 Research Article
Research Article
Anthropometric Effects of Intravenous Methylprednisolone Versus Oral Corticosteroids in the Treatment of Juvenile Dermatomyositis
Xiahong Si1*, Asim Haque2, Linda I. Ray3 and VV Vedanarayanan3,4
1Department of Neurology, Austin VA Clinic, USA
2Department of Neurology, Barrow Neurological Institute, USA
3Department of Pediatrics, University of Mississippi Medical Center, USA
4Department of Neurology, University of Mississippi Medical Center, Jackson, USA
Xiaohong Si, Department of Neurology, Austin VA Clinic, USA.
Received Date: August 18, 2019; Published Date: August 27, 2019
Abstract
Background: Oral corticosteroids (OCS) and intravenous methylprednisolone (IVMP) remain the backbone of therapeutic intervention for juvenile dermatomyositis (JDM).
Methods:We analyzed anthropometric (height, weight, and BMI) data, including percentile measures from growth charts, along with resolution of functional disability, as measured by the modified Rankin scale (MRS), to determine differences in effects between OCS or IVMP treatment.
Results:Over a 15-year period, 25 JDM patients had complete or near-complete resolution of symptoms and returned to baseline function based on MRS. Within groups, increases in weight and BMI percentiles were only significant in the OCS group (p = 0.009 and 0.011, respectively). Between groups, pretreatment height (p = 0.031), and posttreatment weight (p = 0.048) and BMI (p = 0.048) were significantly different. Percentile changes post- and pretreatment compared to 50th percentile weight for given height and age, along with changes based on a 50th percentile agematched individual were likely to be lower for the IVMP group as compared to the OCS group (p = 0.008 and 0.028, respectively).
ConclusionBoth OCS and IVMP resolved symptomology, but the results suggest that IVMP had a less significant negative impact on growth measures.
Keywords: Juvenile dermatomyositis (JDM); Corticosteroids (OCS); Anthropometry; Modified Rankin scale; Pulse intravenous methylprednisolone (IVMP)
Abbreviations:BMD: Bone Mineral Densit; BMI: Body Mass Index; CHAQ: Childhood Health Assessment Questionnaire; CI: 95% Confidence Interval; DAS: Disease Activity Score; IQR: Interquartile Range; IVIG: Intravenous Igg Immunoglobulin; IVMP: Intravenous Methylprednisolone; JDM: Juvenile Dermatomyositis; KOSCHI: King’s Outcome Score For Childhood Head Injury; Mrs: Modified Rankin Scale; MTX: Methotrexate; OCS: Oral Corticosteroids; P50 BMI: Value Of BMI for the 50th Percentile P50 Wt For Ht 50th Percentile Weight Given A Certain Height And Age; RCT: Randomized Control Trial; SD: Standard Deviation
Introduction
Although juvenile idiopathic inflammatory myopathies are quite rare, juvenile dermatomyositis (JDM) is by far the most common inflammatory myopathy seen in children1 with a yearly incidence of 3 [1]. children/million [2]. Before the introduction of treatment modalities nearly 50 years ago, the mortality rate of JDM was nearly 35%; it has now been reduced by approximately two-thirds [3]. While the exact etiology and pathogenesis are not fully understood, JDM is known to be a vasculopathy with known capillary lumen obliteration, perivascular atrophy, endothelial inflammation, tubuloreticular inclusions, and muscle degeneration and regeneration [4,5]. An array of factors has been implicated, including genetic predisposition, infectious agents and environmental triggers, complement-induced injury, major histocompatibility complex class expression, and expression of adhesion molecules increasing the inflammatory response [1]. The most widely used diagnostic criteria for JDM, as suggested by Bohan and Peter [6] include 4 parameters regarding muscle disease: elevated enzymes, abnormal electromyogram, abnormal biopsy, and proximal muscle weakness; as well as 1 criterion based on dermatologic features. Oral corticosteroids (OCS) have traditionally formed the backbone of JDM therapy as well as numerous other autoimmune, inflammatory, and neoplastic disorders due to their effects on the pathological inflammatory and immune mediators. Corticosteroids are such a frequently used treatment modality, that one study on asthma therapy suggested that nearly 1 in 10 children in Great Britain had been on OCS therapy at some point in their youth [7]. Unfortunately, along with their benefits, OCS can have significant side effects. Long-term effects include: Cushingoid features, weight gain, osteopenia with associate bone fractures and bone necrosis, hypothalamic-pituitary-adrenal axis suppression, peptic ulcer disease, intestinal perforation, hyperglycemia, hyperlipidemia, lipodystrophy, hypocalcemia, hypokalemia, infections, hypertension, cataracts, glaucoma, steroid-induced myopathies, depression, and psychosis [8,9]. OCS can also attenuate achievement of peak bone mass and stunt growth by alteration of gonadal function [10].
Intravenous steroid injections in supra-physiological “pulse” doses were first used in the treatment of renal graft rejections [11]. Since then, pulse dosing has been useful when rapid anti-inflammatory or immunosuppressive effects are needed [12] in other conditions including lupus nephritis, severe glomerulonephritis, acute asthma attacks, vasculitidies, dermatologic conditions, inflammatory bowel disease, acute spinal cord injuries, and hematologic disorders [12-16]. Pulse intravenous methylprednisolone (IVMP) therapy, either alone or with OCS, is thought to allow earlier remission and thus prevent the adverse, long-term sequelae of OCS usage [1]. Furthermore, a significant number of patients with JDM have bowel vascular complications including thrombus formation and vessel obliteration which may decrease absorption of OCS [17-20]. Miller [21] initially examined the response to IVMP in several juvenile rheumatological conditions, including JDM, and noted that all patients experienced temporary benefits, with the significant majority of patients either completely weaned off steroids, or requiring follow-up pulses every 1-2 weeks or further increased pulse intervals. The longest duration of IVMP pulse treatment was three years with no evidence of toxicity, and regression of Cushingoid features was seen in all patients [21].IVMP has been shown to decrease creatine kinase (CK) levels and increase muscle strength in children [22], with similar results including an increased number of cases of remission in adults [23]. Matsubara et al. [24] also observed a decrease in inflammatory markers in adult muscle biopsies along with an increased number of regenerating fibers. Implementing IVMP in the therapeutic regimen was also shown to decrease the morbidity due to calcium deposition [9] which may have more long-term effects than the original myopathic insult, along with persistent muscle weakness and elevated enzyme activity [25]. Finally, when comparing the economic costs between OCS and IVMP, while OCS therapy was less expensive, the length of disease was considerably longer; the IVMP group had shorter disease durations as well as shorter hospitalizations and “appear[ed] to be cost-effective based on theoretical benchmark criteria” [26].
