 Research Article
Research Article
Clinical and Epidemiological Characteristics of Guillain-Barré Syndrome and its Variants in Tijuana General Hospital
Delgado-Ochoa Martha Azucena*, Bataller-Méndez Mauricio, Delgado-Hernández Carlos, García- Noriega María Victoria and Taboada-Pérez Grecia Carolina
Department of Pediatric Neurology, Tijuana General Hospital, Mexico
Delgado-Ochoa Martha Azucena, Department of Pediatric Neurology, Tijuana General Hospital, Mexico.
Received Date: March 24, 2023; Published Date: April 04, 2023
Abstract
Introduction: Guillain Barré Syndrome is an acute inflammatory polyradiculoneuropathy characterized by progressive, ascending, symmetric muscle weakness. It is a heterogeneous disease that presents different clinical-epidemiological variants. The following study aims to determine the frequency of these variants and their clinical and epidemiological characteristics with respect to that published in the national and international literature.
Material and Methods: An observational, cross-sectional, descriptive and retrospective study was carried out in 25 patients from 1 year to 16 years of age who were diagnosed with Guillain-Barré Syndrome. The variables studied were different epidemiological and clinical characteristics and the clinical-electrophysiological variants identified.
Results: 14 men (56%) and 11 women (44%), ratio 1.2:1. The most affected age group was schoolchildren (48%). Only 52% of the cases presented in a classical manner (Acute Demyelinating Inflammatory Polyneuropathy ADIP 32%, Acute Motor Axonal Neuropathy AMAN 20%), Miller-Fisher Syndrome MFS was identified in 20%, followed by Pharyngeal-Cervial-Brachial FCB in 16% of cases. 3 patients presented overlapping variants.
Discussion: The clinical and epidemiological characteristics of pediatric Guillain-Barré Syndrome at the Tijuana General Hospital are similar to the rest of the Mexican population; however, the distribution of clinical-electrophysiological variants is markedly different from the rest of the world population.
Introduction
Guillain-Barré Syndrome (GBS) is an acute inflammatory polyradiculoneuropathy that is characterized for a progressive, symmetric, commonly ascending weakness of the limbs, which usually presents with tendinous hyporeflexia and may include cranial nerve compromise [1,2]. Pathophysiologically, an autoimmune response has been identified which involves cellular and humoral mechanisms against an apparent molecular mimicry between some antigens and the components of myelinating and glial cells, primarily gangliosides, which in turn evolves into neurotoxicity and demyelination with the corresponding alteration of neuromuscular transmission that presents with the characteristic signs and symptoms [1]. There is a recent history of infectious diseases in up to a third of patients, being the gastrointestinal and upper respiratory tract the most frequent [3,4,5]. Different clinicalelectrophysiological variants of the disease are known. These share physiological mechanisms but have explicit differences that permit their differentiation and might condition its response to treatment [1,6,7]. These variants have a different distribution across the globe. This suggests that geopolitical a sociocultural difference might play an important role in the presentation of this disease.
Guillain-Barré Syndrome Generalities
GBS was first described in 1916 by neurologists George Guillain and Jean Alexander Barré in a couple of soldiers while in a French hospital. Later, their clinical description would be complemented with Andre Strohl´s description of the neurophysiological characteristics and the cerebrospinal fluid (CSF) composition of this patients. During the 50´s, a clinical classification was proposed for the different presentations of the disease. This would encompass the whole clinical spectrum and facilitate investigation in the physiological components of the disease. These would in turn be complemented during the 70´s decade with the description of the Miller-Fisher Syndrome (MFS) and taking us to the current description of the disease [2,3]. It is defined as an immunemediated acute inflammatory polyradiculopathy that manifests as progressive muscular weakness, hyporeflexia, that tends to include sensory symptoms and albuminocytologic dissociation in CSF, as well as multiple electrophysiological alterations.
