 Reseach Article
Reseach Article
Clinical Study of Neuroendoscopic Treatment of Subacute Subdural Hematoma (SSH)
Xuejian Wang, Xiangdong Li* and Zhifeng Wang
Department of Neurosurgery, First Affiliated Hospital of Soochow University, Soochow University, Soochow, Jiangsu, PR China
Department of Neurosurgery, First Affiliated Hospital of Soochow University, Soochow University, Soochow, Jiangsu, PR China
Received Date: July 02, 2020; Published Date: July 21, 2020
Abstract
Background: To investigate the methods of neuro-endoscopic treatment on subacute subdural hematoma (SSH), as well as the related surgical techniques and effects.
Methods: The clinical data of 14 patients with SSH who underwent neuroendoscopic treatment in our hospital from September 2018 to June 2019 were retrospectively analyzed. CT and MRI were performed before operation to confirm the diagnosis. 10 cases underwent general anesthesia, and 4 cases underwent local anesthesia. The hematoma was cleared under neuroendoscopy.
Results: All cases were successfully operated. The effective rate was 100%. At the time of discharge, 12 cases showed complete disappearance of hematoma, and 2 cases showed a small amount of remaining liquid and gas, as examined by CT scan. The muscle strength of hemiplegic limb significantly recovered after operation, and the patient’s self-care ability was obviously improved. No surgical complication occurred, and no recurrence happened in 3 months.
Conclusion: The application of neuro-endoscopy in treating subacute subdural hematoma (SSH) is clinically effective, with less trauma, good efficacy, and better safety. Therefore, this method should be widely applied in clinical practice.
Keywords: Subacute subdural hematoma; Neuro-endoscopy; Small bone window
Abbreviation: SSH: subacute Subdural Hematoma; CT: Computerized Tomography; MRI: Magnetic Resonance Imaging
Background
Subacute subdural hematoma (SSH) is a common disease in neurosurgery. The pathogenesis of this disease is complex, and it does not have characteristic clinical manifestations. Some of the patients have a history of trauma. In worse cases, the patient may have consciousness disorder and limb hemiplegia, which severely affect the patient life quality [1]. So far, there are several treatment methods for SSH, including trepanation and drainage, opening bone flap to remove hematoma, and conservative treatment until the disease becomes chronic, followed by surgical removal [2-5]. With the recent advancement in neuro-endoscopy technology, it is possible to clear intracranial hematoma under direct vision and with minimal invasion. From September 2018 to June 2019, we treated 14 SSH patients using neuro-endoscope. The clinical effect was significant, with no complications occurred. The detailed report is as follows.
Clinical data
A total of 14 patients were included in this study, with 9 males and 5 females. The average age was 57 years old, ranging from 43 to 76 years old. Among these patients, 11 had a history of trauma, 9 had headache, 5 had hemiplegia, and 4 had disordered consciousness. All patients received CT or MRI examination: all 14 cases were diagnosed as subacute subdural hematoma (SSH), 1 case was considered as subdural effusion; moreover, 11 cases had unilateral hematoma, and 3 cases showed bilateral hematoma.
Methods
Ethics approval and consent to participate
This research has been approved by the ethics committee of our department. Informed consent has been obtained and this investigation has been conducted according to the principles expressed in the Declaration of Helsinki. And the authors have obtained written informed consent of all the patients. Clinical trial registration number: ChiCTR-POC-16010237.
