 Research Article
Research Article
Effect of Memantine on Cognitive Impairment in Patients with Epilepsy
Mohamed Mostafa Shehab*, Esam Saad Darwish, Salma Mohamed Fahmy and Amal Mohamed Tohamy
Department of Neurology, Faculty of Medicine, Assiut University, Egypt
Mohamed Mostafa Shehab, MD, Department of Neurology, Faculty of Medicine, Assiut University, Egypt.
Received Date: September 21, 2020; Published Date: October 15, 2020
Background:b> Epilepsy is a complex disorder, which involves much more than seizures, encompassing a range of associated co-morbid health conditions that can have significant health and quality-of-life implications. Of these co-morbidities, cognitive impairment is one of the most common and distressing aspects of epilepsy. Cognitive and behavioral impairments have been observed as a consequence even of single seizure. Memantine is approved for the treatment of cognitive impairment in Alzheimer’s dementia and used to treat other neurodegenera0tive dementias. In addition to its benefits on cognition, function, and global status, memantine treatment may also help alleviate behavioral symptoms.
Methods: A randomized, double-blind, placebo-controlled, parallel study was carried over 100 patients with idiopathic epilepsy associated with mild to moderate cognitive impairment divided into two groups each group 50 patients. Group (A) received memantine while group (B) received placebo. A battery of psychometric tests was utilized.
Results: Memantine group showed significant gradual improvement in cognitive and memory functions by WAIS, MAS and BVMT-R after 3 and 6 months in comparison to baseline assessment. While, in Placebo group there was no significant improvement in cognition and memory functions after 3 and 6 months in comparison to baseline assessment.
Conclusion: Memantine (10 mg) once daily is a safe and effective drug in improving cognitive and memory functions in patients with idiopathic epilepsy associated with mild to moderate cognitive impairment.
Keywords: Epilepsy; Cognitive impairment; Memantine
Introduction
Epilepsy is one of the most common neurological disorders and major health problems that affect people in every country throughout the world. The exact mechanism of epilepsy is unknown [1], but a little is known about its cellular and network mechanisms. In epilepsy, the resistance of excitatory neurons to fire during this period is decreased. This may occur due to changes in ion channels or inhibitory neurons not functioning properly. This then results in a specific area from which seizures may develop, known as a “seizure focus” [2]. Another mechanism of epilepsy may be the upregulation of excitatory circuits or down-regulation of inhibitory circuits following an injury to the brain [2,3].
Epilepsy is a complex disorder, which involves much more than seizures, encompassing a range of associated comorbid health conditions that can have significant health and quality-of-life implications. Of these comorbidities, cognitive impairment is one of the most common and distressing aspects of epilepsy [4]. Cognitive and behavioral impairments have been observed as a consequence even of single seizure [5]. In people with epilepsy, cognitive difficulties that compromise educational progress and achievement throughout life are common [6]. The exact cause of cognitive impairment in epilepsy has not been fully explored. Cognitive impairments in epilepsy are a consequence of complex interactions among the etiologies of the epilepsy, the seizures themselves, interictal discharges, and antiepileptic drugs [7-10]. Excitotoxicity, mediated by glutamate acting on N-methyl-D-aspartate (NMDA) receptors in the hippocampus, can cause memory dysfunction in epilepsy [11,12]. All antiepileptic medications have the potential to have the detrimental side effect of slowing cognition. This is by nature of the medication stopping brain “over-activity” like during a seizure [8]. AEDs not only reduce neuronal irritability but also may impair neuronal excitability, neurotransmitter release, enzymes, and factors critical for information processing and memory. Older AEDs have higher cognitive disturbances than newer AEDs [9,10]. There is no specific treatment available for the cognitive impairment of patients with epilepsy [13]. Memantine is a lowaffinity voltage-dependent uncompetitive antagonist of the NMDAtype glutamate receptor [14,15]. It also has antioxidant property and increases production of brain derived neurotropic factor (BDNF) [16,17]. Memantine is used to treat moderate-to-severe Alzheimer’s disease, especially for people who are intolerant of or have a contraindication to acetyl cholinesterase (AChE) inhibitors [18,19].
