 Research Article
Research Article
The Anatomy of the Long Thoracic Nerve Origin, Course, And Branching Pattern: Implications for Long Thoracic Nerve Palsy and Serratus Anterior Dysfunction
Adam Pasquinelly1, Megan Busch1, Patrick Frank2,3, David Giovannucci2,3, Hamoun Delaviz1,2 and Adel Maklad2,3*
1College of Medicine and Life Sciences, College of Medicine and Life Sciences, University of Toledo, Toledo, USA
2Department of Medical Education, College of Medicine and Life Sciences, University of Toledo, Toledo, USA
3Department of Neurosciences, College of Medicine and Life Sciences, University of Toledo, Toledo, USA
Adel Maklad, Department of Neurosciences, College of Medicine and Life Sciences, University of Toledo, Toledo, USA.
Received Date:January 19,2024; Published Date:January 24, 2024
Abstract
Introduction: The long thoracic nerve typically originates from ventral primary rami at the C5, C6, and C7 levels at the root of the neck, and follows a long and superficial course before innervating the serratus anterior muscle. When the long thoracic nerve suffers an insult, it can lead to serratus anterior dysfunction and ensuing scapular winging. However, many cases of serratus anterior dysfunction have been reported without a clear etiology of long thoracic nerve damage; these cases appear to be most common in certain athletes with exaggerated arm movements at the limits of the shoulder’s range of motion and do not appear to have any correlation to athlete traits. To date, no studies have attempted to identify a connection between any specific variant long thoracic nerve anatomy and risk for nerve injury during shoulder movements. Methods: This study describes the origin and branching patterns of 50 long thoracic nerves across 25 cadavers. Serratus anterior dimensions and morphology was also recorded. Results: We describe seven distinct patterns of the long thoracic nerve’s emergence from neck musculature and its branching thereafter, and propose links between certain patterns and their potential susceptibility to nerve injury. The morphologies of the serratus anterior muscle slips are also described, and direct muscular injury to the superior slip is suggested as another potential cause of dysfunction in these athletes. Conclusions: Several etiologies for atraumatic serratus anterior dysfunction are described in this study, with a focus on anatomical variations that may predispose individuals to this pathology especially in the setting of sports with repetitive exaggerated arm movements.
Keywords:Long thoracic nerve; Serratus anterior; Peripheral nerve palsy
List of Abbreviations:AS: Anterior Scalene; IT: Inferior Trunk; LTN: Long Thoracic Nerve; MS: Middle Scalene; MT: Middle Trunk; PS: Posterior Scalene; SCA: Subclavian Artery; ST: Superior Trunk; SSSA: Superior Slip of Serratus Anterior
Introduction
The long thoracic nerve (LTN) typically originates from ventral rami at the C5, C6, and C7 spinal levels and innervates one mus cle - the serratus anterior [1]. Prior anatomical studies report that in most cases, the C5 and C6 roots pierce the middle scalene (MS) muscle, joining with the C7 root contribution after it passes between the anterior (AS) and middle scalene (MS) muscles [1–3]. Variations in the LTN’s course through the upper thoracic wall have been well documented due to potential for iatrogenic injury during thoracotomy, mastectomy, and other procedures of the chest wall [2–7]. These previous studies used both cadaveric dissection and in vivo surgical and radiographic studies to identify variant LTN origins, terminations, and relationships with other thoracic structures. In addition to traumatic and iatrogenic damage to the LTN, cases of idiopathic LTN dysfunction and palsy have been recorded. There is a lack of evidence supporting a clear etiology for atraumatic isolated LTN palsy, which can lead to significant disability by serratus anterior paralysis and subsequent scapular winging. Several conflicting theories about the etiology of this neurological disorder have been studied, including neuralgic amyotrophy (Parsonage- Turner syndrome), inflammatory neuritis, nerve traction injury, and entrapment of the nerve in the scalene muscles of the neck.
Some of the more interesting cases of LTN dysfunction are those observed in athletes in certain sports such as archery, swimming, weightlifting, javelin, tennis, golf, and others [8]. The question of how these sports cause insult to the LTN is open and there is no clear or definitive answer that can be drawn from the current available literature. Many other peripheral nerve injuries are well documented and more clearly understood; often there is a clear etiology which is easy to localize. Common examples of these etiologies include traumatic radial nerve injury with midshaft humerus fractures, anterior interosseous nerve traction injuries seen with pediatric supracondylar humerus fractures, ulnar nerve compressive neuropathies at the wrist by bicycle handlebars [9–11]. Other examples include entrapment of nerves by muscles on either side of its course, as seen in scalene syndrome, or muscles though which the nerve passes, as seen in pronator syndrome, supinator syndrome or coracobrachialis syndrome, or non-muscular compressive neuropathies like carpal and cubital tunnel syndromes [12]. In these classical examples of peripheral nerve injury, there are clear direct insults to the nerve. However, the etiology in case of LTN injury with certain sports is not as clearly defined or understood. Furthermore, in these sports which may be associated with LTN injury, only relatively small percentages of athletes are affected, raising the question as to why certain individuals are more susceptible to the LTN injury and subsequent serratus anterior dysfunction.
