 Mini Review Article
Mini Review Article
Understanding Rasmussen’s Encephalitis: A Review of Current Diagnostic and Management Strategies
Mariam Mahmoud Hassan*
Department of Paediatrics, University Putra Malaysia, Malaysia
Mariam Mahmoud Hassan, Department of Paediatrics, Faculty of Medicine and Health Sciences, University Putra Malaysia 43400 UPM Serdang, Selangor, Malaysia.
Received Date:May 29,2025; Published Date:June 11,2025
Introduction
Rasmussen’s encephalitis (RE) is a rare chronic neurological disorder characterized by unilateral inflammation of the cerebral cortex and presents with intractable seizures and progressive neurological and cognitive deterioration [1]. RE was first described by Theodore Rasmussen in 1958 as a focal encephalitis characterized by epilepsia partialis continua (EPC) with or without other forms of focal onset seizures, and progressive unilateral neurological deficits and MRI evidence depicting contralateral hemispheric atrophy [2] The annual incidence is estimated to be about 2.4 cases/10 million people [1,3] However, there is no worldwide published statistics on sex, geography, or ethnic predilection [4]. The disease mainly affects children, with an average age of onset of 6–7 years [5]. However, there have also been reports that adolescents and adults account for 10% of all RE cases [6]. Patients with RE can have multiple seizure semiologies. Granata et al. revealed that 58% of patients developed multiple seizure types within three months of initial presentation [7]. Epilepsia partialis continua (EPC) is a rare seizure disorder with particular clinical associations, including RE. EPC may emerge at any point in the course of RE, with rates of 37 to 92%, within the first 3 to 5 years [8]. EPC is described in the International League Against Epilepsy (ILAE) (https://www.epilepsydiagnosis.org/, accessed on 2 April 2024) diagnostic manual as “recurrent focal motor seizures (typically affecting hand and face, although other body parts may be affected) that occur every few seconds minutes for extended periods (days or years)”.
Risk Factors
RE is an immune-mediated disease, featured by consistent T-cell involvement [9]. There are various mechanisms related to RE. Adaptive immunity, innate immunity and viral infection are all involved in the development of RE. Immunopathological studies have shown that cytotoxic CD8+ T lymphocytes are the most common T lymphocyte subgroup in the brains of patients with RE [10]. The intensity of peripheral CD8+ T cell expansion is related to the severity of the disease [11]. Although the adaptive immune response is an important effector of central nervous system (CNS) damage, the innate immune response mediated by the activation of microglia and astrocytes is also the core of the pathogenesis of RE. The degree of microglial activation follows the progression pattern of RE, parallels the degree of T lymphocyte infiltration, and is observed in the early stages of cortical involvement [12].
Over the years, several studies have reported the detection of a number of viruses, including enteroviruses, Epstein Bar Virus (EBV), human cytomegalovirus (HCMV), human papillomavirus and herpes simplex virus (HSV), in the brains of RE patients [13]. Furthermore, antigens of various human herpesviruses (HHVs), including HCMV, EBV and HHV6, were detected in RE brain tissues with positive rates ranging from 50 to 88.5% [14]. In addition, it was found that the interferon-induced transmembrane protein-3 (IFITM3) polymorphism rs12252-C is associated with the high detection rate of HCMV and rapid disease progression in RE patients with the IFITM3 rs12252-CC genotype. This result indicates that IFITM3 rs12252-C is related to the disease progression of RE patients by promoting persistent HCMV infection in brain tissue [15]. The triggering factors of the adaptive immune response against brain tissue in RE cases may be single nucleotide variants (SNVs) related to antigen presentation and antiviral infection when facing the challenge of viral infection. Congenital abnormalities in adaptive immunity increase neuropathy damage [16].
Diagnostic Criteria
Diagnostic criteria were based on a European consensus panel for Rasmussen’s encephalitis 2005 [17]. These criteria are grouped in two parts with three criteria for each (Table 1). Part A contains clinical, electromyography (EEG) and radiological criteria The typical form of the disease usually starts in children (80% before the age of 10 years) and evolves in three stages: a prodromal stage, an acute stage and a residual stage [18]. The prodromal stage is nonspecific with infrequent epileptic seizures and few or no neurological deficit (e.g. mild hemiparesis) or behavioural/ cognitive disorders. After a few months, all patients experience an acute stage with frequent intractable seizures. During the acute stage, patients develop progressive, irreversible neurological deficit (e.g. hemiparesis), and/or cognitive decline and/or behavioural changes. Aphasia can occur if the dominant hemisphere is affected. The residual stage is marked by less frequent seizures, but persistent and stable neurological deficits associated with severe intellectual impairment. In RE, deterioration of background electroencephalography (EEG) activity, progressive atrophy on magnetic resonance imaging (MRI) and extensive cerebral hypometabolism 18F-fluorodeoxyglucose positron-emission tomography (PET-FDG) over the affected hemisphere are usually observed [1] (table 1).