Few efforts have examined the effects of either method of therapy with respect to adverse outcomes measured via anthropometric parameters, along with scoring any change in functional status and abilities to carry out the activities of daily living. Specifically, we wanted to use continuous measures – height, weight, and body mass index (BMI) – to compare with age-adjusted normal children, as well as each other, along with a simple, objective measure, the modified Rankin scale [27,28] (MRS), to assess the level of functional disability in each patient. Although commonly used as an assessment of stroke disability in adults [29] the Rankin scale also has been previously utilized to categorize disability in children with JDM [30]. It is similar in ranking methodology to the KOSCHI scale (King’s Outcome Score for Childhood Head Injury) which has been validated for use in prospective studies in children [31]. Based on anecdotal clinical experience, we hypothesized that there would be fewer adverse anthropometric outcomes in children treated with IVMP, yet similar outcomes in terms of recovery from flare-ups and subsequent functional disability. The data shown here were initially presented at the 2010 American Academy of Neurology annual meeting [32].
Methods
In the present study, we retrospectively examined the records of the pediatric neurology and rheumatology clinics from January 1995 until April 2009 at the University of Mississippi Medical Center (UMMC). Only patients with confirmed juvenile dermatomyositis, either via muscle biopsy or imaging studies, and less than 18-years old at treatment onset were included in this study. They were followed until either administrative censoring at age 19 (the agelimit for patients in the pediatric clinics) or clinically they had entered remission of their disease based on their symptoms (see Rankin scale below). The standard treatment protocol included steroid therapy (OCS or IVMP) and methotrexate (MTX). Based on the clinical course, patients were further treated with intravenous IgG immunoglobulin (IVIG) if improvement wasn’t noted after initial therapy. Because variations in treatment occurred before the study began, many of the patients in our database had received multiple courses of treatment, including both OCS and IVMP, during relapses. For all patients, treatment group assignment was based on their latest round of treatment. Around the year 2000, the treatment protocol shifted in our clinic from using OCS as first-line therapy to using IVMP and MTX as first-line therapy, and then adding IVIG if additional pharmacological intervention was needed to induce remission of symptoms. Parameters of interest gathered from each patient included gender, age at which last treatment began, length of treatment, along with pre- and posttreatment weight and height. Furthermore, a categorical measure of disability (modified Rankin score, mRS) was assigned pre- and posttreatment, and also served as an indication of remission when pharmacotherapy was stopped. Specifically, zero denotes no symptoms; 1 represents no disability despite present symptomology and ability to perform daily activities; 2 corresponds to mild disability; 3 designates moderate disability which requires some assistance though able to walk without any help; 4 symbolizes moderately severe disability and an inability to perform daily tasks or walk without external assistance; and 5 comprises severe disability requiring constant nursing and confined to the bed [27]. Calculated parameters included BMI, along with height (ht), weight (wt), and BMI percentiles from standardized growth formulas from the Centers for Disease Control (CDC). Since height, weight, and BMI increase with age in the pediatric population, if the distribution of subjects’ ages differed significantly between the two groups, this issue would obviously confound interpretation. To somewhat “adjust” for this, each individual was compared with an age-appropriate measure using the BMI for the 50th percentile for that age (p50 BMI) and the 50th percentile for weight given the patient’s current height and age (p50 wt for ht). Changes in these latter two parameters were then calculated using the following: change in p50 BMI = (posttreatment BMI - p50 BMI) - (pretreatment BMI - p50 BMI) and change in p50 wt for ht = (posttreatment wt – p50 wt for ht) - (pretreatment wt – p50 wt for ht).
Statistical analysis was performed using Stata v.10.1 (Stata Corp., College Station, TX). Due to the extremely low prevalence of JDM in the pediatric population and thus a likely low sample size, across-group comparisons were performed using the nonparametric Wilcoxon rank-sum test with the null hypothesis that there was no difference in the distribution of ranks between the two groups, along with Fisher’s exact test for 2-by-2 contingency tables comparing OCS versus IVMP. For within-group measures, pre- and posttreatment, the non-parametric Wilcoxon signed-rank test (matched pairs) was utilized to determine association for percentile levels and obesity. Plots showing Epanechnikov kernel densities (to ease illustration in differences) were constructed using bin widths of 20 and area multipliers of 400 for visualization of distributions. To allow for computation of confidence intervals (CI) with Wilcoxon rank-based statistics, the Somer’s D statistic using the somersd option for Stata (RB Newson, Imperial College London) was utilized [33,34] Medians and interquartile ranges (IQR) were used for describing the data since they are more robust against outliers, especially for small sample sizes. Kaplan-Meier survival analysis comparisons were performed using the Wilcoxon- Breslow-Gehan test. Significance levels for all tests were set as p ≤ 0.05 (2-tailed). Due to the likely small sample sizes (especially in the OCS group), no outliers were dropped from analysis. This investigation was approved by the Institutional Review Board of the University of Mississippi School of Medicine.
Results
As expected, a low number of cases were seen, even at the only statewide tertiary-care referral center during the 14-year period. Our goal in the present pilot study was to monitor the efficacy of therapy by attempting to quantify the side effects of OCS versus IVMP and serve as an impetus for future prospective or randomized studies, given these limitations. Of 39 possible cases, 14 patients had incomplete medical records, were ultimately given another diagnosis, or were lost to follow-up, and thus excluded from analysis (Figure 1).