Due to the controversial and incomplete nature of the available literature on the role of LTN course in serratus anterior palsy, more data on the LTN’s course through the thoracic wall is warranted. It seems plausible that anatomical variants of the LTN and its course as it relates to other thoracic anatomy could theoretically increase the risk for athletes in these sports to develop palsy. Currently, there are no reports correlating LTN course variations with risk of LTN palsy. This study aims to provide baseline data on different anatomical variants of the LTN, using a larger sample than previously used and more thorough nerve course analysis. We hope this data may be used in the future alongside patient imaging to identify any correlation between certain anatomical variants of the LTN and risk of LTN injury in these sports, ideally helping to guide clinical decision making in the management of serratus anterior palsy.
Methods
In this study, 50 long thoracic nerves in 25 cadavers (right and left LTN in each cadaver) were identified and dissected from surrounding tissue from their origins at the cervical spinal nerve roots to their terminal innervations of the serratus anterior muscle. Data recorded for each nerve included: the course of the C5, C6, and C7 nerve roots relative to the anterior, middle, and posterior scalene muscles; and the location of convergence of the C5-C6 and C7 roots to form the main trunk of the LTN relative to skeletal landmarks. Cadaver dissection was conducted by the authors in a routine fashion to prepare for upper extremity and brachial plexus study by preclinical medical students. As the root and proximal part of the LTN are found deep to the trunks of the brachial plexus, these trunks were transected and reflected posteriorly proximally and anteriorly distally. Great care was taken during cleaning axillary fat to expose the LTN roots without inadvertently cutting roots. In addition to LTN anatomy, we also report a detailed description of the first slip of serratus anterior, which we believe may play a significant role in preventing scapular winging. After complete dissection of the LTN and its root, photographs were taken at different magnifications and angles. The appropriate images were imported into CorelDraw software to make composite figures and adjust brightness, contrast and labels.
Disclaimer regarding use of human donor patients
This study was performed in the Laboratory of Anatomical Sciences at the University of Toledo College of Medicine and Life Sciences. The cadavers examined in this case were acquired through the University of Toledo Anatomical Donation program, and the donor patients had given written informed consent for use of their remains in research and medical education at the time of enrolling in the program. All human tissue dissection and specimen preparation was performed following the appropriate established protocols endorsed by the University of Toledo Department of Graduate Medical Education. The authors wish to sincerely thank those who donated their bodies to science so that anatomical research could be performed. Results from such research can potentially improve patient care and increase mankind’s overall knowledge. Therefore, these donors and their families deserve our highest gratitude.
Results
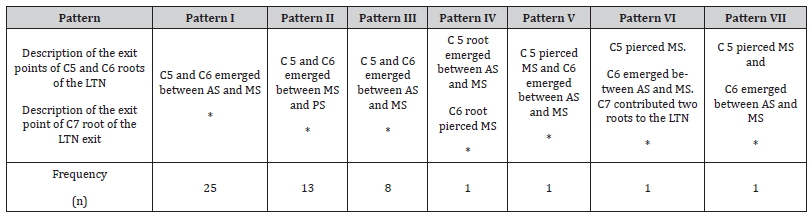
Systematic dissection of the brachial plexus in 21 cadavers (42 sides) revealed 7 different patterns of long thoracic nerve root origin, root course relative to the scalene muscles, nerve course through the axilla, and location of root convergence to form the main trunk of the LTN (Table 1).
Table 1:Different patterns of C5, C6, and C7 roots of the LTN in relations scalene muscles. Asterisks indicate that C7 always emerged between and AS and MS in all patterns.