Table 1:Clinical criteria for Rasmussen encephalitis, adapted from Bien et al., 2005.
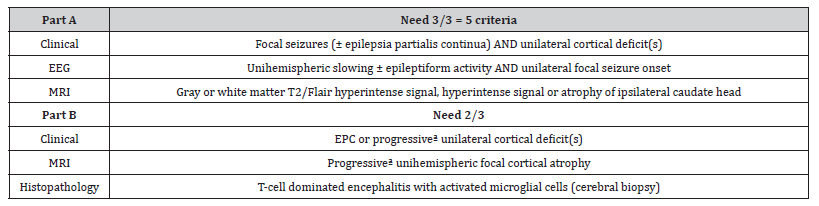
*Note: Patients has to meet A and B Criteria
aProgressive means that at least two sequential clinical examinations and MRI studies are necessary to conclude.
Atypical forms of RE correspond to RE in which epilepsy does not precede motor and or cognitive deficits. Rare cases have been reported with motor impairment (chorea, athetosis, hemiparesis) or cognitive impairment. Epilepsy starts later or is absent. MRI reveals damage to the caudate nucleus, the lenticular nucleus, and or hemispheric atrophy which may precede the onset of epilepsy [19,20]. The characteristic MRI features include cortical swelling in the early phase of RE along with hyperintensity on T2-weighted and fluid-attenuated inversion recovery (FLAIR) images. Previous studies found preferential cerebral atrophy in the frontal lobe and the insula, followed by volume loss of the caudate nucleus as the disease progresses; eventually, the atrophy spreads to the entire ipsilesional cerebral hemisphere [21].
Fluorodeoxyglucose-positron emission tomography (FDG-PET) has been extensively applied in the preoperative evaluation of intractable epilepsy, with irregular metabolism suggesting neural network dysfunction. The relevance of FDG-PET to the localization value of ictal epileptic foci has been established [22].
EEG analyses in RE show that there is asymmetric slowing of the background activity, with unilateral paroxysmal abnormalities on the same side. The EEG may show one or more foci on the same side of seizure onset. The paroxysmal abnormalities may be bilateral [23].
Longitudinal CSF analysis in patients with RE have shown initial increased levels of IgG, CD4+ and CD8+ T-cells, TNF-a, IFN-g, IL-12 and granzyme B. CD8+ T-cells, IFN-g and IL-12 levels decline gradually along the progressive stage, whereas production of granzyme B, TNF-a and CD4+ levels remain elevated [24].
Management
The goal of disease management in RE patients is to reduce the frequency and severity of seizures, and to improve long-term functional outcomes. Antiseizure medications, immunotherapy and surgery are three mainstays of treatment for patients with RE [1]. Early Immunomodulatory therapy can result in seizure freedom, preservation of neurological function and delays surgery, but may potentially be associated with loss of the functions represented by the affected hemisphere [25]. Seizures are managed by antiepileptic drugs (AED). Long-term corticosteroids, intravenous immunoglobulins, plasmapheresis, or protein A immunoadsorption; T-cell inactivating medicines, tacrolimus and azathioprine; and surgery (hemispherectomy or hemispherectomy) have all been used to treat people with RE [26].
Surgery represents the only option to control seizures and stop neurological deterioration in patients with RE [1]. Surgical techniques mainly aim at obtaining a functional disconnection of the affected hemisphere. This has functional consequences because homonymous hemianopia and hemiplegia are inevitable. Recovery of independent walking is expected although fine motor movement in the hand is not. Among surgical procedures of hemispheric disconnection, hemispherectomy techniques exhibit the best results in terms of seizure outcome [27].
In the early stages of the disease process, immunotherapy is used to ‘‘arrest” the progression. This includes, intravenous (IV) methylprednisolone, IV immunoglobulin, plasma exchange, and tacrolimus [28]. Rituximab is a chimeric mouse-human monoclonal anti-CD20 antibody. It has direct effects on B cells and has been used in less than twenty patients with RE albeit with promising results for some patients [29,30]. It is possible to propose a treatment combining corticosteroids followed by immunoglobulins every month. Specific immunotherapy: adalimumab 24 mg/m2 with a maximum of 40 mg via subcutaneous injection every seven or 14 days [31]; rituximab 375 mg/m2 two infusions 15 days apart [32]; tacrolimus starting dose of 0.1 mg/kg/day (for children) with dose escalation after two months, depending on blood levels of tacrolimus (5 and 20 ng/ml) [33,34].
