Depression, Neurology, Psychiatry, Neurovascular disorder, Brainstem
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






