 Research Article
Research Article
GBA Mutation Analysis in Turkish Parkinson’s Disease Patients: Comparison of Clinical and Cognitive Performance
Özlem Anlaş1*, Göksemin Demir2 and Gülseren Bağcı3
1Department of Medical Genetics, University of Health Science Adana City Training and Research Hospital, Turkey
2Department of Neurology, Faculty of Medicine, Pamukkale University, Turkey
3Department of Medical Genetics, Faculty of Medicine, Pamukkale University, Turkey
Özlem Anlaş, Department of Medical Genetics University of Health Science Adana City Training and Research Hospital, Turkey.
Received Date: November 09, 2021; Published Date: January 04, 2022
Abstract
Background: Recent studies have established a substantial relationship between GBA gene and Parkinson’s Disease (PD). Mutations in the GBA play an important role in the pathogenesis of PD.
Methods: 33 young onset and 38 idiopathic PD patients and 76 healthy controls were screened with real time PCR for 11 GBA mutations (N370S, L444P, D409H, 84insGG, IVS2+1G>A, V394L, IVS10-1 G>A, IVS10-4 C>T, R120W, V460V and R496H). Leukocyte GBA expression levels were also investigated. All patients underwent a comprehensive assessment based on the Unified Parkinson’s Disease Rating Scale (UPDRS), H&Y scale, Hamilton’s Depression Scale (HDS), Non-Motor Symptom Questionnaire, Mini Mental State Examination (MMSE) and Addenbrooke’s Cognitive Examination (ACE-R).
Results: Mutation screening showed that 15.5% of PD patients and 0% of controls had GBA mutations (p=0,000). The most common GBA mutations were R496H and L444P mutations. Leukocyte GBA expression was significantly lower in patients with respect to the control (p=0,04). There was no difference in terms of clinical manifestations between mutation positive and negative PD patients.
Conclusions: This study is the first study to investigate the relationship between Parkinson’s disease and GBA mutations in Turkey. The results show that R496H mutation is frequent (14%) among Turkish PD patients. This mutation is related with neither early onset nor clinical manifestations. Regardless from mutation presence most of the PD patients (84.5 %) have cognitive impairment when tested with ACE-R.
Keywords:Parkinson’s disease; Glucocerebrosidase; GBA mutation; ACE-R
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease, characterized by resting tremor, bradykinesia, rigidity and postural instability. These symptoms result predominantly from selective loss of dopaminergic neurons in the substantia nigra pars compacta and subsequent depletion of dopamine in their projections. The pathogenesis of PD remains unclear. An interaction between environmental factors and genetic predisposition is thought to contribute to disease development. Causal mutations in the genes for α-synuclein, parkin, DJ-1, PTEN induced kinase 1 and leucine-rich repeat kinase-2 have been identified [1]. However, mutations of these genes do not account for the occurrence of PD in all patients. Therefore, identifying novel PD genetic risk factors is important to understand its pathogenesis.
Mutations and rearrangements in β-glucocerebrosidase (GBA) gene cause Gaucher disease (GD), a autosomal recessively inherited deficiency of the lysosomal enzyme, glucocerebrosidase [2]. Clinically, GD is characterized by vast phenotypic heterogeneity and is classified into three types based on the severity of associated neurological symptoms. There are multiple independent studies reporting the association between GBA mutations and parkinsonism [3-5]. Although the molecular pathogenic mechanism causing PD in GBA mutation carriers remains unclear, clinical observations, neuropathologic evidence, and genetic studies have implicated mutations in the GBA gene in parkinsonian phenotypes and in Parkinson disease (PD) susceptibility. Together with family studies revealing a significant frequency of parkinsonian symptoms in obligate or confirmed GBA mutation carrier relatives of patients with GD [6-8] and Parkinsonian manifestations reported in genotypically heterogeneous patients with GD [6,7,9], suggested an association between GD and PD. Furthermore, brain samples from autopsy-confirmed PD cases revealed significantly higher carrier frequencies than the estimated GBA mutation carrier frequency in the general population [10]. These findings strongly suggest that heterozygosity for mutations in the glucocerebrosidase gene may be a risk factor for parkinsonism.
