 Review Article
Review Article
Altered Whole Brain Gray Matter Volume in High Myopia Patients - A Narrative Review
Koka Gogichashvili1,2*, Mirza Khinikadze1, Onyekachi Emmanuel Anyagwa2, Radhika Prashant Patil2, Mostafa Yassin2, Shashwat Sandeep Phade2, Aishwarya Ninad Bhuta2, Kumar Saurabh2, Vismaja Vijayan2, Srushti Kishor Jamdar2, Oluwatoyin Adalia Dairo2 and Reza Badrnejad2
1Caucasus Medical Centre, Tbilisi, Georgia
2School of Medicine, New Vision University, Georgia
Koka Gogichashvili, Caucasus Medical Centre, Tbilisi, Georgia.
Received Date: February 17, 2023; Published Date: March 06, 2023
Abstract
High myopia (HM) is a refractive error caused by axial elongation, with its prevalence impacting the quality of life and the socioeconomic well-being of nations. Due to the intimate ties between the brain’s visual-related areas and the eye’s associated structures, aberrant visual function alters the brain’s functional connections and morphology. This narrative review, which contains pertinent publications and studies from 2018 to 2022, outlines the advancement of research connecting changes in gray matter volume in patients with extreme myopia and other microstructural alterations caused by this condition. Numerous techniques, including optical coherence tomography (OCT), voxel-mirrored homotopic connectivity (VMHC), and DKI, were employed in the studies, but research confirmed that functional magnetic resonance imaging (fMRI), is the preferred imaging technique for examining spontaneous brain activity. Gray Matter Volume (GMV) values in High Myopic patients (HM) were significantly lower, according to a study that used MRI data that was processed using the SPM8 software. In contrast, increased GMV values were found in the brain stem, left Para hippocampal gyrus/thalamus, right Para hippocampal gyrus/thalamus and the left and right putamen. Furthermore, spectral domain OCT has demonstrated that excessive myopia causes thinning of the choroidal layer. Different dReHo values in various brain regions are observed in HM patients, which may indicate that HM significantly alters brain activity. The potential ramifications of this should highlight the need for additional study and therapeutic management of high myopia, to showcase the important pathological effects of this condition on the brain and its functional roles amongst society.
Keywords:Gray matter volume; high myopia; fMRI; Magnetic resonance imaging; Voxel-based morphometry; White matter
Abbreviations:HM: High Myopia; OCT: Optical Coherence Tomography; VMHC: Voxel-Mirrored; Homotopic Connectivity; DKI: Diffusion Kurtosis Imaging; Fmri: Functional Magnetic Resonance Imaging; GMV: Gray Matter Volume; Cu: Cuneus; Hcs: Healthy Controls; HM: High Myopia: LG: Lingual Gyrus; Parahg: Parahippocampal Gyrus; Put: Putamen; Tha: Thalamus; AD: Axial Diffusivity; DKI: Diffusion Kurtosis Imaging
Introduction
One of the common causes of vision impairment in the world is myopia. Myopia is alarmingly on the rise which negatively affects both the economic well-being of nations and the quality of life around the globe mainly due to high academic pressure and indoor time spent [1,2,3]. The high prevalence of myopia leads to an increased incidence of high myopia as these two are closely related diseases [3].
High myopia (HM) is a refractive defect of -6 diopters or worse due to axial elongation and may cause other pathological changes such as cataract formation, glaucoma, retinal detachment and ischemia, choroidal neovascularization, macular changes and hemorrhage, and myopic foveoschisis [3,4]. High myopia is a multifactorial medical condition but taking genetic profile into consideration, a number of genes have been identified to have a prominent role in the prognosis and progression of high myopia with diverse inheritance patterns [5]. Some genes identified including COL1A1 (Collagen Type I Alpha 1 Chain) not only play a role in connective tissue disorder but also are associated with high myopia [6]. Furthermore, it is necessary to mention that early therapies with contact lenses, eyewear, and lifestyle adjustments that slow the progression of myopia can considerably lessen its burden due to their intimate alignment with the eye [7,6].
Following changes in the axial length of the eye, the retina nerve fibres thin, and the optic disk deforms [1]. With very close relations between eye structures, optic nerves, and brain visualrelated areas, abnormal visual function leads to changes in the functional connectivity and morphology of the brain [1,3]. There are some methods such as Functional magnetic resonance imaging (fMRI), Voxel-Mirrored Homotopic Connectivity (VMHC), Regional homogeneity, and dynamic amplitude of low-frequency fluctuation to evaluate functional connectivity density and spontaneous neuronal activity in a resting state brain and recent studies show changed activities and impaired cognition in patients with high myopia [1,3].