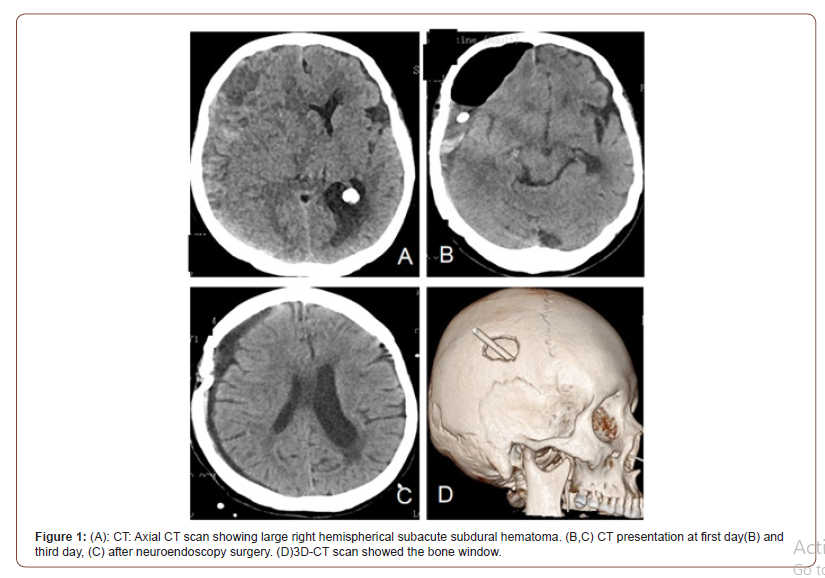
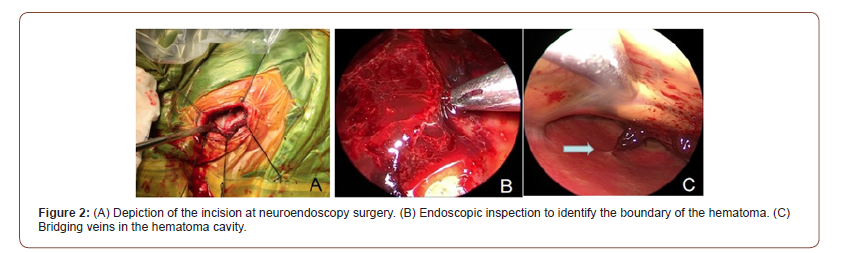
All patients underwent routine head CT examination before surgery, and the hematoma location was marked by scalp MARKER. The incision was designed and planned based on the hematoma position indicated by CT scan (Figure1 A). 10 cases were treated with general anesthesia and 4 cases were treated with local anesthesia. Subcutaneous administration of lidocaine was used for local anesthesia. A 4cm straight incision was made on the thickest hematoma layer; then, a 2 * 3cm bone window was open (Figure 1 D, Figure 2 A). The endocranium was cut open in an “X” shape, from where the old bloody fluids flew out. Then, the hematoma cavity was rinsed with warm saline. After the rinsing liquid was clear, the 0° neuro-endoscope was connected and introduced into intracranial cavity under direct visual observation (Figure 2 B-C). The subdural space was then examined, and the aspirator was placed to absorb hematoma. After bleeding spot was determined, the bleeding vessel was treated (Figure 3).
Then, the separated small cavity area was open, and the subdural hematoma cavity was repeatedly flushed to make sure it was clean (Figure 2 B-C). After that, the subdural drainage tube was placed under the endoscope vision to ensure its safe placement and suitable position. The drainage bag was fixed and placed at pillow side in a suitable location (not too low). After the operation, the patients were supplied with large amount of liquid to expand the brain tissue and accelerate the blood outflow. After 1-2 days of drainage, brain CT was performed again to observe the drainage of hematoma (Figure 1 B-C). If the hematoma disappeared, and the drainage volume was significantly reduced, with liquid color becoming more yellowish and lighter, then the drainage bag could be pulled out (Figure 1 B-C). If the drainage fluid was still a lot and the color was dark, then 50,000 units of urokinase was injected and rinsed; the drainage tube was removed until the drainage fluid became less, and its color turned lighter.

Results
The neuro-endoscopic hematoma clearance was successfully conducted on all 14 patients. The hematoma removal was satisfying. Nearly all the hematoma in vision field was removed. The flushing fluid was cleared, and the drainage tube was successfully placed. Postoperative drainage was performed on all 14 patients, among which 11 cases were extubated after 2 days, and 3 cases were extubated after 4 days (2 cases) or 5 days (1 case) due to less ideal brain expansion and more fluid or gas accumulation. The effective rate of the operation was 100%. The CT scan at 7-14 days post-operation showed that, in 12 cases, the midline structure, brain ditch, and cerebral cistern returned to normal, and 2 cases had a small amount of hydrops and gas left. The headache symptom was greatly improved in 9 patients, and 7 of them was completely recovered; also, the 5 patients with hemiplegia showed significant recovery in limb muscle strength; the 4 cases with decreased consciousness were also improved. The self-care ability of these patients was significantly enhanced. There was no surgery complication or recurrence within 3 months.