In this case-control study, we aimed to investigate the possible association between the GBA gene mutations and PD in Turkey. We examined the frequency of most common 11 mutations in GBA gene in PD patients and healthy controls.
Materials and Methods
Here we report the results of mutation analysis of GBA in a series of 71 Turkish patients with PD, collected sequentially in Pamukkale University Hospital in Turkey and 76 healthy controls. All patients were Caucasian and of apparent Turkish ancestry. Patients were diagnosed according to the United Kingdom Brain Bank criteria. Inclusion criteria for patients were; (1) having the diagnosis of idiopathic PD, (2) responsive to L-dopa, (3) no history of recurrent stroke, encephalitis or head trauma (4) no history of neuroleptic or toxic exposure. The inclusion criteria for the control group was; (1) no history or finding suggesting parkinsonism, (2) no history of other neurological diseases, (3) no family history of PD.
The study is approved by the local ethics committee and all of the patients and controls gave their informed consents.
Clinical evaluation
All of the patients were evaluated by the study neurologist (G.D.) A full history, including clinical data, family history, and neurologic examination, was recorded for each patient. All patients underwent a comprehensive assessment comprising motor rating based on the Unified Parkinson’s Disease Rating Scale (UPDRS), clinical stage based on Hoehn and Yahr scale. Hamilton’s Depression Scale (HDS), Non-Motor Symptom Questionnaire (NMS), Mini Mental State Examination (MMSE) and Addenbrooke’s Cognitive Examination (ACE-R) were also applied. The cut off values for MMSE were 18/19 and 22/23 for patients having less and more than 5 year education, respectively [11]. The cut off values for ACE-R were 72 for patients who had less than 5 years of education; 82 for 5-12 years of education and 88 for more than 12 years of education [12-14]. The physical examination and tests were applied during “on” periods.
Methods
Genomic DNA was extracted from peripheral blood by using Roche High Pure PCR Template Preparation Kit as specified in the manufacturer’s protocol. All subjects were investigated for 11 common GBA mutations [N370S (rs76763715), L444P (rs421016), D409H (rs1064651), 84insGG (rs387906315), IVS2+1 G>A (rs104886460), V394L (rs80356769), IVS10- 1 G>A (rs189380051), IVS10-4 C>T (rs755265316), R120W (397515515), V460V (1135675) and R496H (rs7582236)] with real-time PCR using LightCycler 480 Real Time PCR system, Roche Diagnostics. Melting curve analysis was used to detect genotypes as wild type, heterozygous mutant or homozygous mutant. Genomic RNA was extracted from peripheral blood by using QIAamp RNA Blood Mini Kit, QIAGEN as specified in the manufacturer’s protocol. GBA expression analysis was performed on leukocyte RNA from patients who were positive for any of these GBA mutations and from healthy controls with real-time PCR using LightCycler 480 Real Time PCR system, Roche Diagnostics.
The raw data are evaluated in 3 steps. First, we identified the rate of 11 GBA gene mutations in Parkinson’s patients and compared with the healthy controls. In the second step, the mutation rates are compared between patients with young onset PD (≤ 50 years of age) (EOPD) and late onset PD (>50years of age) (LOPD). In the third step, independent from age at disease onset, we compared the clinical parameters such as onset symptom, disease subtype, disease progression (H & Y score), family history, DBS requirement and cognitive performance between mutation positive and negative patients.
Statistical analysis
The data were analyzed by SPSS software program. Continuous variables were expressed as mean ± standard deviation, median (minimum and maximum values), and categorical variables as number and percentage. Student-t test was used for comparing parametric test assumptions and independent group differences; Mann-Whitney U test was used to compare independent group differences when parametric test assumptions were not provided. Differences between categorical variables were analyzed by Chi square analysis. In all analyzes p <0.05 was considered statistically significant.