Clinical Manifestations
The disease spectrum shares some characteristics, as are symmetrical weakness of the limbs and/or cranial nerves, a monophasic course of disease and a later plateau. It is usually associated with recent infectious history, paresthesia before the start of the weakness and albuminocytologic dissociation in CSF. Nevertheless, other characteristics exist that may differentiate the variants for the disease [2]. Other symptoms that may appear in initial phases of the disease include behavioral changes, irritability, paresthesia, limb or back pain and gait disturbance. Physical exam may reveal symmetric weakness with diminished or abolished reflexes. Nadir tends to occur between 2 to 4 weeks, followed by a slow recovery process. Children usually have a shorter course than adults, with a faster recovery time and good prognosis. [2,8-12]. An important complication is autonomic disfunction. This may include arrythmias, orthostatic hypotension, systemic arterial hypertension, non-mechanic intestinal obstruction, vesical disfunction and diaphoresis [5,8].
Electrophysiological Clinical Variants of Guillain- Barré Syndrome
GBS is a heterogenic disease. Differences in symptom appearance may present a diagnostic challenge for the clinician.
Two main subtypes have been identified, as well as other less frequent variants, according to its clinical manifestations and electrophysiological and laboratory findings:
• Acute Demyelinating Inflammatory Polyneuropathy (ADIP): It is considered the classical variant, and it is the most prevalent in western world [1,6,8]. It is mainly mediated by molecular mimicry after an infection. The microorganism´s epitopes are presented to T cells by activated neurotropic macrophages in the peripheric nervous system. There exists a cross-reaction that results in cytokine production and free radicals release, affecting the blood neuro barrier and destroying the myelin sheat, whit its corresponding effect in neural conduction speed, presenting as conduction blockade, diminished distal muscular compound action potential and augmented distal motor latency. It presents in 69 to 90% of patients [1,2,3,10,12].
• Acute Motor Axonal Neuropathy (AMAN): Its main feature is axonal degeneration with lymphocytic infiltration and less degree of inflammation. There is also a molecular mimicry component to this presentation, evidenced by the morphological similitude between Campylobacter jejuni antigens and host´s gangliosides, primordially expressed in the motor axolemma surface. B-cell antibodies cross react with the axonal gangliosides and perpetuate axonal degeneration by macrophage and complement activation. Electrophysiological findings include distal muscular compound potential reduction in absence of other demyelination markers. Clinically, it is known for having a purely motor presentation, which tends to have a faster progression with less frequent cranial nerve involvement or autonomic disfunction. However, sensorymotor presentations aren’t uncommon. It has been reported in up to 70% of cases [1,2,3,10,12].
Less frequent variants [2,10,12] include:
> Acute Motor and Sensory Axonal Neuropathy (AMSAN): A similar variant to AMAN, however, this is accompanied by sensory symptoms and usually has a more severe and prolonged course. Axonal injury affects both motor and sensitive fibers. Studies report a prevalence of less than 22% of cases [5,12]
> Miller-Fisher Syndrome (MFS): It is defined by the triad of ophthalmoplegia, ataxia and hypo/areflexia. It usually presents with hypersomnolence and an absence of limb weakness. Antiganglioside antibodies have been identified in most of the cases. Prevalence of this variant can reach up to 25% of cases [1,2,9,10,12]
> Pharyngeal-Cervial-Brachial (PCB): Symptoms include oropharyngeal, neck and symmetric or asymmetric arm weakness, with arm areflexia and no leg involvement. It may present with bulbar disfunction, or as a superposition with other variants, as is MFS or Bickerstaff Encephalitis. Antiganglioside antibodies can be detected in this variant. Its frequency is reported below 5% of cases [2,10,12].