Pattern I
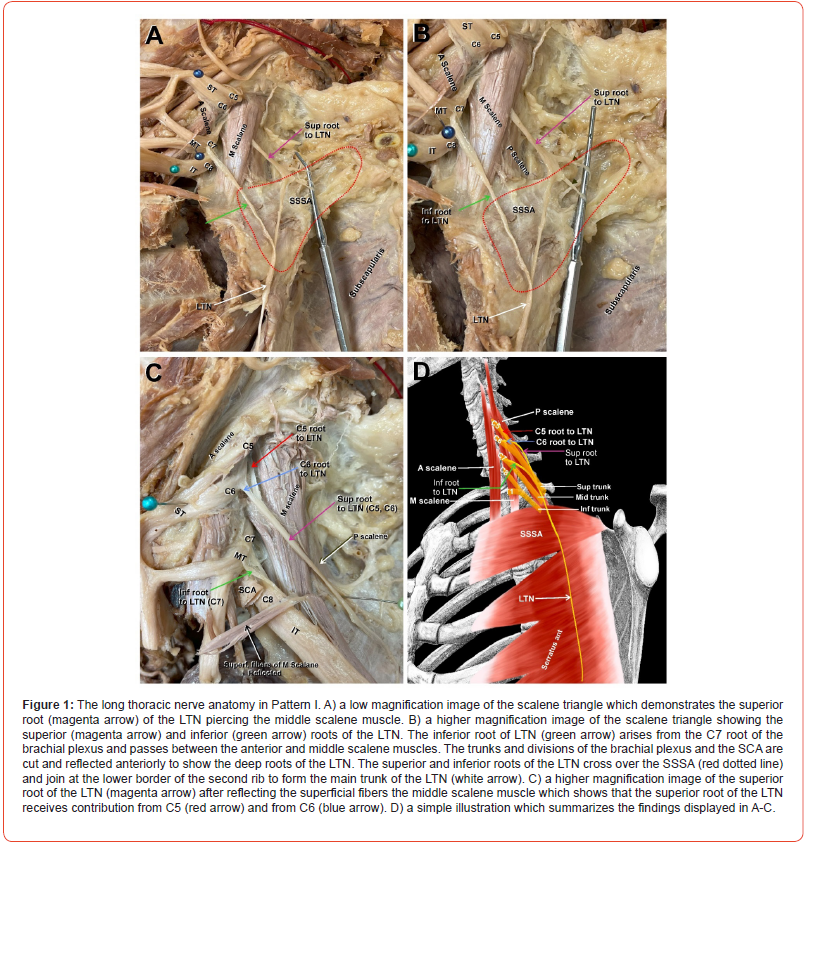
(Figure 1) In this pattern, the C5 and C6 roots of the LTN pierced the MS muscle and emerged as one nerve trunk (Figure 1A, 1B, magenta arrows), hereinafter referred to as the superior root of the LTN. The superior root of the LTN then ran deep to the trunks and divisions of the brachial plexus (Figure 1A, 1B, cut and reflected to better visualize the superior root of the LTN). The C7 root to the LTN (Figure 1A, 1B, green arrows) emerged from the posterior aspect of the C7 anterior primary ramus, which continues to form the middle trunk (MT) of the brachial plexus. We refer to this C7 nerve root to the LTN hereafter as the inferior root of LTN. The inferior root of the LTN coursed under the inferior trunk (IT) of the brachial plexus and subclavian artery (SCA) (Figure 1A, 1B, green arrows). The superior and inferior roots of the LTN (Figure 1A, 1B, magenta and green arrows, respectively) ran over the superior slip of serratus anterior (SSSA, outlined in red dashed line in Figure 1A, B) and united to form the main trunk of the LTN (Figure 1A, 1B, white arrows) at the lower border of the 2nd rib. To further elucidate that the superior root of LTN, which was presumably originating from the C5 and C6 roots, is in fact arising from C5 and C6, we reflected the superficial fibers of the middle scalene muscles to show the precise root contributions. The superior root was in fact receiving contributions from C5 and C6 (Figure 1C, red and blue arrows, respectively). A simple illustration of pattern I is depicted in Figure 1D.