Results
GBA Mutation Rate in PD patients
The demographic characteristics of the patient and control groups are summarized in Table 1. All subjects were investigated for 11 most common GBA mutations. A total of 11/71 (%15,5) PD patients were heterozygous for two common GBA mutations as shown in Table 1. As shown in Table 1 compared with the PD patients there were no GBA mutations in healthy control subjects (p=0,000). The most remarkable finding in GBA mutation positive patients was the high rate of R496H mutation detected in 10 of 11 mutation positive PD patients. Ten out of 11 GBA mutation positive PD patients only 1 (%9) had L444P mutation. Leukocyte GBA expression was significantly lower in patients with respect to the control individuals (p=0,04) (Table 2).
Table 1: The demographic characteristics and GBA mutation rates of the patient and control groups.
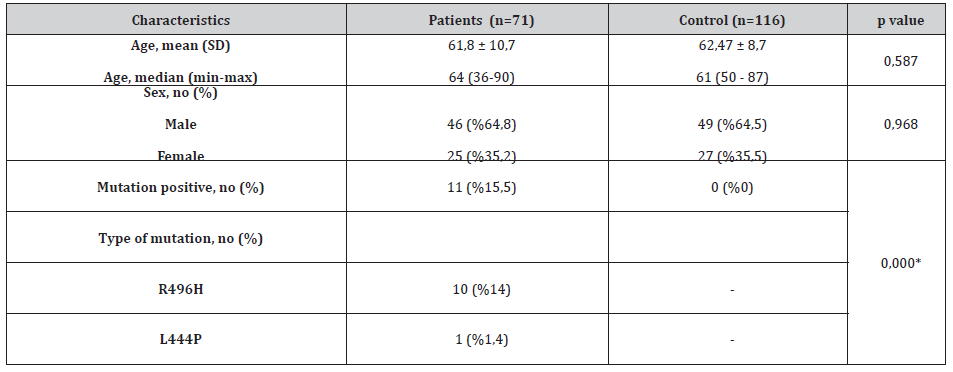
Table 2: GBA ΔCT values of the patients and healthy control subjects.

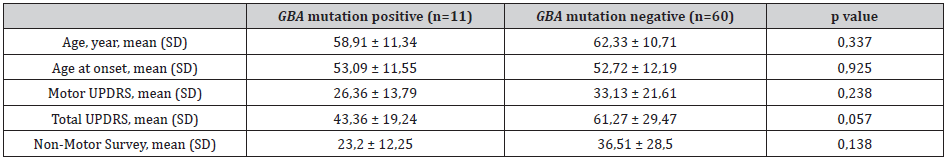
Table 3: Comparison of parameters of GBA mutation-detected and non-mutated patients.
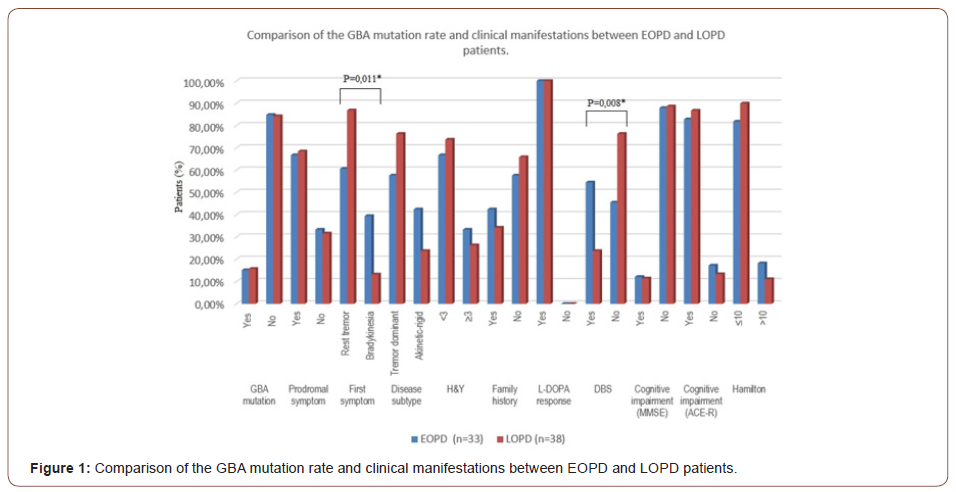
GBA Mutation and Clinical Manifestations in Early and Late Onset PD
As shown in Figure 1, there was no difference in GBA mutation rate between EOPD and LOPD patients. Our comparisons revealed that clinical manifestations were also similar in both groups. Asymmetric rest tremor was the first symptom in both EOPD and LOPD patients, and it was more frequent than bradykinesia as the initial symptom in LOPD patients. (p=0,011). There was no difference in positive family history and cognitive impairment or depression symptoms between EOPD and LOPD patients.