> Paraparetic: Paresis occurs only in inferior limbs. There usually is sensory involvement and anti-GM1 and anti-GD1b antibodies may be identified [10,12]
> Bilateral Facial Palsy with Paresthesia (BFPP): It presents as bilateral facial weakness accompanied by paresthesia and diminished reflexes without limb weakness. It does not fulfill classic GBS criteria, nonetheless, electrophysiological findings are similar to those in ADIP [10,12]
> Pure Sensory: There is no motor involvement, and only sensory symptoms can be identified. It has been associated with anti-GD1b. Less than 1% of cases have reported this variant [10,12]
> Bickerstaff Brainstem Encephalitis (BBE): It has a similar presentation to MFS, even sharing the same antiganglioside antibodies, however, BBE develops into a brainstem encephalopathy with hypersomnolence, reduced conscience, hyperreflexia and, in some cases, it may simulate brain death [2,10,12].
During The Covid-19 Pandemic
Some triggers that have been identified include, besides infection, vaccination, surgery, cancer and pregnancy [6]. Similarly, there have been cases associated to COVID-19 infection, including children. The most frequent variant in these cases has been ADIP with 75%, followed by AMAN with 11% and AMSAN in 9% [13]. This takes relevance in the fact that there has been a rise in incidence after viral epidemics and recent vaccination campaigns, in which GBS has been found as the most common severe complication in the recent SARS-CoV-2 vaccination in Baja California, or in the rise in incidence after the Zika epidemic in 2014 [4,14]. During the time period between April 2020 and March 2021, Tijuana General Hospital suffered conversion into a Covid-19-only hospital, including the Pediatric department. Because of this, no patients with GBS were admitted during this time. This, in itself, presented as an opportunity to compare the disease features before and after the exposition of the virus to the population. Besides, geopolitical characteristics of a border city, and especially the most transited international border in the world, causes a cultural shock in regards to the rest of the country due to the adherent qualities of each population group, as well as migrant floating population [14,15].
Evolution and Treatment
Treatment includes immunomodulation and support, sometimes with the need of invasive mechanical ventilation due to respiratory muscle or protective airway reflexes compromise [3,9]. In children, the main line of treatment is human intravenous immunoglobulin (IVIG), at a dose of 2g/kg divided in 5 days. An IGIV dose of 0.4 g/kg over 5 days is preferred because of a diminished risk of Treatment Associated Fluctuations (TAF), which means a worsening after the initial stabilization within 8 weeks of treatment, which is reported in 6 to 16% of patients [10,12]. Plasma exchange has also shown good effectivity; however, this is an invasive and riskier process not available in many hospitals. Steroid treatment has not proved beneficial [3,5]. Some of the mechanisms of action that have been proposed for IGIV include: Fc receptor block in macrophage, inhibiting myelin attack mediated by antibodies; autoantibody and cytokine regulation through anti-idiotypic and anti-cytokine ways; up-regulation of Fc-gamma IIB inhibitory receptor in B-cells or Down-regulation of B-cell activation factor; interference with activation of the complement cascade or acceleration of endogenous immunoglobin breakdown. Adverse effects include erythema, headache, myalgia, hypotension, fever, hepatic or kidney injury [3]. The disease has a good prognosis, reaching complete recovery in most patients. However, it presents with important disability during the acute and subacute phases of the disease, which in turn can give place to other complications and, in some cases, develop into chronic disfunction and neurological sequels. Early diagnosis and treatment enhance prognosis and decreases complications risks during hospital stay [1,3,6,7]. In pediatric studies, mortality has been described between 3 and 5% [3,5]. Although GBS during infancy tends to be of a better prognosis than in adults, some rare cases may be resistant to standard treatment. This may be due to secondary axonal damage after a severe and prolonged autoimmune attack. It has been postulated that elevated blood levels of IGIV at 2 weeks of administration could be associated with adequate recovery [6,7,9].
Background in Mexico and the Rest of the World
Globally, incidence has been estimated between 0.16 – 3 per 100,000 people in every age group, being a little less common in children, with an incidence of 0.34 and 1.34 per 100,000 people [2,5,10,16]. In the western world, ADIP has been recognized as the most common variant with up to 90% of cases [2,9], while in eastern countries AMAN tends to be the most common variant [3,5,6,17]. This may be due to a different exposure to certain infectious pathogens, such as Campylobacter jejuni. In Mexico, according to the Center for Epidemiological Vigilance, in a period of 24 years there were 400 to 600 annual cases reported in children, with an average incidence of 1.5/100,000 habitants, of which 18.7% were in the age group of 5 – 14 years old [8].