Pattern II
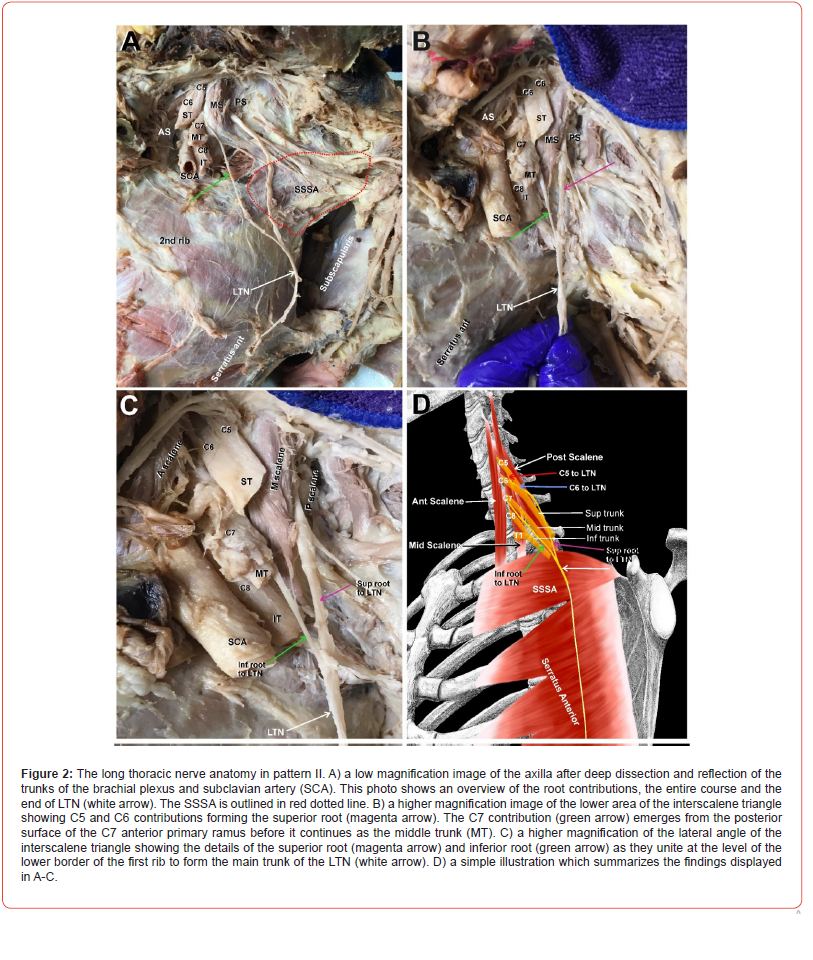
(Figure 2) In this pattern, the C5 and C6 roots form the superior root of the LTN (Figure 2A-C, magenta arrows) and emerged between the MS and PS muscles. The superior root passed laterally, then curved sharply downward crossing over the SSSA (Figure 2A, SSSA outlined in red dotted line). The inferior root to the LTN (Figure A-C, green arrows) emerged from the posterior surface of the C7 anterior primary ramus of the brachial plexus between the AS and PS and passed directly downward to join the superior root of the LTN (Fig 2. A-C, magenta arrows) at the lower border of SSSA to form the main trunk of the LTN (Figure 2A-C, white arrows). A simple illustration of pattern II is depicted in Figure 2D.
Pattern III
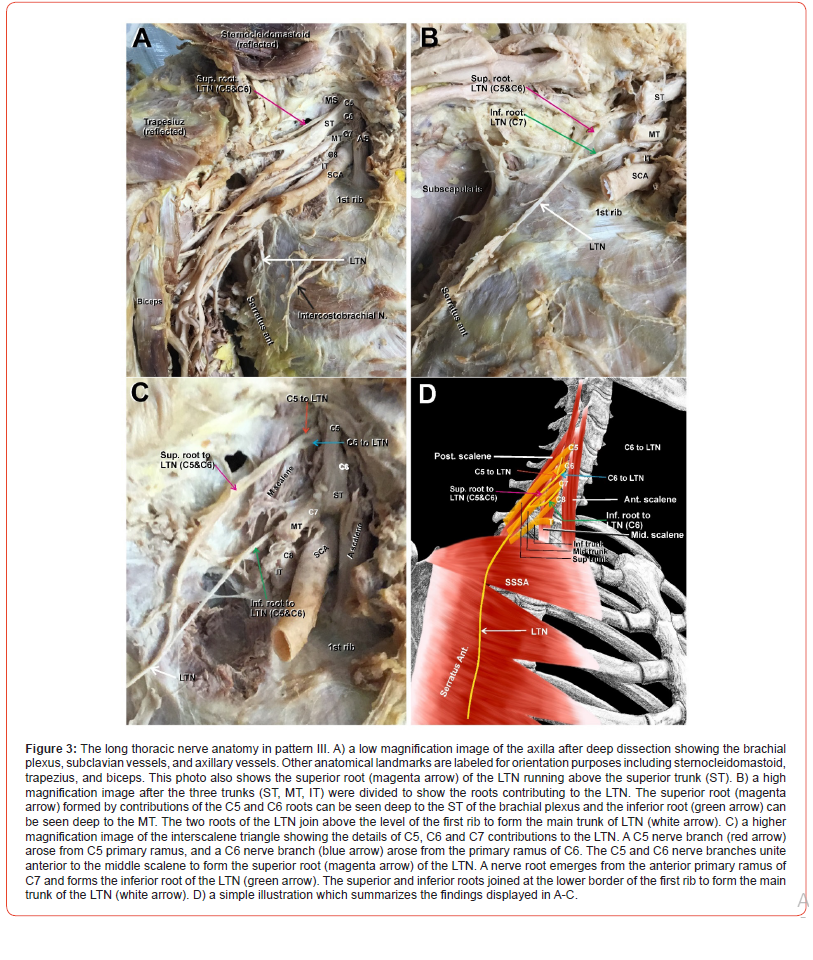
(Figure 3) In this pattern, the C5 and C6 roots (Figure 3 B-D, red and blue arrows) united in the gap between the MS and PS and emerged as one nerve, the superior root of the LTN (Figure 3A-C, magenta arrows). The superior root of the LTN passed behind the superior trunk of the brachial plexus, then above and parallel to it, and finally dove beneath all trunks of the brachial plexus (Figure 3A, magenta arrow). Only after the trunks of the brachial plexus were cut and reflected could the superior root of the LTN be seen again turning caudally (Figure 3B-C, magenta arrows). The inferior root of the LTN emerged from the posterior surface of C7 anterior ramus of the brachial plexus (Figure 3B-C, green arrows). The superior and inferior roots of the LTN (Figure 3B-C, magenta and green arrows) joined high up above the level of the first rib to form the main trunk of the LTN (Figure 3B-C, white arrows). A simple illustration of pattern III is depicted in Figure 3D.