Aim of the work
This study aimed to evaluate the efficacy of memantine on improving cognitive and memory impairment inpatients with epilepsy.
Patients and Methods
This study was double-blinded and included 100 patients with epilepsy recruited from the outpatient epilepsy clinic of Assiut University Hospital. Patients selected had idiopathic epilepsy, aged 18-55 years, with normal brain imaging. Patients were on AEDS more than 6 months with no more than two fits per month. Patients had MMSE score of 10-24 (mild and moderate cognitive impairment) and had no other medical disorders.
Patients with progressive neurological diseases, abnormal brain imaging, more than two fits per month, severe cognitive impairment (score of MMSE less than10), severe medical disorders or aging less than 18 years or more than 55 years were excluded.
Every patient underwent the following:
1. Complete clinical evaluation regarding the onset, type and frequency of seizures and therapeutic history of the patient.
2. Computerised tomography of the brain (CT brain) to exclude symptomatic epilepsy.
3. Baseline cognitive assessment
i. Mini-mental state examination: was performed for screening of cognitive affection in patients with epilepsy [20].
ii. Wechsler Adult Intelligence Scale (WAIS): intelligence quotient (IQ) tests are the primary clinical instruments used to measure adult and adolescent intelligence [21].
iii. Memory Assessment Scale (MAS): This comprehensive battery assesses short-term, verbal and visual memory functioning [22].
iv. Brief Visuospatial Memory Test–Revised (BVMT-R): has been commonly used to evaluate visuospatial memory abilities in neuropsychological populations [23].
Procedure
All data performed in accordance with the Declaration of Helsinki. This study was approved by the ethical committee of Faculty of Medicine, Assiut University, Assiut, Egypt. Those 100 patients were classified into two groups; group (A) received memantine while group (B) received placebo drugs. Memantine hydrochloride tablet was started at the dose of 5 mg once daily orally, and the same dose was given for 3months and dose is titrated to 10 mg once daily orally for the remaining 3months. Placebo was given once daily orally for 6months. Patients were instructed to take the study medication at the same time every day. Follow up was done over 3and 6 months by Wechsler Adult Intelligence Scale (WAIS), Memory Assessment Scale (MAS) and Brief Visuospatial Memory Test–Revised (BVMT-R). Comparison was done between the results of these tests at baseline, three months and six months and between the two groups.
Statistical Analysis
Data was collected and analyzed those using SPSS (Statistical Package for the Social Science, version 20, IBM, and Armonk, New York). Continuous data was expressed in form of mean ± SD or median (range) while nominal data was expressed in form of frequency (percentage). Chi²-test was used to compare the nominal data of different groups in the study while student t-test was used to compare mean of different two groups and paired t test was used to compare baseline date with follow up data. Level of confidence was kept at 95% and P value was considered significant if < 0.05.
Results
(Table 1) Data was expressed in form of frequency (percentage), mean (SD). P value was significant if < 0.05.GTCC: generalized tonic clonic convulsions; GCC: generalized colonic convulsion; GTC: generalized tonic convulsion. Study group showed significant increase in IQ at 3rd and 6th month of follow up in comparison to baseline data. Deterioration index (DI) was significantly decreased at 3rd and 6th month of follow up in comparison to baseline DI in the study group (Table 2). Data was expressed in form of, mean (SD). P value was significant if < 0.05. P1, compared between both groups while P2, compared baseline data with 3rd and 6th data of the same group. MAS showed significant improvement in short term memory, visual memory and total scale memory at 3rd and 6th month of follow up in comparison to baseline data (Table 3).
Table 1:Demonstrates the clinical and demographic data of studied groups.
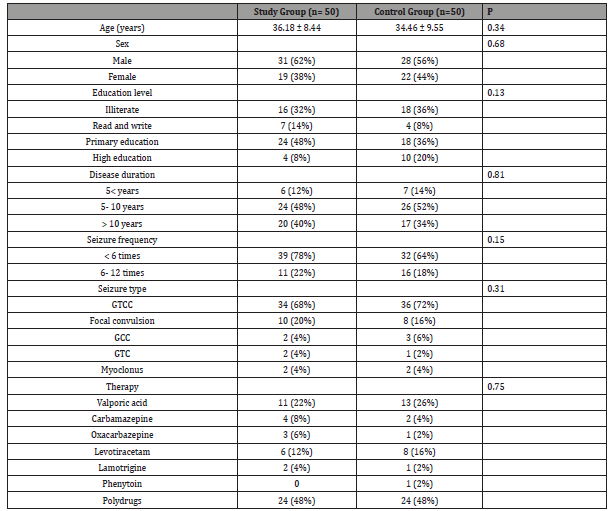
Table 2:Demonstrates intelligence quotient and deterioration index in studied groups.