Discussion
Subacute subdural hematoma (SSH) is the clinical manifestation of a special craniocerebral trauma. It is the process of subdural chronic hemorrhage after trauma, and usually occurs after brain tissue laceration or subarachnoid hemorrhage. The cause of SSH is not fully understood. So far, there are mainly a few theories, including the high-permeability of hematoma fluid theory, increased fibrin degradation products theory, and traumatic subdural hydrops theory [6-8].
The patient’s early clinical manifestations were mild. As time progressed, the subdural hematoma gradually increases, with chronic and progressive increase in intracranial pressure, which causes pressures on local blood vessels and brain tissue. The body on the opposite side of hematoma becomes uncomfortable and the discomfort progressively aggravates, which seriously affects the patient’s life quality [9]. At this time, the treatment method is determined based on the patient’s consciousness and the amount of bleeding. If the patient’s hematoma begins to increase significantly, and the midline structure is significantly offset, with cerebral palsy, then early surgical treatment is recommended. The surgical treatments mostly use minimally invasive treatment, which drills the crania and drains fluid from a small hole. A small number of patients need the operation of opening bone flaps to decrease the pressure. However, there is blindness in the method of crania drilling drainage, so the hematoma is not very efficiently removed. Moreover, the decompression treatment via opening bone flaps has the disadvantage of creating large trauma [2-5]. Thus, all current surgical procedures can cause complications such as residual blood, intracranial gas accumulation, and recurrence [10,11]. In recent years, neuro-endoscopy has been widely used. From the initial nasal surgery, to intracranial surgery, neuro-endoscopy has shown obvious advantages over the traditional methods [12-14].
The disease course of SSH exceeds 3 days. The small blood vessels at the hemorrhage location have been mechanized, and the hematoma has been stabilized. Thus, the possibility of re-bleeding is small. The main damages of hematoma to brain are: 1. Damages from hematoma compression and brain tissue displacement; 2. The toxic substances in hematoma cavity stimulates the surrounding brain tissues, which then leads to cerebral edema damage; the longer the hematoma compression persists, the more severe and more extensive the damages to surrounding brain tissues will be. Therefore, subacute subdural hematoma should be treated with minimally invasive surgery at the early stage, in order to effectively remove the hematoma, drain harmful substances, and reduce the damage to patients.
The most common problems in surgical removal of SSH are the inability to completely remove hematoma and the risk of recurrence. The hematoma mass is not completely liquid, and most of it is crumby. Thus, simple drainage cannot efficiently remove it. If the blood clots are not fully absorbed, local hematoma or incomplete hydrops drainage are more likely to occur, resulting in partially relieved clinical symptoms or recurrence. In the past, SSH patients usually need a secondary surgery or a large bone flap craniotomy surgery to remove hematoma. Neuro-endoscopy has obvious advantages for this disease. The operation purpose is to relieve the hematoma compression and reset the brain tissue location in a minimally invasive way, in order to decrease the secondary damage of hematoma to brain tissue.
Based on the clinical results of this group of patients, we conclude that: 1 neuro-endoscopy can improve the safety of rinse, drainage and blood mass removal during surgery; it also helps the safe placement of drainage tube, which reduces the recurrence rate. In particular, the exploration of bleeding vessels and removal of blood mass are under direct vision, which is superior to the conventional “blind” process. 2. The incision site should be made on the thickest part of hematoma, where the space is larger and easier for endoscopic operation, so that the possibility of brain damage is reduced. 3. When the endoscope is placed, if the endoscope field is not clear, try not to rush to deep areas, in order to avoid accidental injury on bridge vein, cortex or arachnoid. After the field is rinsed clear, the operation can go deeper to remove the blood clot and residual hematoma. 3. The endoscope provides two-dimensional images, which affect the judgment of depth. Thus, the operation should be gentle and careful, and the probe should be gradually entered under direct observation, in order to avoid damage caused by endoscope. 4. The drainage tube should be inserted under endoscope, in order to prevent it from entering the brain or subarachnoid space. 5. The drainage time after neuro-endoscopic surgery is shorter than traditional methods, because the hematoma has been adequately absorbed and rinsed during surgery, leaving very few residuals. This greatly reduces the incidence of infection caused by drainage tube.