Many brain regions are subject to changes in high myopia patients namely the fusiform, inferior temporal, postcentral, and precentral gyri in addition to the Rolandic operculum, posterior cingulate cortex, and lenticular nucleus [1,3]. The methods mentioned earlier above are also used in pattern recognition and studying brain alteration and morphology in a variety of conditions such as optic neuritis, Alzheimer’s disease, Parkinson’s disease, stroke, and many more [1,3,8]. In this review, we discuss the advancement in studies linking the alterations in gray matter volume in patients with high myopia and other micro structural alterations that occur with this pathology.
Methods of Literature Search
A PubMed engine search was carried out by using the keywords “gray matter volume, high myopia, magnetic resonance imaging, voxel-based morphometry, white matter volume”. All the research articles and studies published in English between 2018 - 2022 were included in this paper for review and other relevant articles and studies were included in this narrative review.
Imaging Methods
A study by Huang et al. applied usage of Optical coherence tomography (OCT) to gain insight on the affected parts of the brain due to high myopia. OCT is a non-invasive method used to get insight on myopic fundus lesions. The difference in thickness of the retinal fibre layer was comparable both in HM and low myopia patients using OCT, [4]. The studies conducted by Huang et al., Cheng et al., and Ji et al. utilized functional magnetic resonance imaging (fMRI), which has the advantages of being used as a multi-sequence, multiparameter, non-ionizing radiation, routine, and functional imaging technique [1,3,8]. In the study conducted by Cheng et al., a technique called voxel-mirrored homotopic connectivity (VMHC) was used to explore the differences in functional connections between voxels in bilateral hemispheric symmetrical systems and quantify their coordination. It is also an automated method for interpreting neuro morphological MRI data that is frequently used to analyze changes in the gray and white matter volume. Utilizing resting functional connections as a basis, this functional magnetic resonance imaging technique examines the patterns of brain tissue [1]. Resting-state functional magnetic resonance imaging (rs-fMRI), which investigates the connection between spontaneous brain activity and clinical symptoms, was also applied in the Ji et al. study [3]. The advantages of rs-fMRI over conventional fMRI techniques include direct signal acquisition and the identification of functional areas in a range of patient demographics. Most employed in rs-fMRI analysis, dynamic regional homogeneity displays the dynamic temporal consistency of spontaneous brain activity between adjacent voxels, describes similarities in local brain activity, and investigates the functional coordination of spontaneous neural activity. The data from fMRI often has the inescapable limitations of the fMRI environment, such as heartbeat, muscle beat, and respiratory movements [3]. Wang et al. applied Diffusion tensor imaging (DTI) as its major MRI method to provide quantitative measures of microstructural integrity and organization in vivo [9]. DTI has been widely used to assess microstructural abnormalities in the brain’s white matter because it analyses water diffusion based on the Hypothesis that water molecules move in a way consistent with a Gaussian distribution. In biological tissues, barriers such as organelles and cell membranes cause water molecules to diffuse non-Gaussianly more frequently. As a result, the DTI model’s applicability and sensitivity might not be ideal. Therefore, Diffusional kurtosis imaging (DKI), as a natural extension of the DTI model, permits non- Gaussian diffusion quantification and can be used to measure the microstructural integrity and tissue complexity of WM even in the presence of crossing fibers. Additionally, compared to traditional DTI, it has shown enhanced sensitivity and specificity for detecting developmental and degenerative changes in brain tissues [9].
Huang et al. [8] HM groups used CIRRUS HD-OCT (optical coherence tomography), which was not done prior fMRI in any of the studies we evaluated, to calculate the mean retinal nerve fiber layer (RNFL) thicknesses of eyes. A 3-T MRI scanner with an 8-channel head coil was used for the MRI scanning. VBM was used to preprocess all MRI data, and MATLAB’s toolbox was used to analyze it (version 2013a) [10]. In the work by Wang et al., [11] MRI data were collected using a 3 T MRI scanner equipped with an 8-channel head coil and analyzed using the same data processing and analysis of brain imaging toolbox implemented in MATLAB (Version R2013a) [8] as by Huang et al., [8]. These findings unequivocally show that fMRI is the preferred imaging method.