It has been published that, in Mexico, the most common history of infectious disease included diarrhea, while the most common variant has been found to be AMAN, associated with a campylobacter jejuni infection in 25% of patients, also presenting a seasonal distribution with a peak during summer. This may be due to the geographic characteristics of the region, as well as the circulating pathogenic flora. Studies completed in pediatric hospitals in the center of the country have found an average age of 6 years, with a discreet prevalence in boys, and finding high disability scores at admission [8]. AMAN was identified in up to 57% of patients, followed by ADIP in about 40% and MSF in 3%. Other variants are considered rare in children, and weren’t described in said studies. (4, 8, 16). Nonetheless, it is of notice a study conducted in northwestern Mexico that reported a prevalence of MFS in 17% [8]. Up to 15% of pediatric patients will need invasive respiratory support due to respiratory mechanical failure during the course of the disease [16].
Materials and Methods
An observational, transversal, retrospective and descriptive study was conducted in 25 patients age 1 year to 16 years who were diagnosed with GBS in the Pediatric department from Tijuana General Hospital (HGT) between August 2018 and December 2022, excluding those diagnosed between April 1 2020 and March 1 2021, corresponding to the Covid-19 pandemic hospital transformation in which only SARS-CoV2-positive patients were accepted for treatment. Studied variables included different epidemiological and clinical features, as well as identified variants. Relevant data was recovered from the clinical files of each patient using a tool previously designed for this investigation by the authors. Results were processed through Microsoft Excel software, analyzing variables with descriptive statistics and comparing results for both temporal groups. Variable association was assessed with Chi Squared Test or Student´s T Test, as appropriate.
Results
30 GBS patients were reported, of whom 25 met inclusion criteria for this study.
> Epidemiological Findings
Out of all patients, 14 were male (56%) and 11 female (44%) for a 1.2:1 ratio. Most affected age group was that of middle childhood (6 – 12 years) with 48%, followed by early childhood (2 – 5 years) and adolescents (13 – 16 years) in 20% of cases. Less affected were toddlers, with only 12%.
56% of patients received care in the time after hospital conversion. We failed to find a statistically significant difference for disease frequency before and after this event (p=0.4891).
Up to 52% of the patients had no identifiable history in the weeks prior to diagnosis; while 36% presented upper respiratory tract infections, only 8% presented gastrointestinal infections and only two cases were reported with an infection in another site, in both cases urinary, one of which was accompanied by a gastrointestinal infection.
The breakdown of the data can be seen in (Table 1)
> Clinical Findings
Table 1:Epidemiological Findings.