Conclusion
(RE) is a severe, rare, chronic inflammatory brain disease resulting in drug-resistant epilepsy and progressive destruction of one hemisphere with loss of neurological function. This will be associated with a deterioration of background (EEG) activity, a progressive atrophy on (MRI) imaging and an extensive positron emission tomography. The course can be variable; some patients may experience significant disability, while others may have relatively stable conditions with appropriate management. Long-term outcomes are influenced by the age of onset, response to treatment, and the extent of brain involvement. Ongoing researches are aiming to understand the underlying pathophysiology, improving diagnostic criteria, and developing more effective treatment modalities. Overall, while RE poses significant challenges due to its complex nature and effects on quality of life, advancements in medical and surgical treatments offer hope for better management and outcomes for affected individual.
Acknowledgement
None.
Conflict of Interest
No Conflict of interest.
References
- Varadkar S, Bien CG, Kruse CA, Jensen FE, Bauer J, et al. (2014) Rasmussen’s encephalitis: clinical features, pathobiology, and treatment advances. Lancet Neurol 13: 195–205.
- Rasmussen T, Olszewski J, Lloyd-Smith D (1958) Focal seizures due to chronic localized encephalitis. Neurology 8(6): 435–435.
- Orsini A, Foiadelli T, Carli N, Costagliola G, Masini B, et al. (2020) Rasmussen’s encephalitis: from immune pathogenesis towards targeted- therapy. Seizure Eur J Epilepsy 81: 76–83.
- Chamlagain R, Shah S, Thapa S, Kandel B, Dhital R, et al. (2022) Different modalities of the treatment of Rasmussen encephalitis: a systematic review of case reports of a rare disease. F1000Research, 11: 1049.
- Andermann F, Farrell K (2006) Early onset Rasmussen’s syndrome: a malignant, often bilateral form of the disorder. Epilepsy Res 70(Suppl. 1): S259–S262.
- Villani F, Pincherle A, Antozzi C, Chiapparini L, Granata T, et al. (2006) Adult-onset Rasmussen’s encephalitis: anatomical-electrographicclinical features of 7 Italian cases. Epilepsia 47(Suppl. 5): 41–46.
- Granat T, Gobbi G, Spreafico R, Vigevano F, Capovilla G, et al. (2003) Rasmussen’s Encephalitis: Early Characteristics Allow Diagnosis. Neurology 60: 422–425.
- Longaretti F, Dunkley C, Varadkar S, Vargha-Khadem F, Boyd SG, et al. (2012) Evolution of the EEG in Children with Rasmussen’s Syndrome. Epilepsia 53: 1539–1545.
- Schwab N, Bien CG, Waschbisch A, Becker A, Vince GH, et al. (2009) CD8+ T-cell clones dominate brain infiltrates in Rasmussen encephalitis and persist in the periphery. Brain 132: 1236–46.
- Pardo CA, Nabbout R, Galanopoulou AS (2014) Mechanisms of epileptogenesis in pediatric epileptic syndromes: Rasmussen encephalitis, infantile spasms, and febrile infection-related epilepsy syndrome (FIRES). Neurotherapeutics 11: 297–310.
- Schneider-Hohendorf T, Mohan H, Bien CG, Breuer J, Becker A, et al. (2016) CD8(+) T-cell pathogenicity in Rasmussen encephalitis elucidated by large-scale T-cell receptor sequencing. Nat. Commun 7: 11153.
- Troscher AR, Wimmer I, Quemada-Garrido L, Kock U, Gessl D, et al. (2019) Microglial nodules provide the environment for pathogenic T cells in human encephalitis. Acta Neuropathol 137: 619–635.
- Pardo CA, Nabbout R, Galanopoulou AS (2014) Mechanisms of epileptogenesis in pediatric epileptic syndromes: Rasmussen encephalitis, infantile spasms, and febrile infection-related epilepsy syndrome (FIRES). Neurotherapeutics 11: 297–310.
- Zhang Y, Wang Y, Chen S, Chen S, Guan Y, et al. (2017) Expression of human cytomegalovirus components in the brain tissues of patients with Rasmussen’s encephalitis. Virol Sin 32: 115–121.
- Wang YS, Luo QL, Guan YG, Fan DY, Luan GM, et al. (2021) HCMV infection and IFITM3 rs12252 are associated with Rasmussen’s encephalitis disease progression. Ann. Clin Transl Neurol 8: 558–570.
- Ai J, Wang Y, Liu D, Fan D, Wang Q, et al. (2021) Genetic factors in Rasmussen’s encephalitis characterized by whole-exome sequencing. Frontiers in Neuroscience 15: 744429.