Patient Age
Age is an important aspect when it comes to comparing the effect of high-grade myopia on the gray matter volume on the brain. This is as changes occur throughout the ageing process that affect the normal functioning of the brain. This is mentioned in the Wang et al. study as it states that changes in cerebral blood flow to gray matter are common and although controversial, there is evidence of reduced cerebral blood flow to gray matter in patients that are older [11]. Although other studies opted for adults there were no specific references made to the patients’ ages or variation in the mean age of the patients included with Huang et al. reporting a mean age of 23.29 ± 1.36 and 22.42 ± 1.47 for the control group and the myopia patients respectively [8]. Cheng et al. also had a similar mean age with the study reporting a mean age of 26.235 ± 0.462 and 25.783 ± 0.102 years for the myopia and control group respectively [1]. Wang et al. suggests that there is no relation between the patient’s ages and the development of microstructural abnormalities in high myopia patients [11]. Thus, there is no significant conclusion to be drawn concerning the direct effect of the ages of the patients and the relationship between myopia and change in the gray matter volume of the brain.
Handedness
The existence of Handedness and its consideration in the development of the human trait is one which is fully discussed in the neurological field, however, the assessment of handedness has not universally agreed on scale of measurement [12]. The correlation between handedness and myopia is a field of study scarcely considered or researched on adequately. Polarizing results have led to much confusion as to if there is a link between handedness and myopia. A study in 2003 by Mansour et al. [13] found no relationship between these two components and this was further supported by a study by Cheng et al. in 2014 [14], which found no connection between handedness and ocular refraction. However, research studied by Yong-Feng Y., insists on a correlation between myopia and handedness, and supported by a 2001 study undertaken by Needlam R [13,15]. which proposes a lateralization theory towards this agreement between these components. It should be stated that even though the association between handedness and myopia factors influencing brain and ocular development in-utero might suggest plausible connection [16,17]. With the primary focus being high myopia, gray matter alterations and its interaction with handedness, it becomes tasking as most studies, especially between 2018-2022, omit reasons for handedness choice in the sample population observed.
In an experimental study by Wang et al. for Characterizing Brain Microstructural Abnormalities in High Myopia Patients [9], a total of 35 High myopia (HM) patients were recruited from outpatient clinics of Beijing Friendship Hospital, Capital Medical University between January 2017 to December 2018. It also included, thirty-five age- and sex-matched healthy controls (HCs) with uncorrected visual acuity >/= 1.0 were also recruited. All subjects were right-handed as an inclusion criterion. Similar to the previous investigation, Cheng et al. conducted an fMRI-based cohort study using voxel-mirrored homotopic connectivity (VMHC) on 89 patients with HM and 59 HCs without ametropia between September 2018 and September 2020 albeit published in 2022 [3]. The same character, i.e., Presence of Right Handedness has also been listed as a common demographic character of all subjects, both suffering from HM as well as HCs in a study by Huang et al. 2018 [4]. Although Ji et al. did not refer to handedness in population sampling in the 2022 study exploring dynamic spontaneous brain activity in patients with high myopia [3], Wang et al. and Huang et al. also included right handedness in their 2021 and 2018 study respectively [8,9].
The study of Huang et al. concluded that HM patients showed significantly decreased GMV values in the right cuneus/lingual gyrus and the right thalamus in addition to, higher GMV values in the brain stem, right Para hippocampal gyrus/thalamus, left Para hippocampal gyrus/thalamus, as well as the right and left putamen as compared with HCs [8]. With little to no study properly outlining the correlation between brain structure in right hand dominance and the role it plays in high myopia development, it creates a difficulty in seeing a connection in sample population selection. Also, None of these studies provide evidence on the effect of handedness of the subjects either on grey matter volume or development of high myopia, which clearly shows lack of sufficient work in this field.