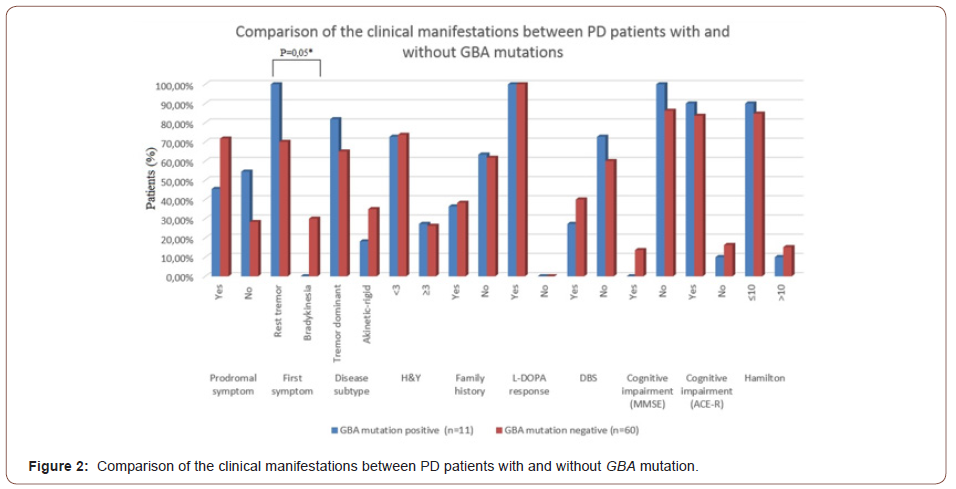
Clinical Manifestations in GBA Mutation Positive and Negative PD Patients
In the third step of the analysis, independent from age at disease onset, we compared the prodromal symptoms, symptom at disease onset, disease subtype, disease severity (H & Y score), positive family history, depression, non-motor symptoms, DBS requirement and cognitive performance between mutation positive and negative patients.
Prodromal symptoms such as mask face, constipation and anosmia, were present in 45.5 % of mutation positive and 71,7% of mutation negative patients. In all GBA mutation positive PD patients, asymmetric rest tremor was the initial symptom of the disease. However, the first symptom was rest tremor in 70% of the PD patients who were negative for GBA mutations (p=0,05). The family history for PD was similar in both mutation positive and negative patients (Figure 2). Despite the high and consistent mutation rate, there was a remarkable similarity in age at disease onset, “on” UPDRS scores, non-motor symptoms scores, depression scores and family history between mutation positive and negative patients (Table 3 and Figure 2).
In cognitive evaluation we used MMSE and ACE-R tests. According to MMSE results only 6.7% of PD patients had cognitive impairment, however with ACE-R test we identified that 84.5% of the PD patients had cognitive impairment. In other words, MMSE missed 63% (45 of 71 patients) of patients who had cognitive impairment (Table 4). Mutation positivity was not a significant factor on cognitive performance.
Table 4: Comparison of MMSE and ACE-R tests.