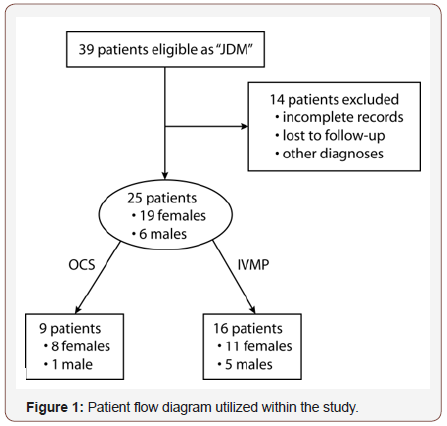
Of the remaining 25 patients, 9 patients (8 females and 1 male) were assigned to the OCS treatment arm while 16 were given IVMP therapy (11 females and 5 males). Please note that data for the study focused on each patient’s last “regimen history” of therapy in determining whether they were placed in the OCS or IVMP group. Table 1 shows demographic and parameters obtained from medical records. Mean age at treatment onset for the entire group was 9.2 yrs ± 4.6 (SD) and mean treatment length was 24.4 mos ± 14.9 (SD). Treatment of two patients was stopped in the study due to reaching the age limit for inclusion in our clinic; this, however, coincided with remission of their symptoms (id = 4, 21). Within the OCS group, 7 patients had received MTX treatment and 1 had received IVIG, whereas in the IVMP group, 14 patients had received MTX and 4 patients had received IVIG. Thus, it was not possible to isolate only pure cases of OCS or IVMP usage (see Table 1).
Table 1: Demographic data for patients in the study. ID – identifier for purposes of the study only. Tx – treatment. Tx arm: IVMP (intravenous methylprednisolone) or OCS (oral corticosteroid). Tx supplement: MTX – methotrexate, IVIG – intravenous immunoglobulin, Pred – short course of oral steroids, Pulse x n – IVMP treatment n times. Percentiles based on age from standard CDC growth charts for particular growth parameter.
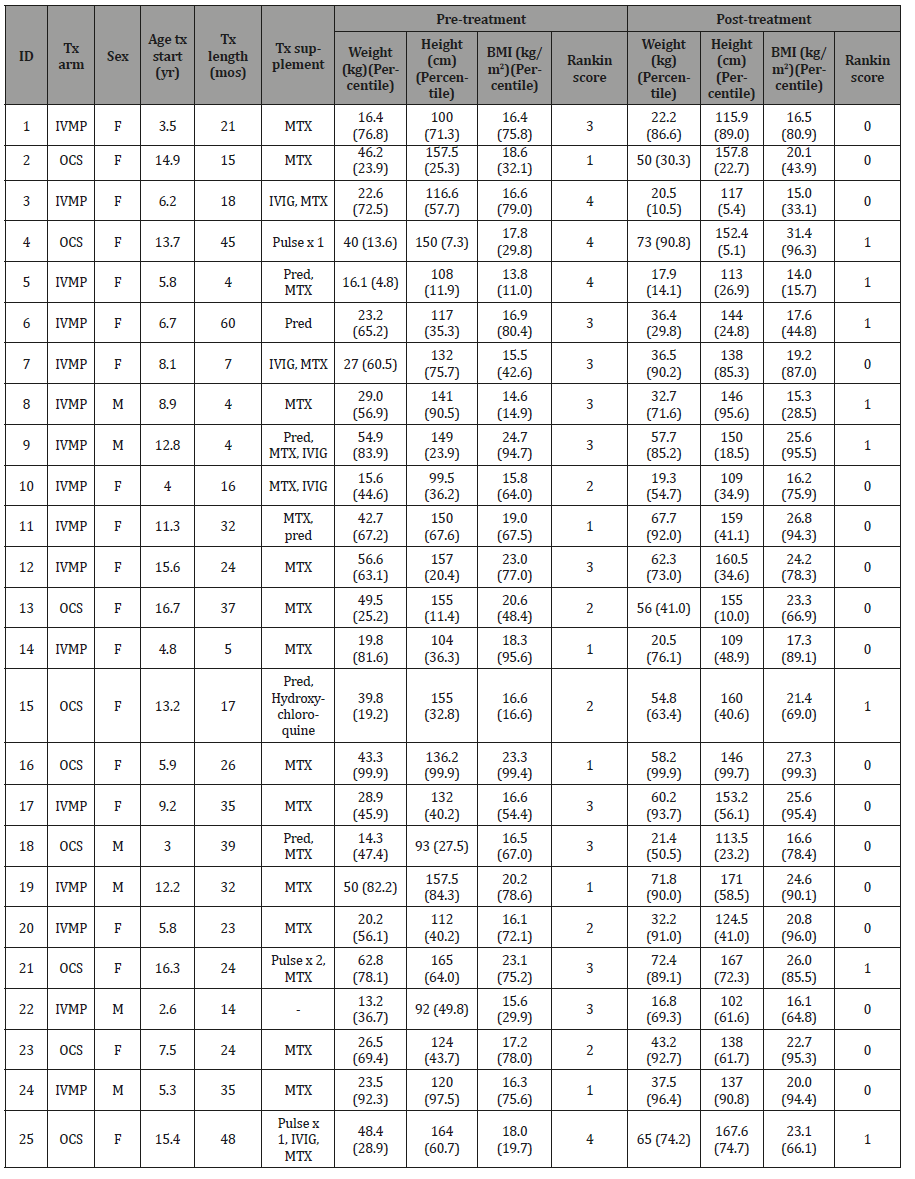
Three patients in the OCS arm had to be given IVMP pulses in order to help minimize symptoms, whereas 2 patients in the IVMP arm were given a brief course of oral corticosteroids. Only 1 patient in the study received no other adjunct therapy besides IVMP, and 1 patient received an additional medication, hydroxychloroquine, to help control symptoms. Based on the MRS, pretreatment functional disability ranged from mild symptomology to moderately severe disability, whereas posttreatment improvements ranged from none to minimal symptomology, thus all individuals from both groups were considered to be in remission. No patient in either group qualified for the WHO’s definition of short stature (< 3rd percentile), but id = 3 (IVMP) and 4 (OCS) were both near this threshold. The former’s negative shift in height percentile was particularly marked, going from 57.7 to 5.4. The maximum increase seen in either group was 18 percentile points. For weight percentiles, the aforementioned individual (id = 3) decreased 62 percentile points, while the maximum increases in either group were 77 (OCS) and 48 (IVMP) percentile points. With respect to BMI percentiles posttreatment, 3/9 OCS patients would be considered obese by CDC and WHO standards (≥ 95th percentile), with 1 additional individual labeled as at risk for being overweight (≥ 85th percentile), whereas only 1 individual was obese pretreatment (p = 0.157). Similarly for the IVMP group, only 1/16 was obese pretreatment whereas 3/16 were obese posttreatment (p = 0.317). Finally, comparing obese individuals between the two groups, the differences were insignificant for both pretreatment (p = 0.600) and posttreatment (p = 0.630) (Table 2).