Number Of High Myopia Patients Vs Number Of Control Patients
In the Huang et al. study, a total of 82 HM patients (52 men and 30 women) were enrolled from the Ophthalmology Department of the First Affiliated Hospital of Nanchang University. The diagnostic criteria were as follows: refractive diopter less than 6.00; absence of any other ocular diseases (strabismus, cataracts, glaucoma, retinal degeneration, etc.); history of ocular trauma or eye surgery; psychiatric disorders; and cardiovascular system diseases [8]. The difference in number of HM and Healthy Control (HC) patients (58 subjects) could be questioned as it may call for numerical bias; however, a large sample size plays a role in result analysis. With the Cheng et al. study, the patients were chosen from a pool of candidates between September 2018 and September 2020 [1]. The cohort had 59 HCs and 89 patients with HM. Criteria for patients with myopia were as follows: aged 18–60 years, meeting the diagnostic criteria of high myopia and right-hand dominance, and having normal MRI scans. The exclusion criteria were: other diseases, abnormal brain structure due to the presence of hematomas or tumours, and presence of mental disorders [1]. In a study by Ji et al., at the Department of Ophthalmology at Nanchang University, 82 patients with HM and 59 HCs were assessed from August to December 2021. Binocular vision of -6 diopters or worse, corrected decimal visual acuity of at least 1.0, completion of MRI-related tests, optical coherence tomography, ultrasonography, and other ophthalmic examinations were considered as the inclusion criteria for patients with HM [3]. Exclusion criteria for patients with HM included binocular vision better than -6 diopters, maculopathy, choroidal neovascularization, retinal pigment epithelial disease, history of ocular trauma or ophthalmic surgery, neurological disease, and/or cerebral infarction. In Nanchang City, healthy controls were chosen at random based on their age, sex, and level of education [3]. While Wang et al., fixes the numeral issue with their study on cerebral blood flow alterations in HM by having 35 HM and 35 HC study subjects, This could be argued for accuracy if the small sample size is called into question [9].
Duration Of High Myopia (Disease Duration)
Another important factor to consider is the duration of the high myopia in the patients. This is an important factor to consider since the aim of the study is to ascertain the relationship between high myopia and the gray matter volume of the brain. A study by Wang, H. et al. states a great deal of changes occurs such as the suspected reduction of the cerebral blood flow to the gray matter [11]. Therefore, Wang et al. [9] using 3T diffusion kurtosis imaging (DKI) technology were to study microstructural damage in high myopia (HM) patients , where the mean HM duration was 23.8 ± 11.8 years, DKI can be used as a sensitive tool for detecting microstructural injury in WM tracts also and can provide useful information for detecting microstructural injury and monitoring disease progression, according to the mean AD values in the right ILF and IFOF (Figure 1) which showed significantly positive correlations with disease duration among diffusion parameters. Hence, duration of high myopia can be related to microstructural damage in certain WM tracts in HM patients but whether the variable has an effect on volume of grey matter, still calls for research in this area (Figure 1).
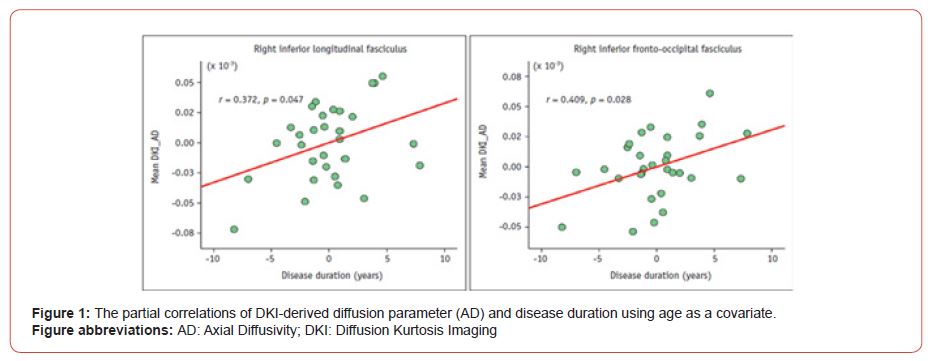
The same variable, i.e., duration of high myopia, with the mean HM duration was 8.38 ± 1.23 years has also been listed as a common demographic character of HM subjects in a study by Huang et al. [8] but the significance of the HM duration has not been discussed in relation to its effect on grey matter volume. Huang, X. et. al and Wang, H. et al both state that due to their study structure and sample size, no significant result can be concluded with regards to the effect of the duration of myopia and changes in the brain [8,11]. Thus, more studies are required to investigate this issue.
Gray Matter Concentration /Volume
The Huang et al. study majorly focused on grey matter volume in HM patients, with all study subjects undergoing an MRI scan [8]. The obtained MRI data were processed using the SPM8 software and hence, the relationship between the mean GMV values of the brain regions and clinical features, including refractive dioptre and the mean retinal nerve fibre layer thickness, in the HM group were analyzed using Pearson’s correlation [18].