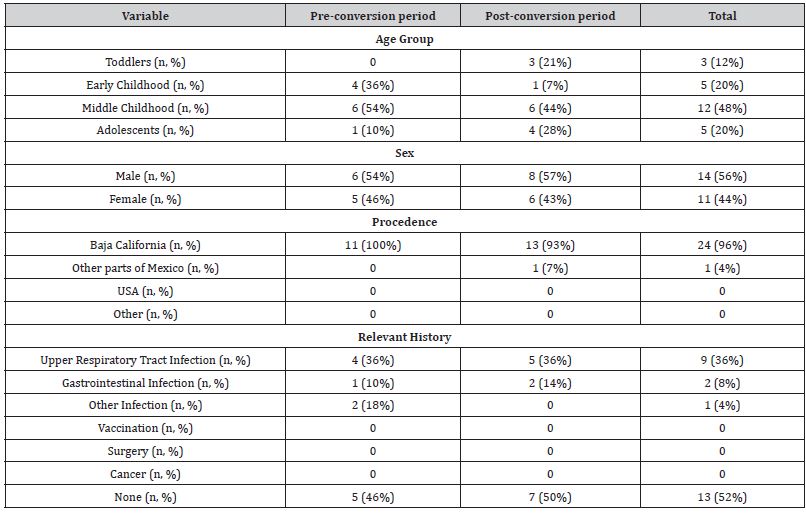
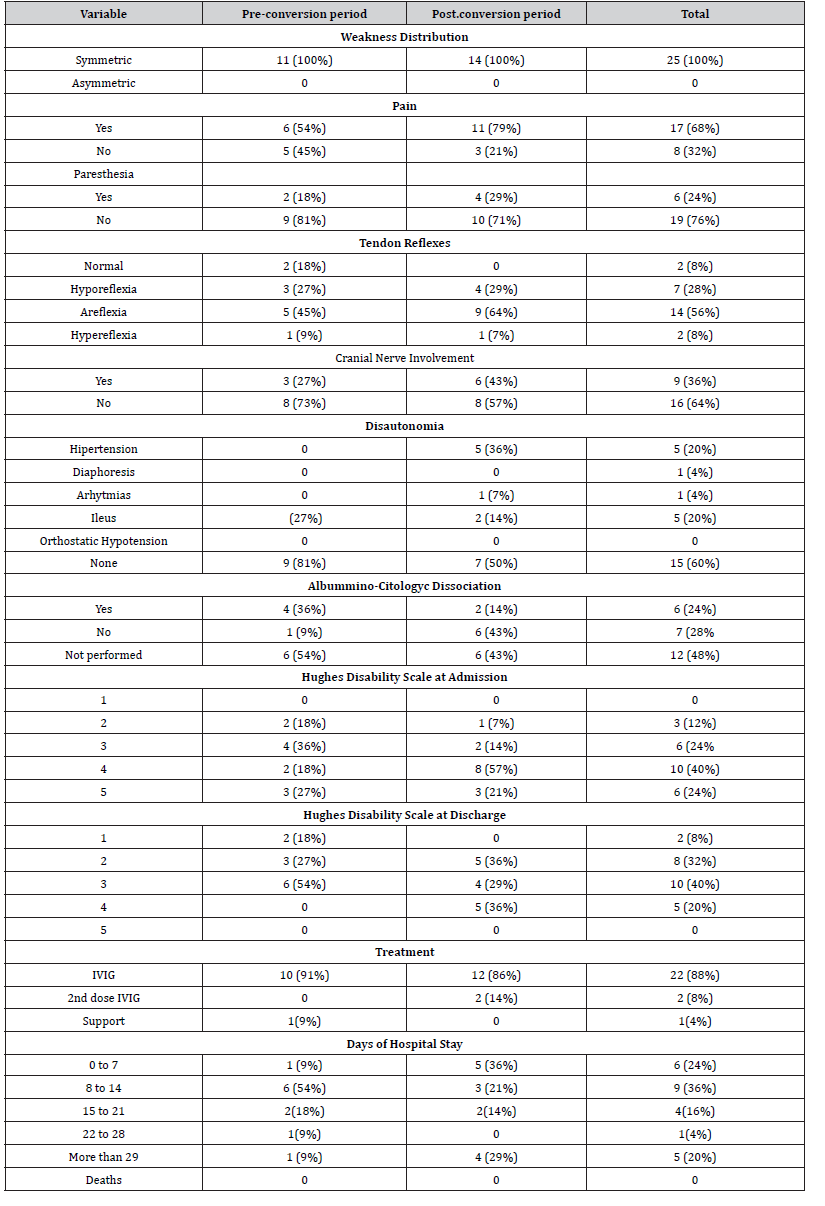
In this regard, it was evidenced that 100% of the patients had progressive, symmetrical muscle weakness. 68% of the patients reported pain, while only 24% presented paresthesias. Pain reports were increased by 25% after the hospital reconversion. The vast majority of patients (86%) presented decreased tendon reflexes, with areflexia of the affected limbs standing out in 56% of cases. Cranial nerve involvement was noted in 1 out of 3 patients. We highlight the 100% increase in frequency after hospital conversion. In the same way, an increase in the frequency of dysautonomia was evidenced, going from 19 to 50%, highlighting the presence of hypertension and ileus in 20% of the patients. Regarding the Hughes scale of disability, at admission a median of 4 was recorded with 64% of the patients confined to a bed upon arrival. 24% of the patients required invasive mechanical ventilation to protect their breathing. At discharge, a median of 3 was evidenced, with only 20% of patients confined to a bed. Hospital stay was mostly two weeks or less, with a median of eight to fourteen days. 20% of the patients had a stay of more than twenty-eight days, associated with mechanical ventilation and higher levels on the disability scale on admission. No deaths were recorded during the study period. The rest of the data obtained is broken down in (Table 2).