Table 2: Parameters of interest based on treatment arm. * denotes significance at p ≤ 0.05.
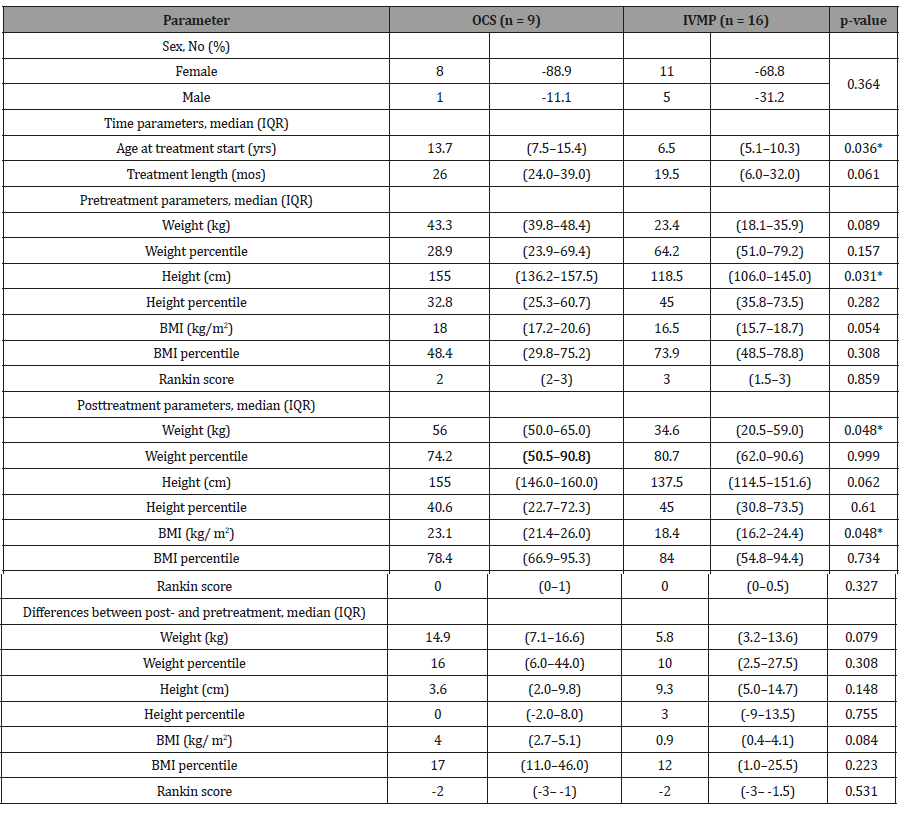
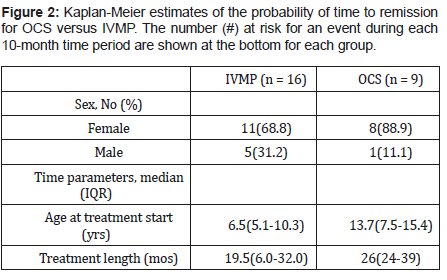
Table 2 shows comparisons of parameters of interest between the IVMP and OCS groups. As expected based on the femalepredominance seen with this condition, JDM was seen more often in females than males (3.17: 1), but the difference was not statistically significant. The distribution for median age at treatment onset (unadjusted for previous treatment, see Discussion) was significantly less for the IVMP than OCS, 6.5 yrs versus 13.7 yrs. Median treatment length to remission of symptoms was borderline significant between the two groups, with IVMP requiring 19.5 mos versus 26 mos for OCS (p = 0.061). This is further illustrated in Figure 2.
Because the median ages between the two groups were significantly different (see Table 2), differences in height, weight, and BMI would not be unexpected. Pretreatment, significant differences were noted in height with borderline differences in BMI. No significant differences were noted in the pretreatment percentiles for weight, height, or BMI, nor the initial MRS. Posttreatment, there were significant differences in weight and BMI, along with borderline differences in height. There were no differences in any of the percentile measures nor the final MRS. With respect to comparison of changes post- and pretreatment, weight and BMI were borderline significant, with the IVMP group gaining less weight and less of an increase in BMI. Of primary interest was not only the change across groups, as noted above, but also the change seen within each arm of the study, pre- and posttreatment (Figure 3).