Discussion
In recent years researchers have found a strong association between PD and the GBA gene, responsible for Gaucher’s disease which is a lysosomal storage disease. Sidransky and colleagues found that parents of Gaucher patients and their second-degree relatives were PD patients. When they performed GBA mutation analysis on these family members with PD, they found that they carried heterozygous GBA mutation [7]. After this study, following studies have revealed a strong relationship between PD and GBA that is not exclusive to a specific ethnic group or a specific GBA mutation [5,15,16]. Although there are differences between populations, GBA mutations are seen in approximately 5-10% of PD cases [17,18]. The high frequency of mutations among ethnically diverse, heterogeneous series of PD patients makes this gene the most common genetic risk factor for PD. Mutation detection rates in the healthy control group also differ between populations [15]. GBA mutation rate in PD is highest in Ashkenazi Jews with 10.7- 31.3% [19]. In this population, about 3% of GBA mutation carriers are also found in healthy individuals. GBA mutation carriers have 5 times higher risk for PD than in the normal population [20]. GBA mutation rates in early-onset patients were two-fold higher than late-onset patients [21].
We found a frequency of 15.5 % (11 of 71) pathogenic GBA mutationin Turkish PD series and no mutation in healthy controls (P=0,000). The most common mutation identified was R496H. Ten out of 11 GBA mutation positive PD patients (91 %) had R496H mutation and only 1 (%9) had L444P mutation. These results represent a significantly higher frequency of R496H mutation in GBA in Turkish PD patients. Additionally leukocyte GBA expression was significantly lower in patients with respect to the control individuals (p=0,04). Chiasserini et al. have found that GCase enzyme levels were normal despite low expression levels [22]. They have found low GBA expression levels at frontal cortex, caudate nucleus, hippocampus, substantia nigra and cerebellum in GBA mutation positive PD patients. However GCase enzyme levels at these brain regions were normal, except substantia nigra and cerebrospinal fluid. Another study showed a negative association between with GCase activity in leucocytes and measurement of oligomeric alpha-synuclein in plasma of GD patients, postulating that the reduced GCase activity might be linked to the peripheral accumulation of toxic alpha-synuclein species [23].
Previously, R496H mutation was reported as a frequent mutation in Ashkenazi population, but a large multi-center analysis with 16 participating centers revealed that L444P and N370S are the most common GBA mutation among Ashkenazi Jewish subjects and either mutation was found in 15% of patients and 3% of controls [15]. Hence there is no previous data about GBA mutation in PD among Turkish patients, the frequency of most common GBA mutations is unknown. Many studies in the literature report results about genotype-phenotype relationship among the L444P and N370S mutation carriers with PD. Some studies state that R496H mutation is one of the frequent “mild” mutations but this mutation is not separately evaluated in terms of clinical parameters.
To understand the genotype-phenotype relationship, we compared prodromal symptoms, age at disease onset, initial symptom at onset, disease subtype, motor impairment, presence of family history, non-motor symptoms and cognitive performance in mutation positive and negative PD patients. Our results revealed that PD patients with R496H mutation did not differ from patients without mutation in terms of these clinical findings and scores, except initial symptom at onset.
All of the patients with R496H mutation had asymmetric hand tremor at onset. Despite the fact that the onset symptom was rest tremor in 100% of the GBA mutation positive PD patients, 81.8% was with tremor predominant subtype. In other words, the subtype of the disease might have evolved in the later stages of the disease, regardless of the onset symptom.
Previous studies report that GBA mutations predispose to a younger age at motor onset which occurs about 1.7-6.0 years earlier than in PD patients without GBA mutation [3,24,25]. Furthermore, studies with early age at onset PD strengthened this finding [26,27]. However our results are different from previous reports which have found the above mentioned results in mostly L444P and N370S mutation carriers. In our series, R496H mutation which is a mild mutation according to definitions of Beutler et al, was the only predominant mutation. Clinical correlation analysis revealed that it is not possible to differentiate R496H mutation carriers clinically from non-carriers. Several studies have demonstrated the differential effects of severe versus mild GBA mutations on the risk and age at onset of Parkinson’s disease [28,29]. Carriers of severe GBA mutations tend to have an earlier age at onset, however phenotypic presentation of mild mutations may not be differentiated from sporadic PD.