> Clinical-electrophysiological variants
Table 2:Clinical Findings.
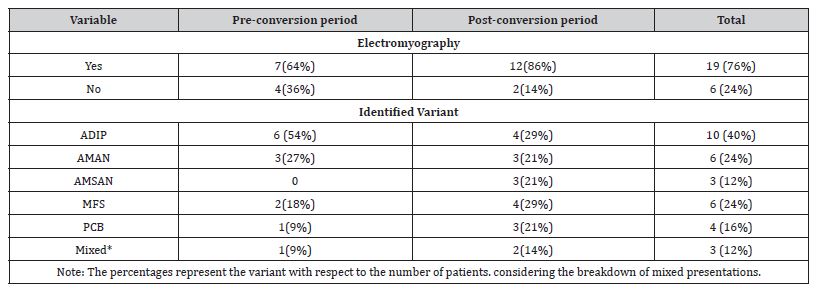
The high frequency of variants classically classified as atypical stands out. Only 52% of the cases presented in a classical manner (ADIP 32%, AMAN 20%), while among the rest the MFS stood out with 20% of the cases, followed by the PCB in 16% of the cases. 3 patients presented overlapping variants for 12%, same number as AMSAN. No cases of pure sensory, paraparetic, BFPP or Bickerstaff encephalitis were reported.
The difference in the distribution of variants before and after hospital conversion is striking. Prior to it, typical presentations were reported in 64%, subsequently decreasing to 43%. Among the less classic variants, PCB presented a significant increase, reaching 21% of cases after reconversion.
The complete data is summarized in Table 3:
Table 3:Clinical-Electrophysiological Variants.
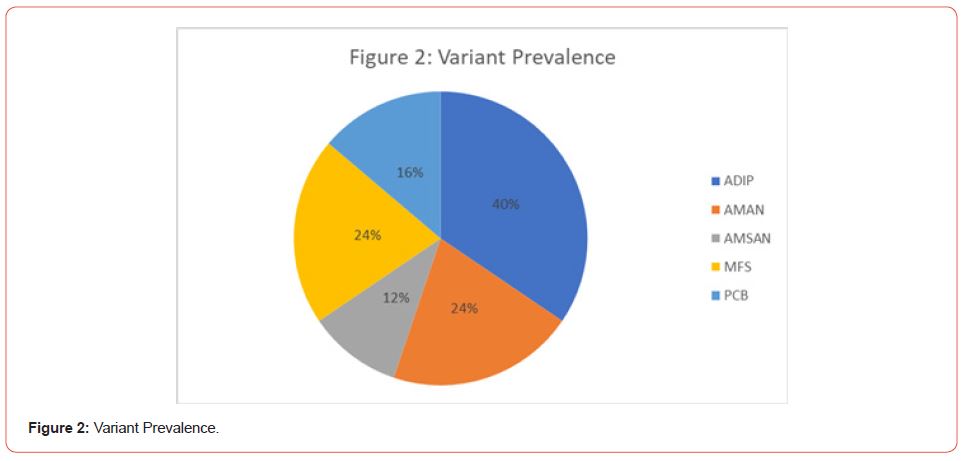
The presentation in patients is illustrated in Figure 1. When performing a breakdown of all the variants detected during the study, a higher prevalence of PDIA is found with 40% of the cases. The breakdown of variants can be seen in Figure 1 and Figure 2.