The disadvantage of this procedure is that it depends on the proficiency and experience of the doctors who perform neuro-endoscopic surgery. The biggest problem of introducing a rigid endoscope into a narrow operating space is that the cortex may be damaged; thus, the passage for introducing endoscope should be sufficiently spacious. When the endoscope is placed, the hand grip needs to be stable and the endoscope should be handled under direct vision in order to avoid damages to cortex and drainage vessels.
Conclusion
The application of neuro-endoscopy in the treatment of subacute subdural hematoma (SSH) has a significant clinical effect. It has the advantages of small trauma, better effect, direct visualization, and good safety. Therefore, this method should be widely used in clinical practice.
Declaration of Patient Consent
The authors have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published, and outstanding efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Funding
This work was supported by a grant from: Traditional Chinese medicine science and technology project in jiangsu province( YB2015113); The science and technology program of Nantong City (JC2018101); The science and technology program of Nantong Health Committee MA2019003; The science and technology program of Nantong City(HS12018002). The funding source(s) had no more involvement.
Competing Interest
None.
References
- Ueba T, Yasuda M, Inoue T (2015) Endoscopic burr hole surgery with a curettage and suction technique to treat traumatic subacutesubdural hematomas. J Neurol Surg a Cent Eur Neurosurg 76(1): 63-5.
- Endo H, Fukawa O, Mashiyama S, Kawase M (2004) Single burr hole surgery for acute spontaneous subdural hematoma in the aged: patient reportsof three cases. No Shinkei Geka 32(3): 271-276.
- Kenning TJ, Dalfino JC, German JW, Drazin D, Adamo MA (2010) Analysis of the subduralm evacuating port system for the treatment of subacute and chronic subdural hematomas.J Neurosurg 113(5): 1004-1010.
- Asfora WT, Schwebach L (2003) A modified technique to treat chronic and subacute subdural hematoma: technical note. Surg Neurol 59(4): 329-332.
- Lollis SS, Wolak ML, Mamourian AC (2006) Imaging characteristics of the subdural evacuating port system, a new bedside therapy for subacute/chronic subdural hematoma. AJNR Am J Neuroradiol 27(1):74-75.
- Kim BW, Jung YJ, Kim MS, Choi BY (2011) Chronic subdural hematoma after spontaneous intracranial hypotension: a case treated with epidural blood patch on c1-2. J Korean Neurosurg Soc 50(3): 274-276.
- Almenawer SA, Farrokhyar F, Hong C, Alhazzani W, Manoranjan B, et al. (2014) Chronic subdural hematoma management: a systematic review and meta-analysis of 34,829 patients. Ann Surg 259(3): 449-457.
- Chan DY, Chan DT, Sun TF, Ng SC, Wong GK, et al. (2017) The use of atorvastatin for chronic subdural haematoma: a retrospective cohort comparisonstudy. Br J Neurosurg 31(1): 72-77.
- Kim SB, Kim MK, Kim KD, Lim YJ (2014) Unintended complication of intracranial subdural hematoma after percutaneous epidural neuroplasty. J Korean Neurosurg Soc 55(3): 170-172.
- Ohba S, Kinoshita Y, Nakagawa T, Murakami H (2013) The risk factors for recurrence of chronic subdural hematoma. Neurosurg Rev 36(1):145-149; discussion 149-150.
- Kim SH, Lee JH, Joo W, Chough CK, Park HK, et al. (2015) Analysis of the risk factors for development of post-operative extradural hematoma after intracranial surgery. Br J Neurosurg 29(2): 243-248.
- Turhan T (2018) Dry-field maneuver for controlling the massive intraventricular bleeding during neuroendoscopic procedures. Childs Nerv Syst 34(3): 541-545.
- Schroeder HW, Oertel J, Gaab MR (2004) Incidence of complications in neuroendoscopic surgery. Childs Nerv Syst 20(11-12): 878-883.
- Oertel J, Linsler S, Csokonay A, Schroeder HWS, Senger S (2018) Management of severe intraoperative hemorrhage during intraventricular neuroendoscopic procedures: the dry field technique. J Neurosurg 1: 1-9.
-
Xuejian Wang, Xiangdong Li, Zhifeng Wang. Clinical Study of Neuroendoscopic Treatment of Subacute Subdural Hematoma (SSH). 8(3): 2020. ANN.MS.ID.000687.
-
Acute meningoradiculoneuritis, Sjögren syndrome, Lymphocytic meningitis, Polyradiculopathy, Neuro-Sjögren
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