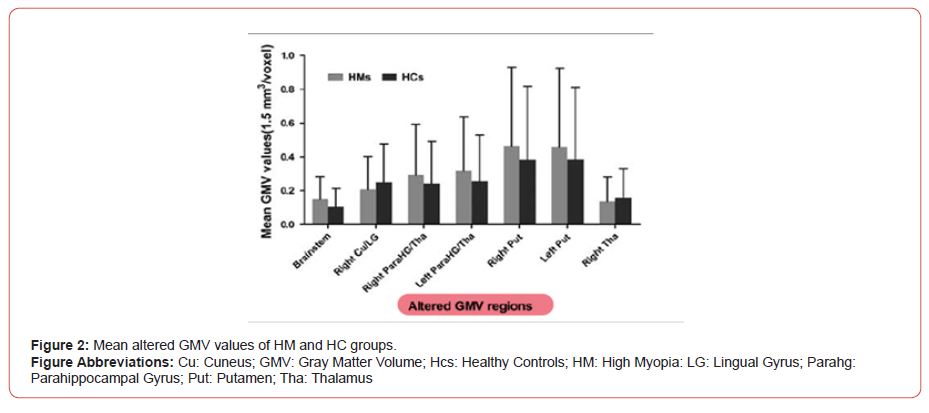
As a result, in comparison with HCs, HM patients showed significantly reduced GMV values in the right cuneus/lingual gyrus and the right thalamus. In contrast, HM groups showed higher GMV values in the brain stem, right parahippocampal gyrus/thalamus, left Para hippocampal gyrus/thalamus, as well as the right and the left putamen (Figure 2).
As HM patients showed increased GMV values in the bilateral para hippocampal gyrus/thalamus and that increased GMV values were correlated negatively with the mean RNFL of the left eye; the study goes on to conclude that that may be interpreted as dysfunction in the functional area associated with memory in the brain [8]. Furthermore, pathogenic abnormalities in HM may be connected to structural alterations in the thalamus and bilateral Para hippocampal gyrus of the brain. This study, however, does not identify the core cause of the elevated GMV values in certain brain structures in the HM group. Huang et al. also states that there is an increase in the gray matter volume of the putamen which may be interpreted as the compensation of the motor response in high myopia patients [8]. This is corroborated by Cheng et al. as it found that there is increased Voxel-mirrored homotopic connectivity values in the putamen as well which indicates increased activity in that area compared to surrounding regions [1]. Wang et al. suggest that the changes caused by high myopia in fact are actually related to the white matter. The paper continues to elaborate that the axonal damage in the thalamus, corticospinal tract, right inferior longitudinal fasciculus and right inferior fronto-occipital fasciculus is noted in high myopia patients [11].
Alterations In Gray Matter Determined By Gender
The role of the patient’s gender, to assess the effect of myopia and its structural changes on the brain’s volume of gray matter, was explored across a number of studies. However, the results from such analysis were statistically poor in quality. Two studies categorized patients into a high myopia group (HM) or a healthy control group (HC), with both groups containing randomized numbers of adult males and females. The ratio of male to female participants in the study by Huang et al., was presented as 52:30 within the HM group and 28:30 within the HC group [8]. Whilst a one-sample t test was used to extrapolate and quantify gender as a variable worthy for explaining the topic in question, gender was excluded from the final analysis of this study as there was no significant evidence present to suggest that gender can affect gray matter in those with high myopia. A similar study conducted by Wang et al., also compared the differences in results across males and females, though an insignificant P-value of 0.641 was reported and thus, meant that once again, gender had no significant impact within the conducted study [11]. Across both studies, it was clear that there was no significant alteration in the volume of gray matter in patients with high myopia, which could be quantified by the differences in the gender assigned to the participants at birth.
Area of Brain Affected
High Myopia [HM] or degenerative myopia, can progress to damage neurosensory retina and choroid, which can also extend to structures of the brain. In order to identify and evaluate changes in brain activity functional magnetic resonance is very much used in different studies. Mirzajani et al. identified decreased functional activity in visual cortex regions in patients with HM [19]. Also, Huang et al. in 2018 demonstrated that there are certain changes in the visual pathway and limbic system structure using Voxel mirrored homotopic connectivity [VMHC]. Voxel mirrored homotopic connectivity [VMHC] explored the differences in functions and relationships between voxels in systems with bilateral hemispheres and helped evaluate their coordination [4].