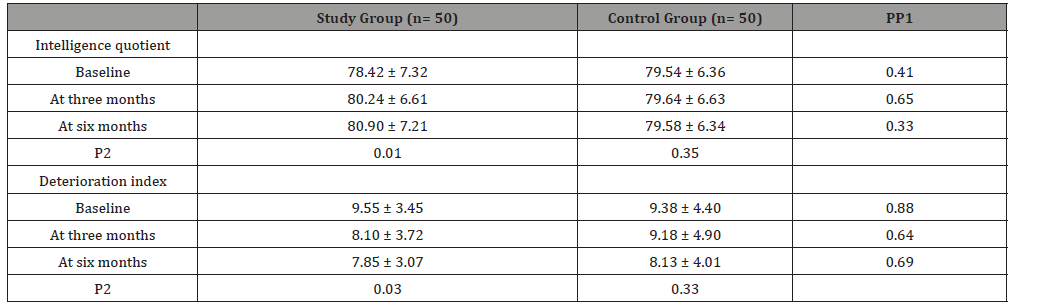
Table 3:Demonstrates Memory assessment scale in studied groups.
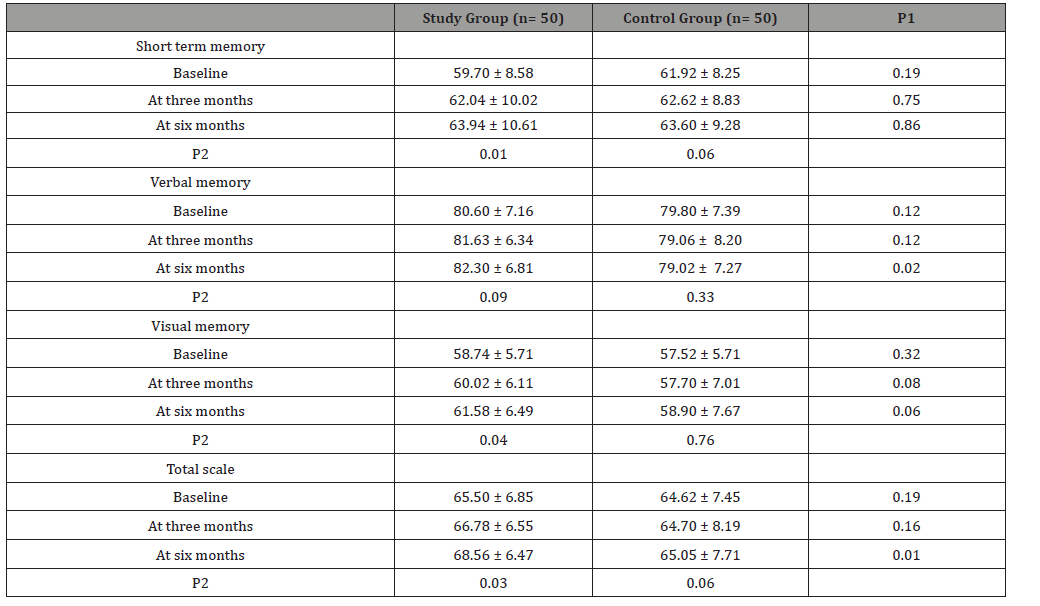
Table 4:Demonstrates Brief visuospatial memory test in studied groups.

As regarding brief visuospatial memory test, study group had significant higher discrimination index, significant improvement in percent retained, and lower response bias at 3rd month and 6th month in comparison to baseline data (Table 4).