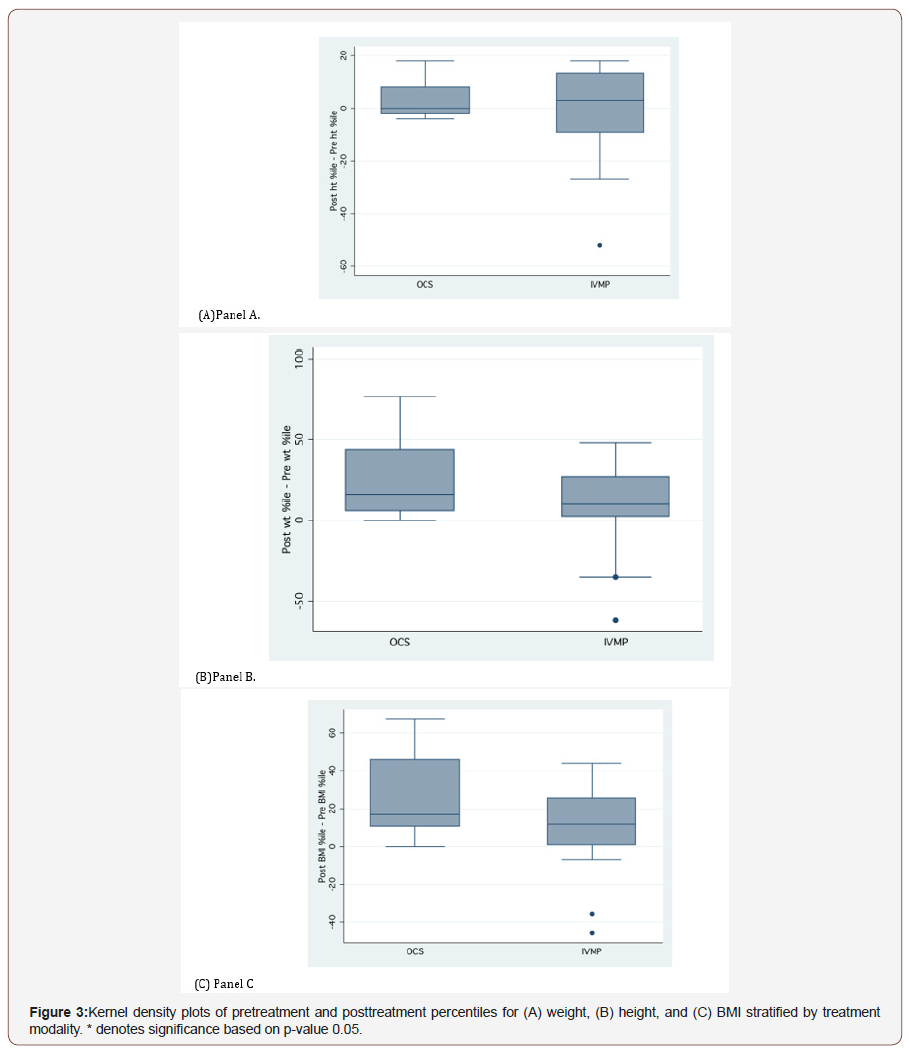
Figure 3 shows kernel density plots for the anthropometric percentiles. In Panel A, while the weight distributions and medians changed (see also Table 2) from pre- to posttreatment for both the OCS (28.9 percentile to 74.2 percentile) and IVMP (64.2 percentile to 80.7 percentile) groups, the change was significant only for the OCS group (p = 0.009). Neither group showed a significant change in height percentile over the course of treatment (Panel B). Finally, significant increases in BMI percentiles (Panel C) were noted for the OCS group (48.4 percentile to 78.4 percentile, p = 0.011), but not for the IVMP group (73.9 percentile to 84.0 percentile).
Next, using the Somers’ D parameter, 95% confidence intervals were then calculated for the most “age-adjusted” parameters of interest as shown in Table 3.
Table 3: Change in various parameters between treatment groups using Somer’s D parameter to calculate confidence intervals. The reference group for comparison for each parameter is OCS. See text for further explanation. * denotes significance at p ≤ 0.05.
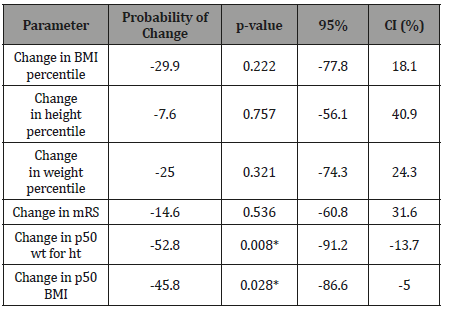
Given a randomly-chosen individual in the OCS group and another randomly-chosen individual in the IVMP group, the IVMP patient was 29.9% less likely to have a greater positive change in BMI percentile than vice versa, with confidence limits from 77.8% less to 18.1% more. However, this result was not significant. Similar negative predictors were calculated for height and weight percentiles along with mRS, but also were not significant as note by the p-values and Cis (Figure 4).
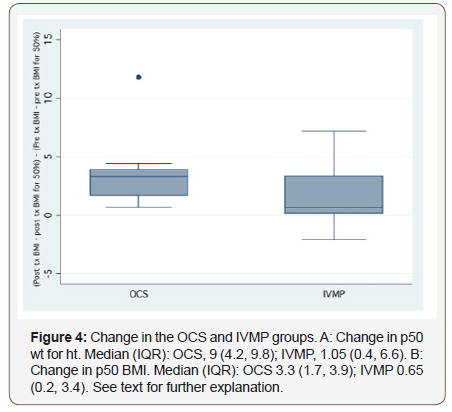
Figure 4 shows box plots for the changes in p50 weight for height and p50 BMI. From both panels, it’s evident that the IVMP group had a greater spread of values from the positive and negative whiskers along with a larger IQR as compared to the OCS group. Given a randomly-chosen individual from each group, the change in p50 weight for height is 52.8% less likely to have a greater positive change in the IVMP group than vice versa, with confidence limits ranging from 91.2% less to 13.7% less. Similarly, the change in p50 BMI is 45.8% less likely to have a greater positive change in the IVMP group than vice versa, with confidence limits ranging from 86.6% less to 5% less.
Discussion
In this retrospective study, we observed that both IVMP and OCS were efficacious in inducing remission, yet there were several important differences between the two groups. Clear differences were noted in the distributions of age at start of current treatment, along with pretreatment height, and posttreatment weight and BMI, all of which need to be considered within the context of age (see below). Borderline significant differences were seen in the distributions of treatment time, pretreatment weight and BMI, and change in weight and BMI from pretreatment to posttreatment. With respect to age, because differences were noted in the distributions of age at the start of current treatments, absolute heights, weights, and even BMIs between the two groups cannot be directly compared because each of these parameters increases with age in adolescents. Instead, examining growth percentiles more accurately assesses changes in each individual. Generally, as “normal” children develop, they should not deviate greatly from earlier percentile measurements. In fact, significant changes above and below earlier percentile measurements can alert pediatricians to possible abnormalities in growth and development. Presently, significant increases were seen in both weight and BMI percentiles for the OCS group but not for any of the measures for the IVMP group. While we examined only the endpoints, Ramanan and colleagues noted that the addition of MTX to OCS therapy compared to just OCS alone caused an increase in height velocity and less of an increase in BMI (see below) in a 1-year period.
Two “age-adjusted” references for comparison used in our study were the 50th percentile weight, given a certain height and age, as well as the 50th percentile BMI for a given age. The change in the differences from the corresponding values for post- and pretreatment were then used to determine, if given a randomly-chosen individual from each group, the likelihood of a greater or lesser change in a parameter in the IVMP individual with respect to the OCS individual as compared to vice versa. The likelihood of negative predictors in an IVMP-chosen individual were significant for both the aforementioned parameters, implying the posttreatment weights and BMIs were likely higher in the OCS group. With respect to disease duration, it was difficult to compare directly with other studies due to variations in endpoint definitions. Santiago et al noted a mean disease duration of 46.5 mos (SD 31.5 mos) with no distinction made between the two treatments [35], whereas Collison et al noted a mean steroid treatment duration of 53.1 mos (SD 40.5 mos) [36]. It was unclear, however, if this latter measure36 was for just this episode or was a lifetime cumulative dosage, and whether this involved OCS, IVMP, or both. Pachman and colleagues directly compared OCS with IVMP in 25 patients and noted significantly shorter periods of functional impairment between the two groups, 3.6 yrs versus 0.8, respectively [22]. Al- Mayouf et al noted a mean treatment duration of 23.5 months for 12 patients on combined OCS, MTX, and intermittent IVMP therapy.