- Bien CG, Granata T, Antozzi C, Cross JH, Dulac O, et al. (2005) Pathogenesis, diagnosis and treatment of Rasmussen encephalitis: a European consensus statement. Brain 128(Pt 3): 454–71.
- Bien CG (2002) The natural history of Rasmussen’s encephalitis. Brain 125: 1751–9.
- Garg D, Agarwal A, Dash D, Swati Mahajan, Rajesh Kumar Singh , et al. (2019) Rasmussens encephalitis presenting as progressive parietal dysfunction sans seizures. Seizure 71: 219–21.
- Noordin NS, Deyo LJ, Ryon CW, Anderson WT (2021) A typical presentation of cerebral palsy and seizures: a case report on Rasmussen’s encephalitis in an adolescent. Cureus 13(3): 1–4.
- Yamazaki E, Takahashi Y, Akasaka N, Fujiwara T, Inoue Y (2011) Temporal changes in brain MRI findings in Rasmussen syndrome. Epileptic Disord 13: 229–239.
- Strohm T, Steriade C, Wu G, Hantus S, Rae-Grant A, et al. (2019) FDG-PET and MRI in the Evolution of New-Onset Refractory Status Epilepticus. AJNR Am J Neuroradiol 40: 238–244.
- Granata T, Gobbi G, Spreafico R, Vigevano F, Capovilla G, et al. (2003) Rasmussens encephalitis: early characteristics allow diagnosis. Neurology 60: 422–5.
- Takahashi Y, Mine J, Kubota Y, Yamazaki E, Fujiwara T (2009) A substantial number of Rasmussen syndrome patients have increased IgG, CD4+ T cells, TNFa, and Granzyme B in CSF. Epilepsia 50:1419–31.
- Hoffman CE, Ochi A, Snead 3rd OC, Widjaja E, Hawkins C, et al. (2016) Rasmussen’s encephalitis: advances in management and patient outcomes. Childs Nerv Syst 32(4): 629–40.
- Granata T (2013) Andermann F: Rasmussen encephalitis. Handbook of Clinical Neurology. Elsevier B.V: pp 511–519.
- Fallah A, Lewis E, Ibrahim M, Kola O, Tseng H, et al. (2021) Comparison of the real world effectiveness of vertical versus-lateral functional hemispherotomy techniques for pediatric drug resistant-epilepsy: A post-hoc analysis of the HOPS study.
- Liba Z, Vaskova M, Zamecnik J, Kayserova J, Nohejlova H, et al. (2020) An immunotherapy effect analysis in Rasmussen encephalitis. BMC Neurol 20(1): 359.
- Timarova G, Lisa I, Kukumberg P (2016) Long-term effect of rituximab in a case with late-onset Rasmussen’s encephalitis with anti-ganglioside IgGQ1b and anti-GAD antibodies positivity. Case Report. Neuro Endocrinol Lett 37(3): 179–83.
- Sansevere AJ, Henderson LA, Stredny CM, Prabhu SP, Shah A, et al. (2020) Posterior-onset Rasmussen’s encephalitis with ipsilateral cerebellar atrophy and uveitis resistant to rituximab. Epilepsy Behav Rep 21(14): 100360.
- Lagarde S, Villeneuve N, Tre´buchon A, Elsa Kaphan, Anne Lepine, et al. (2016) Anti-tumor necrosis factor alpha therapy (adalimumab) in Rasmussens encephalitis: an open pilot study. Epilepsia 57(6): 956–966.
- Sansevere AJ, Henderson LA, Stredny CM, Sanjay P Prabhu, Ankoor Shah, et al. (2020) Posterior- onset Rasmussens encephalitis with ipsilateral cerebellar atrophy and uveitis resistant to rituximab. Epilepsy Behav Rep 14: 100360.
- Takahashi Y, Yamazaki E, Mine J, Yuko Kubota, Katsumi Imai, et al. (2013) Immunomodulatory therapy versus surgery for Rasmussen syndrome in early childhood. Brain Dev 35(8): 778–85.
- Cho SM, Zeft A, Knight EP, Kotagal P, Wyllie E, et al. (2017) Refractory status epilepticus secondary to atypical Rasmussen encephalitis successfully managed with aggressive immunotherapy. Neurol Clin Pract 7(1): e5–8.
-
Mariam Mahmoud Hassan*. Understanding Rasmussen’s Encephalitis: A Review of Current Diagnostic and Management Strategies. Arch Neurol & Neurosci. 17(5): 2025. ANN.MS.ID.000923.
-
Cerebral Cortex, Seizures, Neurological, Cognitive Deterioration, Sex, Children, Multiple Seizure, Epilepsia Partialis Continua (EPC), Epilepsy, Immunopathological, RE Brain Tissues, Human Herpesviruses (Hhvs), Neural Network
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