Discussion
The difference in the distribution of clinical-electrophysiological variants in our setting is immediately striking. Although most studies do not have specific reports of all the variants studied in this research, the presentations classically considered atypical do present a much higher frequency than that described in the global literature. This could be explained by the sociodemographic and environmental characteristics of our population and geographic region, since the city is located on the busiest international border in the world and has a large multiethnic floating population. Similarly, there is an increase in atypical presentations after hospital conversion (p=0.07) although there is no significant increase in cases of the disease. It should be noted that these results only reflect the difference before and after the hospital conversion period, and not its possible relationship with the COVID-19 pandemic, social isolation and quarantine, or the new hygiene measures derived from it. The results of the study suggest a certain similarity between the epidemiological characteristics of our population with those reported in the rest of the Mexican Republic [8,14]. An infectious history was only detected in 50% of the patients, so we cannot rule out that asymptomatic infections could trigger the disease. Regarding the clinical characteristics, similarity was observed in most of the variables. However, it is noteworthy that 1 out of 3 patients presented cranial nerve involvement. Most of the patients presented in advanced stages of disability, which may suggest both a rapid progression of the disease and little information from caregivers and health personnel about the nature of it, given that they may perform a medical assessment or make a hospital referral until the symptoms are very evident. No deaths were recorded during the study period. The weaknesses of this research include, first of all, the small sample size. Secondly, the lack of inclusion of patients from other health institutions, since their public insurance covers other population groups with different socioeconomic features. Third, in the absence of a specific protocol for institutional approach, complementary diagnostic tests such as electrophysiological tests were not requested or collected in some cases, which would allow for a better characterization of all registered cases.
Despite this, the difference registered in the distribution of clinical-electrophysiological variants is very evident with the rest of the world population, so new, larger inter-institutional studies are necessary to determine if there really is a difference and the causes that could be associated to it.
Conclusions
The clinical and epidemiological characteristics of pediatric Guillain-Barré Syndrome at the General Hospital of Tijuana are similar to the rest of the Mexican population; however, the distribution of clinical-electrophysiological variants is markedly different from the rest of the world population (p=0.003). The demyelinating variant (ADIP) was the most common in our study in 40% of the patients. The prevalence of PCB stands out in 16% of the patients studied when the world literature suggests a prevalence of less than 5% [10,12], while in 12% of the patients there was an overlap of variants. As for MFS, it was presented with the same frequency as AMAN. There does not seem to be a significant difference in the relationship of variants or frequency of the disease before and after hospital conversion.
Acknowledgement
None.
Conflict of Interest
No Conflict of interest.
References
- Soltani ZE, Rahmani F, Rezaei N (2019) Autoimmunity and cytokines in Guillain-Barré syndrome revisited: review of pathomechanisms with an eye on therapeutic options. European cytokine network 30: 1-4.
- Rebolledo-García D, González-Vargas PO, Salgado-Calderón I (2018) Síndrome de Guillain-Barré: viejos y nuevos conceptos. Medicina interna de México 34(1): 72-81.
- Abbassi N, Ambegaonkar G (2019) Guillain-Barre syndrome: a review. Paediatrics and Child Health 29(11): 459-62.
- Arriaga-Nieto L, Hernández-Bautista PF, Vallejos-Parás A, Grajales-Muñiz C, Rojas-Mendoza T, et al. (2022) Predict the incidence of Guillain Barré Syndrome and arbovirus infection in Mexico, 2014–2019. PLOS Global Public Health 2(3): e0000137.
- Ryan MM, Kaplan SL, Shefner JM (2017) Guillain-Barré syndrome in children: Epidemiology, clinical features, and diagnosis. UpToDate. Waltham, Mass.: UpToDate.