In terms of examining functional outcomes of disease, both the Disease Activity Score (DAS) [37] (Bode) and the Childhood Health Assessment Questionnaire (CHAQ) have been suggested as a valid core set to monitor JDM disease activity [38]. However, they either require parents to have filled out a detailed questionnaire a priori [38] or the clinicians to complete an extensive 28-point joint examination [37], and thus were not usable in this retrospective study. Instead, we used the mRS which is a simple measure of assessing functional disability quickly in the clinic and from information available in patient charts for retrospective analysis [30,39]. Although not yet validated in the pediatric population [40,41] it has been utilized in pediatric patients in assessing stroke [42,43] intracranial hemorrhage [44], central venous thrombosis in Bechet’s disease [45] arteriovenous malformations [46] and neurotrauma [47]. Our retrospective assessment of IVMP and OCS therapy showed positive results in that no individual suffered from bone fractures, life-threatening infections, cataracts, calcinosis, or death in either group. These are possible adverse outcomes in JDM flare-ups, especially due to corticosteroid therapy. In fact, all of the patients in this study had either complete or near-complete resolution of their symptoms after treatment as shown by the mRS results, with no difference between the two groups. Our lack of side effects is in marked comparison to other studies, in which calcinosis and fractures were frequently seen [3,35,48,49].
While our study provides useful data to show the possible benefits of using IVMP versus OCS therapy, there are several caveats that need mentioning. Regarding our protocol, an informal change was noted circa 2000 from OCS as primary intervention to IVMP and MTX therapy. We unfortunately cannot rule out a selection bias – patients who appeared sicker at onset in previous flare-ups might have been treated more aggressively with both interventions and other anti-rheumatic treatments (MTX, hydroxychloroquine, IVIG, etc.), along with differences in individual clinician preferences (see below). Furthermore, while the treatment length was generally shorter for the IVMP group, they were also younger in age as compared to the OCS group. While not explicitly noted, the OCS group was more likely to be comprised of individuals who were relapsing, since median age of initial disease diagnosis is near 6 – 7 years of age. While we didn’t see the side effects seen in others, long-term outcomes in these individuals is unknown, especially in those who have shifted out of the pediatric population and are no longer followed by our clinic. Furthermore, we did not stratify by disease subtype, [19,50] i.e., monocyclic, chronic polycyclic, or chronic, active disease – these could respond differently in terms of treatment and outcomes (other schemes of clinicopathological classification exist [1]). It is unlikely, however, that we had any patients with the last subtype since long-term complications are often seen in those individuals.
Other parameters of interest that we did not examine were bone mineral density (BMD) [51,52] and the effects of cumulative glucocorticoid intake, [53] especially since many records from outside referral sources were incomplete. Thus, the extent of prior pharmacotherapy and its additive effects on the present study cannot be ruled out. Sinha and Bagga suggested that sustained lower quantitative doses of OCS are cumulatively more toxic than those of larger, but less frequently occurring doses of IVMP [54]. With respect to BMD, Castro et al [53] only observed a correlation between low BMD and corresponding low weights in Brazilian girls with JDM, and not other variables such as age, stature, Tanner stage, or cumulative glucocorticoid dosage. In fact, they assert that weight is likely the “best predictor of bone mass” [53]. This fact could help explain the lack of bone-related side effects in our patients. Alsufyani et al. [51] reached a similar conclusion with no consistent relationship noted between BMD and steroid therapy. In a study in adult women with DM (mean age 48.4 yrs, standard deviation 16.3 yrs), Haugeberg and colleagues, however, noted that IVMP treatment led to high rates of bone loss, but no similar correlation was shown for OCS therapy [55]. But, the normal female population in that age group has a different basal hormonal state than in adolescents.
Finally, it is understood that our sample size lacks power to draw any definitive conclusions. Moreover, in the OCS group, we had only 1 male individual, precluding any inferences to be made based on gender between the groups, and all our patients were from a single referral center. While the aforementioned are all recognizable limitations of our work, to our best knowledge, ours is the first study to specifically examine one of the main issues of steroid usage – the effects on various growth parameters – and compare them between OCS and IVMP treatments based on an easily usable functional scoring scale, the mRS. The usage of IVMP has increased considerably in the past decade in order to help induce a rapid remission from symptomatic JDM flare-ups. Unfortunately, the incidence of JDM is low enough to preclude any randomized control trial (RCT) to investigate any medications, including corticosteroids, for its treatment [56]. Furthermore, the paucity of standardized measures to assess therapeutic responses have further hindered stringent evaluation of drug therapies [38]. In fact, there is currently no exact protocol for clinicians to follow, although there has been some recent consensus based on retrospective studies and anecdotal evidence to utilize some form of corticosteroid therapy (OCS and/or IVMP) along with MTX [56] Another important point to consider is economic feasibility. While initially the costs versus benefits ratio might seem to forsake initiation of any widespread RCTs, an economic analysis of this disease (along with another study dealing with rheumatological disorders in general [57]) showed that based on theoretical measures, IVMP is the more cost-effective option with OCS treatment being initially cheaper, but lasting considerably longer [26] Even in adults, studies have shown that IVMP led to greater likelihood of remission and better outcomes as compared to OCS [23,58] It is hoped that investigations such as ours will spur the interest for prospective cohort and even RCT.
Acknowledgement
The authors would like to thank Marie Diener-West, PhD and Lori Jordan, MD, PhD for their critical review and guidance with the analysis, along with Kevin Gilmore, RN for assisting in the data collection, and Shaun Wang for editing and formatting.
Conflict of Interest
No conflict of interest.
References
- Compeyrot-Lacassagne S, Feldman BM (2005) Inflammatory myopathies in children. Pediatr Clin North Am 52(2): 493-520.