Similar to our findings, Sidransky et al reported that severity of motor impairment or Hoehn and Yahr staging revealed no significant differences between GBA mutation carries and noncarriers, although one study reported GBA- associated PD patients to show more bradykinesia [15]. PD patients carrying a GBA mutation have a higher prevalence of cognitive impairment compared to nonmutation carriers [25,28-30]. Genotype-phenotype correlations indicated severe mutations (p-L444P) to predispose to more frequent cognitive decline [27]. There is no sufficient data about R496H mutation and cognitive impairment, but our results revealed that there was a high frequency (84.5 %) of cognitive impairment in PD patients regardless from presence of mutation.
Conclusion
In conclusion, this study is the first study to investigate the relationship between Parkinson’s disease and GBA mutations in Turkey. The results show that R496H mutation is frequent (14%) among Turkish PD patients. This mutation is related with neither early onset nor clinical manifestations. Regardless from mutation presence, most of the PD patients (84.5 %) have cognitive impairment. MMSE test may not be adequate, therefore more detailed neuropsychiatric test are required to determine the cognitive deficits in PD patients. The study have some limitations. First of all is that, the sample size is not large enough for represent the Turkish population. The other limitation is that, because of cost effectiveness we didn’t able to evaluate GBA expression levels from all subjects. Limitations of our study should be overcome in future with larger cohorts.
Acknowledgement
This study is conducted by Dr. Özlem Anlaş as a thesis and supported by Pamukkale University. Özlem Anlaş and Göksemin Demir have equally contributed to the study.
Conflict of Interest
No conflict of interest.
References
- T Gasser (2005) Genetics of Parkinsonʼs disease. Curr Opin Neurol 18: 363-369.
- E Beutler (2007) Gaucher disease Curr Opin Chem Biol 129: 17-49.
- J Aharon Peretz, H Rosenbaum, R Gershoni-Baruch (2004) Mutations in the Glucocerebrosidase Gene and Parkinson’s Disease in Ashkenazi Jews. N Engl J Med 351(19): 1972-1977.
- C Sato, A Morgan, AE Lang, S Salehi-Rad, T Kawarai, et al. (2005) Analysis of the glucocerebrosidase gene in Parkinson’s disease. Mov Disord 20: 367-370.
- W Westbroek, AM Gustafson, E Sidransky (2011) Exploring the link between glucocerebrosidase mutations and parkinsonism. Trends Mol Med 17: 485-493.
- B Bembi, S Zambito Marsala, E Sidransky, G Ciana, M Carrozzi, et al. (2003) Gaucher’s disease with Parkinson’s disease. Neurology 61: 99 LP-101.
- O Goker-Alpan, R Schiffmann, ME LaMarca, RL Nussbaum, A McInerney-Leo, et al. (2004) Parkinsonism among Gaucher disease carriers. J Med Genet 41(12): 937-940.
- A Halperin, D Elstein, A Zimran (2006) Increased incidence of Parkinson disease among relatives of patients with Gaucher disease. Blood Cells Mol Dis 36(3): 426-428.
- ZA Neudorfer O, Giladi N, Elstein D, Abrahamov A, Turezkite T, et al. (1996) Occurence of Parkinson’s syndrome in type I Gaucher disease. QJM 89: 691-694.
- A Lwin, E Orvisky, O Goker-Alpan, ME LaMarca, E Sidransky (2004) Glucocerebrosidase mutations in subjects with parkinsonism, Mol Genet Metab 81(1): 70-73.
- TZ Keskinoglu P, Ucku R, Yener G, Yaka E, Kurt P (2009) Reliability and validity of revised Turkish version of Mini Mental State Examination (rMMSE-T) in community-dwelling educated and uneducated elderly., Int J Geriatr Psychiatry 24: 1242–1250.
- E Mioshi, K Dawson, J Mitchell, R Arnold, JR Hodges (2006) The Addenbrooke’s Cognitive Examination revised (ACE-R): A brief cognitive test battery for dementia screening, Int J Geriatr Psychiatry 21(11): 1078-1085.
- A Nieto, I Galtier, E Hernández, P Velasco, J Barroso (2016) Addenbrooke’s Cognitive Examination-Revised: Effects of Education and Age. Normative Data for the Spanish Speaking Population. Arch Clin Neuropsychol.