The 2022 study by Cheng et al. identified decreased VMHC values in putamen and fusiform gyrus in patients with HM [1]. This information correlates to the findings of Huang et al. in 2018, where it mentions that global gray matter volume of bilateral putamen is increased, due to structural changes for compensation for motor functions in HM patients. In this study the authors state a relationship between the difficult visual state in high myopia may lead to changes in structure in the fusiform gyrus and negatively have an effect on its physiological action. Huang et al. also states deceased gray matter volume in right cuneus/lingual gyrus and the right thalamus, with increased volume in brain stem, right Para hippocampal gyrus/thalamus, left Para hippocampal gyrus/ thalamus as well as the right and left putamen [8]. Patients with HM are also known to present lesions affecting white matter and gray matter regions. Ji et al. studied patients with a resting state functional magnetic resonance imaging with dynamic regional homogeneity analysis, finding that patients with HM present higher dReHo values in the left fusiform gyrus, right inferior temporal gyrus, right Rolandic operculum, right postcentral gyrus and right precentral gyrus. Higher order cognitive processes like language and visual comprehension, as well as emotional control, are significantly influenced by the right inferior temporal gyrus. These results imply that there may be a change in functional activity in these different areas of the brain [3].
Wang et al, using measurement of cerebral blood flow, had no identification of cerebrum changes in flow, although cerebellum did present with some areas of increased blood flow. This may be due to cerebellum’s role in vision formation and transmission, or impaired ocular adjustment and reflex, such as, saccade, smooth pursuit movement, fixation, accommodation and vestibulo ocular reflex [11]. Further investigation is needed in this area to determine causality of increased blood flow to bilateral cerebellum [11]. Another study by Wang et al. also states that,” DKI data revealed that microstructural abnormalities in white matter in HM subjects predominantly in the bilateral corticospinal tract (CST), right inferior longitudinal fasciculus (ILF), superior longitudinal fasciculus (SLF), inferior fronto-occipital fasciculus (IFOF), and left thalamus [9].
Microstructural Alterations
According to Huang et al. [8], right cuneus/lingual gyrus and right thalamus Gray Matter Volume (GMV) values in High Myopic patients (HM) were considerably lower than those in control groups (HC). In contrast, HM patients displayed noticeably elevated GMV values in the brain stem, right Para hippocampal gyrus/thalamus, left Para hippocampal gyrus/thalamus, as well as the right and left putamen. Wang et al., [9] has discussed previous studies conducted by Noppeney et al., [6] which demonstrates relative to sighted controls and early blind or high myopic patients, HM patients show decreased gray matter volume (GMV) in early visual areas. Additionally, they exhibit optic chiasm and optic radiation atrophy. These studies hypothesis that since the cuneus is a component of the occipital lobe, which is crucial for visual processing and interacts with the primary visual cortex, therefore HM may also be accompanied by impairment in the visual cortex. Additionally, because the Para hippocampal gyrus and the thalamus are essential components of the limbic system, which is crucial for memory and emotions, HM may also be accompanied by dysfunction in memory. In order to make up for the HM’s motor impairment, the volume of the bilateral putamen may have increased. Furthermore, high myopic eyes show a thinning of the choroidal layer, as has been shown by spectral domain OCT. Focus on specific brain regions can be argued by the study of Ji et al. which revealed that patients with HM had significantly higher dReHo values in the right Rolandic operculum (R-ROL), suggesting that behaviours in these neural connections are strengthened [3]. So, we assume that HM leads to increased R-ROL activity, which may result in deficiencies in the processing of combined exteroceptive and interoceptive information in patients with HM. Patients with HM have different dReHo values in numerous brain regions, which suggests that HM causes significant changes in dynamic spontaneous brain activity.
Conclusion
The importance of further understanding the connection between gray matter volume changes, microstructural alterations to brain structure and the integral role of a prevalent condition such as high myopia cannot be overstated. The implication of such need should open up the need for more research and therapeutic management of high myopia while simultaneously understanding in depth how the pathology of myopia might further affect other brain structures and its implication on their functional roles.
Acknowledgements
We express our thanks to Juan Carlos Ayala Alvarez and Courtney Storm True body for their insight, expertise and support during this study.
Conflict of Interest
The authors declare no conflict of interest. There are no relevant financial or non-financial competing interests to report.