- Mendez EP, Lipton R, Ramsey-Goldman R, Roettcher P, Bowyer S, et al. (2003) US incidence of juvenile dermatomyositis, 1995-1998: results from the National Institute of Arthritis and Musculoskeletal and Skin Diseases Registry. Arthritis Rheum 49(3): 300-305.
- Huber AM, Lang B, LeBlanc CM, Birdi N, Bolaria RK, et al. (2000) Medium- and long-term functional outcomes in a multicenter cohort of children with juvenile dermatomyositis. Arthritis Rheum 43(3): 541-549.
- Banker BQ, Victor M (1966) Dermatomyositis (systemic angiopathy) of childhood. Medicine (Baltimore) 45(4): 261-289.
- Feldman BM, Rider LG, Reed AM, Pachman LM (2008) Juvenile dermatomyositis and other idiopathic inflammatory myopathies of childhood. Lancet 371(9631): 2201-2212.
- Bohan A, Peter JB (1975) Polymyositis and dermatomyositis (first of two parts). N Engl J Med 292(7): 344-347.
- Warner JO (1995) Review of prescribed treatment for children with asthma in 1990. Bmj 311(7006): 663-666.
- Aulakh R, Singh S (2008) Strategies for minimizing corticosteroid toxicity: a review. Indian J Pediatr 75(10): 1067-1073.
- Fisler RE, Liang MG, Fuhlbrigge RC, Yalcindag A, Sundel RP (2002) Aggressive management of juvenile dermatomyositis results in improved outcome and decreased incidence of calcinosis. J Am Acad Dermatol 47(4): 505-511.
- Mushtaq T, Ahmed SF (2002) The impact of corticosteroids on growth and bone health. Arch Dis Child 87(2): 93-96.
- Coburg AJ, Gray SH, Katz FH, Penn I, Halgrimson C, et al. (1970) Disappearance rates and immunosuppression of intermittent intravenously administered prednisolone in rabbits and human beings. Surg Gynecol Obstet 131(5): 933-942.
- Hari P, Srivastava RN (1998) Pulse corticosteroid therapy with methylprednisolone or dexamethasone. Indian J Pediatr 65(4): 557-560.
- Cathcart ES, Idelson BA, Scheinberg MA, Couser WG (1976) Beneficial effects of methylprednisolone "pulse" therapy in diffuse proliferative lupus nephritis. Lancet 1(7952): 163-166.
- Cole BR, Brocklebank JT, Kienstra RA, Kissane JM, Robson AM (1976) "Pulse" methylprednisolone therapy in the treatment of severe glomerulonephritis. J Pediatr 88(2): 307-314.
- Fessel WJ (1980) Megadose corticosteroid therapy in systemic lupus erythematosus. J Rheumatol 7(4): 486-500.
- Neild GH, Lee HA (1977) Methylprednisolone pulse therapy in the treatment of polyarteritis nodosa. Postgrad Med J 53(621): 382-387.
- Pachman LM (1990) Juvenile dermatomyositis: a clinical overview. Pediatr Rev 12(4): 117-125.
- Paller AS (1996) The use of pulse corticosteroid therapy for juvenile dermatomyositis. Pediatr Dermatol 13(4): 347-348.
- Huang JL (1999) Long-term prognosis of patients with juvenile dermatomyositis initially treated with intravenous methylprednisolone pulse therapy. Clin Exp Rheumatol 17(5): 621-624.
- Rouster-Stevens KA, Gursahaney A, Ngai KL, Daru JA, Pachman LM (2008) Pharmacokinetic study of oral prednisolone compared with intravenous methylprednisolone in patients with juvenile dermatomyositis. Arthritis Rheum 59(2): 222-226.
- Miller JJ (1980) Prolonged use of large intravenous steroid pulses in the rheumatic diseases of children. Pediatrics 65(5): 989-994.
- Pachman LM (1994) Juvenile dermatomyositis (JDMS): new clues to diagnosis and pathogenesis. Clin Exp Rheumatol 12 Suppl 10: S69-73.
- Matsubara S, Sawa Y, Takamori M, Yokoyama H, Kida H (1994) Pulsed intravenous methylprednisolone combined with oral steroids as the initial treatment of inflammatory myopathies. J Neurol Neurosurg Psychiatry 57(8): 1008.
- Matsubara S, Hirai S, Sawa Y (1997) Pulsed intravenous methylprednisolone therapy for inflammatory myopathies: evaluation of the effect by comparing two consecutive biopsies from the same muscle. J Neuroimmunol 76(1-2): 75-80.
- Bowyer SL, Blane CE, Sullivan DB, Cassidy JT (1983) Childhood dermatomyositis: factors predicting functional outcome and development of dystrophic calcification. J Pediatr 103(6): 882-888.
- Klein-Gitelman MS, Waters T, Pachman LM (2000) The economic impact of intermittent high-dose intravenous versus oral corticosteroid treatment of juvenile dermatomyositis. Arthritis Care Res 13(6): 360-368.
- Farrell B, Godwin J, Richards S, Warlow C (1991) The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry 54(12): 1044-1054.
- Rankin J (1957) Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scott Med J 2(5): 200-215.
- van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J (1988) Interobserver agreement for the assessment of handicap in stroke patients. Stroke 19(5): 604-607.
- Vedanarayanan V, Subramony SH, Ray LI, Evans OB (1995) Treatment of childhood dermatomyositis with high dose intravenous immunoglobulin. Pediatr Neurol 13(4): 336-339.
- Calvert S, Miller HE, Curran A, Hameed B, McCarter R, et al. (2008) The King's Outcome Scale for Childhood Head Injury and injury severity and outcome measures in children with traumatic brain injury. Dev Med Child Neurol 50(6): 426-431.
- Haque A, Ray LI, Vedanarayanan VV (2010) Using anthropometric measures to monitor efficacy of pulse intravenous methylprednisolone versus oral corticosteroid therapy in the treatment of Juvenile Dermatomyositis. S006-004. 62nd American Academy of Neurology annual meeting. Toronto, Canada.
- Newson RB (2001) Parameters behind "non-parametric" statistics: Kendall's tau-a, Somers' D and median differences. Stata J 1(1): 1-20.
- Somers RH (1962) A new asymmetric measure of association for ordinal variables. Am Sociol Rev 27: 799-811.