Discussion
The exact cause of cognitive impairment in epilepsy has not been fully explored. The mechanism behind cognitive and memory dysfunction in epilepsy may be due to excitotoxic damage caused to the brain by the excitatory neurotransmitter glutamate through NMDA receptor. This glutamate excess affects long term potentiation and learning under pathological conditions which lead to neuronal cell death and free radical formation [12]. Alteration of this excito-toxic pathway can be a novel, potentially safer and more effective, approach to the treatment of memory loss [17,24]. In this study, we used memantine which is uncompetitive antagonist of the NMDA-type glutamate receptor. It can prevent brain damage caused by excito-toxicity by blocking the NMDA receptor channel and by preventing glutamate action. A total of 100 patients were included in the study divided into two groups each group 50 patients, group A received memantine and group B received placebo for 6 months. In our study, we compared between memantine group and placebo group at baseline, 3rd and 6th month regarding their cognitive and memory functions using Wechsler Adult Intelligence Scale (WAIS), Memory Assessment Scale (MAS) and Brief Visuospatial Memory Test–Revised (BVMT-R). We found significant improvement in cognition in memantine group assessed by WAIS including IQ and DI. This effect started at the 3rd month of memantine therapy and highly significant improvement was seen at the 6th month of followup (p=0.01 in IQ, p=0.03 in DI), which corresponds to 10 mg once daily dosage of memantine with no significant improvement in IQ and DI at 3rd and 6th month of follow up in comparison to baseline in placebo group. Our study showed significant improvement in MAS with memantine group as regarding verbal memory and total scale compared with placebo. This effect was significant at the 6th month of follow up ((p=0.02 in verbal memory, p=0.01in total scale). Memantine group also showed significant improvement in short term memory, visual memory and total scale at 3rd and 6th month of follow up in comparison to baseline (p=0.0.1, p=0.04, p=0.03).
Our study also showed statistically significant improvement in memory assessed by Brief visuospatial memory test in memantine group compared with placebo. This effect started at 3rd month of memantine therapy and significant improvement was seen at the 6th month of follow-up (p=0.02, p=0.01, p=0.03 as regarding discrimination index, percent retained and response bias respectively). These results are consistent with the findings of [25] who studied the efficacy of memantine on improving cognitive functions in epileptic patients receiving anti-epileptic drugs. The previous study included 55 epileptic patients 26 memantine group and 29 placebo group for 4 months (the increase in number of patients and longer duration of the present study made the result more reliable). This study was similar to our study in the age of the patient was in between 18-55 and most of them with GTCC and on multiple anti-epileptic medications.
Our results are also consistent with [26] study which studied effect of memantine on improving memory in subjects with focal-onset epilepsy and memory dysfunction but in this study subjects underwent cognitive testing at baseline, after a 13-week randomized, parallel-group, double-blinded phase and following a 13-week open-label extension phase using the selective reminding test (SRT), continuous long-term retrieval (CLTR) score and 7/24 Spatial Recall Test learning score as the primary outcome measures and secondary measures included tests of attention span, fluency, visual construction, and response inhibition and this study revealed no significant effect of memantine on cognition when assessed at the end of the blinded period but pooled data at the end of the open-label phase showed significant improvement over baseline performance in measures of verbal memory, frontal-executive function, and memory-related quality of life.
Conclusion
From this study, we conclude that patients with idiopathic epilepsy associated with mild to moderate cognitive impairment have significantly improved regarding their cognitive and memory functions after taking memantine at dose 10mg once daily orally in comparison to those patients who took placebo.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Parent JM, Kron MM (2012) Neurogenesis and epilepsy. Jasper's Basic Mechanisms of the Epilepsies [Internet] 4th edition: National Center for Biotechnology Information (US).
- McPhee S, Ganong W (2010) Pathophysiology of Disease: An Introduction to Clinical Medicine, diterjemahkan oleh Brahm. U, EGC, Jakarta: 493-500.
- Goldberg EM, Coulter DA (2013) Mechanisms of epileptogenesis: a convergence on neural circuit dysfunction. Nature Reviews Neuroscience 14(5): 337-349.
- Holmes GL (2015) Cognitive impairment in epilepsy: the role of network abnormalities. Epileptic Disorders 17(2): 101-116.
- Smeets VM, Van Lierop BA, Vanhoutvin JP, Aldenkamp AP, Nijhuis FJ (2007) Epilepsy and employment: literature review. Epilepsy & behavior 10: 354-362.