References
- Yi Cheng , Xiao-Lin Chen , Ling Shi , Si-Yu Li , Hui Huang, et al. (2022) Abnormal functional connectivity between cerebral hemispheres in patients with high myopia: A resting FMRI study based on voxel-mirrored homotopic connectivity. Frontiers in Human Neuroscience 16: 910846
- Brien A Holden, Timothy R Fricke, David A Wilson, Monica Jong, Kovin S Naidoo, et al. (2016) Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 123(5): 1036–1042.
- Yu Ji, Qi Cheng, Wen-Wen Fu, Pei-Pei Zhong, Shui-Qin Huang, et al. (2022) Exploration of abnormal dynamic spontaneous brain activity in patients with high myopia via dynamic regional homogeneity analysis. Frontiers in Human Neuroscience 16: 959523.
- Yasushi Ikuno (2017) Overview of the complications of high myopia. Retina 37(12): 2347–2351.
- Wang YM, Lu SY, Zhang XJ, Chen LJ, Pang CP, et al. (2022) Myopia genetics and heredity. Children (Basel, Switzerland) 9(3): 382
- Uta Noppeney, Karl J Friston, John Ashburner, Richard Frackowiak, Cathy J Price (2005) Early visual deprivation induces structural plasticity in gray and white matter. Current Biology 15(13): R488–R490.
- Sankaridurg, P (2017) Contact lenses to slow progression of myopia. Clinical & Experimental Optometry 100(5): 432–437.
- Xin Huang, Yuxiang Hu, Fuqing Zhou, Xiaoxuan Xu, Yifan Wu, et al. (2018) Altered whole-brain gray matter volume in high myopia patients: A voxel-based morphometry study. Neuroreport 29(9):760–767.
- Huihui Wang, Hongwei Wen, Jing Li, Qian Chen, Shanshan Li, et al. (2021) Characterization of brain microstructural abnormalities in high myopia patients: A preliminary diffusion kurtosis imaging study. Korean Journal of Radiology 22(7): 1142–1151.
- Needlam R (2001) Left-handed thinking. Times Retrieved June 7, 2005.
- Huihui Wang, Shanshan Li, Xi Chen, Yanling Wang, Jing Li, et al. (2020) Cerebral Blood Flow Alterations in High Myopia: An Arterial Spin Labeling Study. Neural Plast 2020: 6090262.
- Marietta Papadatou-Pastou, Eleni Ntolka, Judith Schmitz, Maryanne Martin, Marcus R Munafo, et al. (2020) Human handedness: A meta-analysis. Psychological Bulletin 146(6): 481–524.
- Ahmad M Mansour, Zaher M Sbeity, Kassem M Kassak (2003) Hand dominance, eye laterality and refraction. Acta Ophthalmologica Scandinavica 81(1): 82–83.
- Ching-Yu Cheng, May-Yung Yen, Hsin-Yi Lin, Wei-Wei Hsia, Wen-Ming Hsu (2004) Association of ocular dominance and anisometropic myopia. Investigative Ophthalmology & Visual Science 45(8): 2856–2860.
- Yong-Feng, Y (2000) Preliminary research on the relationship between causes of pyopia & left-handedness or right-handedness. Journal of Heze Teachers College: 4.
- Bu D, & Wang, X (2017) Causal effect study of high cholesterol on myopia. IEEE International Conference on Bioinformatics and Biomedicine [BIBM].
- Mathangi Krishnakumar, Shweta Atheeshwar, Mathangi D. Chandrasekar (2014) Myopia and digit ratio in medical college students. Plos One 9(2): e89800.
- SPM8 software - statistical parametric mapping (2022) Wellcome Centre for Human Neuroimaging [n.d.].
- Mirza Jani A, Ghorbani M, and Rasuli B, Mahmoud-Pasha Zadeh, A (2017) Effect of induced high myopia on functional MRI signal changes. Phys Med 37: 32–36.
-
Koka Gogichashvili*, Mirza Khinikadze, Onyekachi Emmanuel Anyagwa, Radhika Prashant Patil, Mostafa Yassin, et al.. Altered Whole Brain Gray Matter Volume in High Myopia Patients - A Narrative Review. 14(4): 2023. ANN.MS.ID.000841.
-
Brain, High Myopia, Gray matter volume; High myopia; fMRI, Thalamus, Axial Diffusivity, Putamen, Parahg, Cuneus, Voxel-Mirrored, Coherence, Tomography, Homotopic Connectivity, Voxel-Mirrored.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