- Santiago RA, Silva CA, Caparbo VF, Sallum AM, Pereira RM (2008) Bone mineral apparent density in juvenile dermatomyositis: the role of lean body mass and glucocorticoid use. Scand J Rheumatol 37(1): 40-47.
- Collison CH, Sinal SH, Jorizzo JL, Walker FO, Monu JU, et al. (1998) Juvenile dermatomyositis and polymyositis: a follow-up study of long-term sequelae. South Med J 91(1): 17-22.
- Bode RK, Klein-Gitelman MS, Miller ML, Lechman TS, Pachman LM (2003) Disease activity score for children with juvenile dermatomyositis: reliability and validity evidence. Arthritis Rheum 49(1): 7-15.
- Ruperto N, Ravelli A, Pistorio A, Ferriani V, Calvo I, et al. (2008) The provisional Paediatric Rheumatology International Trials Organisation/American College of Rheumatology/European League Against Rheumatism Disease activity core set for the evaluation of response to therapy in juvenile dermatomyositis: a prospective validation study. Arthritis Rheum 59(1): 4-13.
- Feliciano CE, Rodriguez-Mercado R (2008) Evaluation of pediatric patients with vascular malformations managed with endovascular and radiosurgical techniques using a modified Rankin Disability Scale. P R Health Sci J 27(1): 27-33.
- Cohen J (2009) Interrater reliability and predictive validity of the FOUR score coma scale in a pediatric population. J Neurosci Nurs 41(5): 261-267; quiz 268-269.
- Ramaswamy V, Mehta V, Bauman M, Richer L, Massicotte P, et al. (2008) Decompressive hemicraniectomy in children with severe ischemic stroke and life-threatening cerebral edema. J Child Neurol 23(8): 889-894.
- Jordan LC, Hillis AE (2007) Hemorrhagic stroke in children. Pediatr Neurol 36(2): 73-80.
- Mathews MS, Sharma J, Snyder KV, Natarajan SK, Siddiqui AH, et al. (2009) Safety, effectiveness, and practicality of endovascular therapy within the first 3 hours of acute ischemic stroke onset. Neurosurgery 65(5): 860-865; discussion 865.
- Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Oleinik A, et al. (2010) The spot sign score in primary intracerebral hemorrhage identifies patients at highest risk of in-hospital mortality and poor outcome among survivors. Stroke 41(1): 54-60.
- Yesilot N, Bahar S, Yilmazer S, Mutlu M, Kurtuncu M, et al. (2009) Cerebral venous thrombosis in Behcet's disease compared to those associated with other etiologies. J Neurol 256(7): 1134-1142.
- da Costa L, Thines L, Dehdashti AR, Wallace MC, Willinsky RA, et al. (2009) Management and clinical outcome of posterior fossa arteriovenous malformations: report on a single-centre 15-year experience. J Neurol Neurosurg Psychiatry 80(4): 376-379.
- Rotarescu V, Ciurea AV (2008) Quality of life in children after mild head injury. J Med Life 1(3): 307-322.
- Seshadri R, Feldman BM, Ilowite N, Cawkwell G, Pachman LM (2008) The role of aggressive corticosteroid therapy in patients with juvenile dermatomyositis: a propensity score analysis. Arthritis Rheum 59(7): 989-995.
- Silverman ED, Myones BL, Miller JJ (1984) Lymphocyte subpopulation alterations induced by intravenous megadose pulse methylprednisolone. J Rheumatol 11(3): 287-290.
- Spencer CH, Hanson V, Singsen BH, Bernstein BH, Kornreich HK, et al. (1984) Course of treated juvenile dermatomyositis. J Pediatr 105(3): 399-408.
- Alsufyani KA, Ortiz-Alvarez O, Cabral DA, Tucker LB, Petty RE, et al. (2005) Bone mineral density in children and adolescents with systemic lupus erythematosus, juvenile dermatomyositis, and systemic vasculitis: relationship to disease duration, cumulative corticosteroid dose, calcium intake, and exercise. J Rheumatol 32(4): 729-733.
- Stewart WA, Acott PD, Salisbury SR, Lang BA (2003) Bone mineral density in juvenile dermatomyositis: assessment using dual x-ray absorptiometry. Arthritis Rheum 48(8): 2294-2298.
- Castro TC, Terreri MT, Szejnfeld VL, Len C, Fonseca AS, Hilario MO (2005) Bone mineral density of Brazilian girls with juvenile dermatomyositis. Braz J Med Biol Res 38(2): 309-313.
- Sinha A, Bagga A (2008) Pulse steroid therapy. Indian J Pediatr 75(10): 1057-1066.
- Haugeberg G, Griffiths B, Sokoll KB, Emery P (2004) Bone loss in patients treated with pulses of methylprednisolone is not negligible: a short term prospective observational study. Ann Rheum Dis 63(8): 940-944.
- Huber AM, Giannini EH, Bowyer SL, Kim S, Lang B, et al. (2010) Protocols for the initial treatment of moderately severe juvenile dermatomyositis: results of a Children's Arthritis and Rheumatology Research Alliance Consensus Conference. Arthritis Care Res (Hoboken) 62(2): 219-225.
- Criswell LA, Such CL (1996) Cost effectiveness analysis of drug therapies for rheumatoid arthritis. J Rheumatol Suppl 44: 52-55.
- Yanagisawa T, Sueishi M, Nawata Y, Akimoto T, Nozaki T, et al. (1983) Methylprednisolone pulse therapy in dermatomyositis. Dermatologica 167(1): 47-51.
-
Xiahong Si, Asim Haque, Linda I. Ray, VV Vedanarayanan. Anthropometric Effects of Intravenous Methylprednisolone Versus Oral Corticosteroids in the Treatment of Juvenile Dermatomyositis. Arch Neurol & Neurosci. 4(4): 2019. ANN.MS.ID.000595.
-
Juvenile dermatomyositis (JDM); Corticosteroids (OCS); Anthropometry; Modified Rankin scale; Pulse intravenous methylprednisolone (IVMP); BMD: Bone Mineral Densit; BMI: Body Mass Index; CHAQ: Childhood Health Assessment Questionnaire; CI: 95% Confidence Interval; DAS: Disease Activity Score; IQR: Interquartile Range
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