- Algahtani H, Shirah B, Alrefaei K, Albassam M, Abdelghaffar N (2020) Are Repeated Cycles of Intravenous Immunoglobulin Justified in Patients With Poorly Responsive Guillain-Barré Syndrome?. The Neurohospitalist 10(3): 224-228.
- Godoy DA, Rabinstein A (2015) Is a second cycle of immunoglobulin justified in axonal forms of Guillain-Barré syndrome? Arquivos de Neuro-Psiquiatria 73: 848-51.
- De La Re AD, Fonseca-Chon I, Sotelo-Cruz N (2016) Guillain-Barré syndrome. Experience with 91 children at a pediatric hospital in northwestern Mexico. Archivos de Neurociencias 21(1): 7-16.
- Korinthenberg R, Trollmann R, Felderhoff-Müser U, Bernert G, Hackenberg A, et al. (2020) Diagnosis and treatment of Guillain-Barré Syndrome in childhood and adolescence: An evidence-and consensus-based guideline. European Journal of Paediatric Neurology 25: 5-16.
- Leonhard SE, Mandarakas MR, Gondim FA, Bateman K, Ferreira ML, et al. (2019) Diagnosis and management of Guillain–Barré syndrome in ten steps. Nature Reviews Neurology 15(11): 671-83.
- López-Hernández JC, Galnares-Olalde JA, Gutiérrez A, Estrada SA, García-Grimshaw M, et al. (2022) Guillain-Barre syndrome in Mexico: clinical features and validation of Brighton Collaboration Group criteria. Rev Neurol: 258-64.
- Shahrizaila N, Lehmann HC, Kuwabara S (2021) Guillain-Barré syndrome. The lancet 397(10280): 1214-1228.
- Das KY, Midhun Raj KT, Samprathi M, Sridhar M, Adiga R, et al. (2021) Guillain–barré syndrome associated with SARS-CoV-2 infection. Indian Journal of Pediatrics 88: 479.
- Mendez-Lizarraga CA, Chacon-Cruz E, Carrillo-Meza R, Hernández-Milán NS, Inustroza-Sánchez LC, et al. (2022) Report of Adverse Effects Following Population-Wide COVID-19 Vaccination: A Comparative Study between Six Different Vaccines in Baja-California, Mexico. Vaccines 10(8): 1196.
- Salazar-Otaola GF, Olivares-Torres CA (2021) Experiencia del Hospital General en la reestructuración de los servicios quirúrgicos por COVID-19. Revista Mexicana de Cirugía Torácica General 1(2): 60-6.
- Nachamkin I, Barbosa PA, Ung H, Lobato C, Rivera AG, et al. (2007) Patterns of Guillain-Barre syndrome in children: results from a Mexican population. Neurology 69(17): 1665-71.
- Yadav S, Jain P, Sharma S, Kumar V, Aneja S (2019) Guillain–Barre syndrome in North Indian children: Clinical and serial electrophysiological features. Neurology India 67(3):724-727.
-
Delgado-Ochoa Martha Azucena*, Bataller-Méndez Mauricio, Delgado-Hernández Carlos, García-Noriega María Victoria and Taboada-Pérez Grecia Carolina. Clinical and Epidemiological Characteristics of Guillain-Barré Syndrome and its Variants in Tijuana General Hospital. Arch Neurol & Neurosci. 14(5): 2023. ANN.MS.ID.000849.
-
Pediatric Neurology, Neuropathy, clinical-electrophysiological, neurophysiological, muscular weakness, hyporeflexia, sensory symptoms, arrythmias, orthostatic hypotension, systemic arterial hypertension, non-mechanic intestinal obstruction, vesical disfunction, diaphoresis.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
- Abstract
- Introduction
- Guillain-Barré Syndrome Generalities
- Clinical Manifestations
- Electrophysiological Clinical Variants of Guillain- Barré Syndrome
- During The Covid-19 Pandemic
- Evolution and Treatment
- Background in Mexico and the Rest of the World
- Materials and Methods
- Results
- Discussion
- Conclusions
- Acknowledgemnet
- Conflict of Interest
- References