- Berg AT, Langfitt JT, Testa FM, Levy SR, DiMario F, et al. (2008) Global cognitive function in children with epilepsy: a community‐based study. Epilepsia 49(4): 608-614.
- Raspall-Chaure M, Neville BG, Scott RC (2008) The medical management of the epilepsies in children: conceptual and practical considerations. The Lancet Neurology 7(1): 57-69.
- Eddy CM, Rickards HE, Cavanna AE (2011) The cognitive impact of antiepileptic drugs. Therapeutic advances in neurological disorders 4(6): 385-407.
- Motamedi GK, Meador KJ (2004) Antiepileptic drugs and memory. Epilepsy & Behavior 5(4): 435-439.
- Meador KJ, Gilliam FG, Kanner AM, Pellock JM (2001) Cognitive and behavioral effects of antiepileptic drugs. Epilepsy & Behavior 2(4): SS1-SS17.
- Packer L, Tritschler HJ, Wessel K (1997) Neuroprotection by the metabolic antioxidant α-lipoic acid. Free Radic Biol Med 22(1-2): 359-378.
- Standaert DG (1996) Treatment of central nervous system degenerative disorders. The pharmacological basis of therapeutics: 503-555.
- Mosaku KS, Fatoye FO, Komolafe M, Lawal M, Ola BA (2006) Quality of life and associated factors among adults with epilepsy in Nigeria. The International Journal of Psychiatry in Medicine 36(4): 469-481.
- Rogawski MA, Wenk GL (2003) The neuropharmacological basis for the use of memantine in the treatment of Alzheimer's disease. CNS drug reviews 9(3): 275-308.
- Jones KC, Han JY, Shah AA (2016) Cognitive Continuum: Areas of Controversy with Cognitive Enhancers. Psychiatric Annals 46(2): 110-117.
- Kumar S (2004) Memantine: Pharmacological properties and clinical uses. Neurol India 52(3):307.
- Gardoni F, Di Luca M (2006) New targets for pharmacological intervention in the glutamatergic synapse. Eur J Pharmacol 545(1): 2-10.
- Mount C, Downton CJNm (2006) Alzheimer disease: progress or profit? 12(7): 780.
- Herrmann N, Chau SA, Kircanski I, Lanctot KLJD (2011) Current and emerging drug treatment options for Alzheimer’s disease: a systematic review 71(15): 2031-2065.
- Folstein MF, Folstein SE, Mchugh PR (1975) “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research 12: 189-198.
- Lichtenberger EO, Volker MA, Kaufman AS, Kaufman NL (2006) Assessing gifted children with the Kaufman assessment battery for children—Second edition (KABC-II). Gifted Education International 21: 99-126.
- Williams JM (1991) Memory assessment scales. Odessa, FL: Psychological Assessment Resources 199.
- Benedict RH (1997) Brief visuospatial memory test--revised: professional manual, PAR.
- Sonkusare SK, Kaul C, Ramarao P (2005) Dementia of Alzheimer’s disease and other neurodegenerative disorders—memantine, a new hope. Pharmacological research 51: 1-17.
- Marimuthu P, Varadarajan S, Krishnan M, Shanmugam S, Kunjuraman G, et al. (2016) Evaluating the efficacy of memantine on improving cognitive functions in epileptic patients receiving anti-epileptic drugs: A double-blind placebo-controlled clinical trial (Phase IIIb pilot study). Annals of Indian Academy of Neurology 19: 344.
- Leeman-Markowski BA, Meador KJ, Moo LR, Cole AJ, HOCH DB, et al. (2018) Does memantine improve memory in subjects with focal-onset epilepsy and memory dysfunction? A randomized, double-blind, placebo-controlled trial. Epilepsy & Behavior 88: 315-324.
-
Qusai Al harahsheh. Ethics of Seclusion Usage among Aggressive Patients in Psychiatric Setting. 9(1): 2020. ANN.MS.ID.000705.
-
Epilepsy; Cognitive impairment; Memantine, seizures, Cognitive, Behavioral impairments; N-methyl-D-aspartate, Neuropsychological populations, cognitive impairment.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