- D Berankova, E Janousova, M Mrackova, I Eliasova, M Kostalova, et al. (2015) Addenbrooke’s cognitive examination and individual domain cut-off scores for discriminating between different cognitive subtypes of Parkinson’s disease, Parkinsons Dis.
- E Sidransky, MA Nalls, JO Aasly, J Aharon-Peretz, G Annesi, et al. (2009) Multicenter Analysis of Glucocerebrosidase Mutations in Parkinson’s Disease N Engl J Med 361: 1651-1661.
- LN Clark, BM Ross, Y Wang, H Mejia-Santana, J Harris, et al. (2007) Mutations in the glucocerebrosidase gene are associated with early-onset Parkinson disease, Neurology 69(12): 1270–1277.
- LV Kalia, AE Lang (2015) Parkinson’s disease. Lancet.
- A Migdalska-Richards, AHV Schapira (2016) The relationship between glucocerebrosidase mutations and Parkinson disease. J Neurochem 77-90.
- E Sidransky, G Lopez (2012) The link between the GBA gene and parkinsonism. Lancet Neurol 11: 986-998.
- LM Chahine, J Qiang, E Ashbridge, J Minger, D Yearout (2013) Clinical and biochemical differences in patients having parkinson disease with vs without GBA mutations. JAMA Neurol 70: 852–858.
- YR Wu, CM Chen, CY Chao, LS Ro, RK Lyu, et al. (2007) Glucocerebrosidase gene mutation is a risk factor for early onset of Parkinson disease among Taiwanese. J Neurol. Neurosurg. & amp; Psychiatry 78: 977–979.
- D Chiasserini, S Paciotti, P Eusebi, E Persichetti, A Tasegian, et al. (2015) Selective loss of glucocerebrosidase activity in sporadic Parkinson’s disease and dementia with Lewy bodies. Mol Neurodegener 10: 15.
- S Pchelina, A Emelyanov, G Baydakova, P Andoskin, K Senkevich, et al. (2017) Oligomeric α-synuclein and glucocerebrosidase activity levels in GBA-associated Parkinson’s disease. Neurosci Lett 636: 70–76.
- ZY Tan EK, Tong J, Fook-Chong S, Yih Y, Wong MC, et al. (2007) Glucocerebrosidase Mutations and Risk of Parkinson Disease in Chinese Patients aucher. Arch Neurol 64: 1056–1057.
- J Neumann, J Bras, E Deas, SS O’sullivan, L Parkkinen, et al. (2009) Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease, Brain 132(Pt 7): 1783-94.
- YR Wu, CM Chen, CY Chao, LS Ro, RK Lyu, et al. (2007) Glucocerebrosidase gene mutation is a risk factor for early onset of Parkinson disease among Taiwanese. J Neurol Neurosurg Psychiatry 78(9): 977–979.
- Z Gan-Or, N Giladi, U Rozovski, C Shifrin, S Rosner, et al. (2008) Genotype-phenotype correlations between GBA mutations and Parkinson disease risk and onset. Neurology 70: 2277–2283.
- K Brockmann, K Srulijes, AK Hauser, C Schulte, I Csoti, et al. (2011) GBA-associated PD presents with nonmotor characteristics, Neurology.
- RN Alcalay, E Caccappolo, H Mejia-Santana, MX Tang, L Rosado, et al. (2012) Cognitive performance of GBA mutation carriers with early-onset PD The CORE-PD study, Neurology 78(18): 1434-40.
- LM Chahine, A Ahmed, Z Sun (2011) Effects of STN DBS for Parkinson?s disease on restless legs syndrome and other sleep-related measures, Parkinsonism Relat. Disord 17: 208-211.
-
Özlem Anlaş, Göksemin Demir, Gülseren Bağcı. GBA Mutation Analysis in Turkish Parkinson’s Disease Patients: Comparison of Clinical and Cognitive Performance. Arch Neurol & Neurosci. 12(2): 2022. ANN.MS.ID.000782. DOI: 10.33552/ANN.2022.11.000781
-
Neurobiology, Consciousness, Nervous Activity, Cognition, Brain.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