Pattern IV
(Figure 4) Pattern IV was rare, occurring once in the 42 specimens, and unusual in that the C5 root of the LTN emerged between the AS and MS (Figure 4A-C, red arrows) as seen in pattern II, whereas the C6 root pierced the MS (Figure 4A-C, blue arrows) as seen in pattern I. The C5 and C6 roots continued for a relatively longer distance lateral to the lateral border of the MS, forming a primary loop before they joined to form the superior root of the LTN (Figure 4B-C, magenta arrow). The inferior root of the LTN emerged from the inferior surface of the anterior primary ramus of C7 (Figure 4A-C, green arrows). The superior and inferior roots joined (Figure 4, magenta and green arrows) at the lower border of SSSA (Fig 4A, red dotted line) to form the main trunk of the LTN (Figure 4 B-C, white arrows) at the lower border of the second rib. A simple illustration of pattern IV is depicted in Figure 4D.
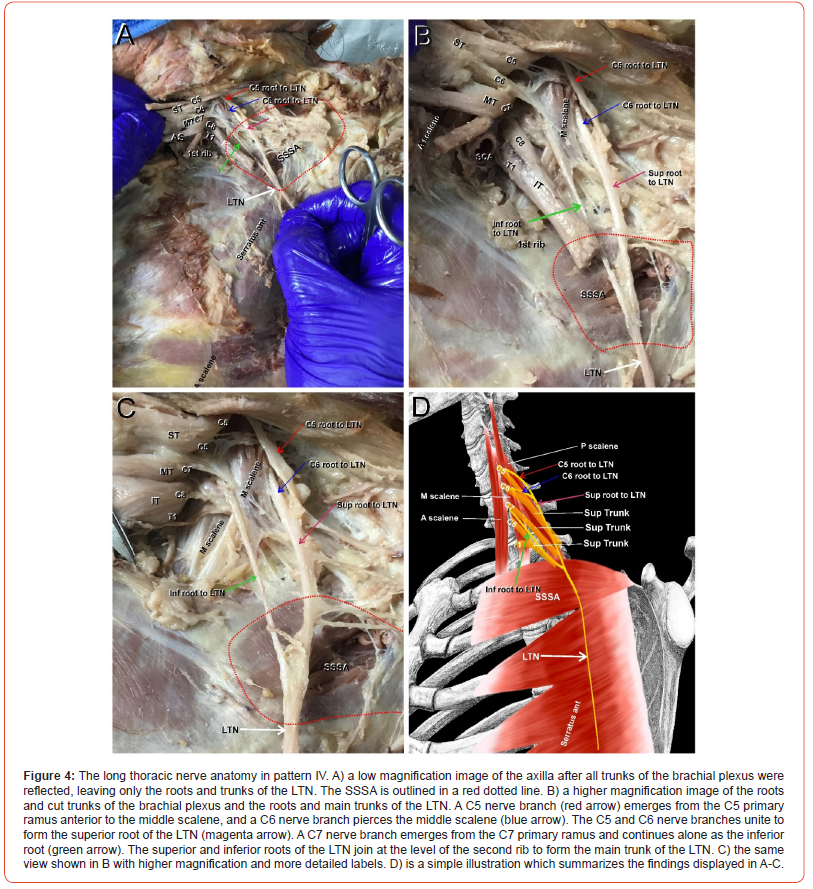
Pattern V
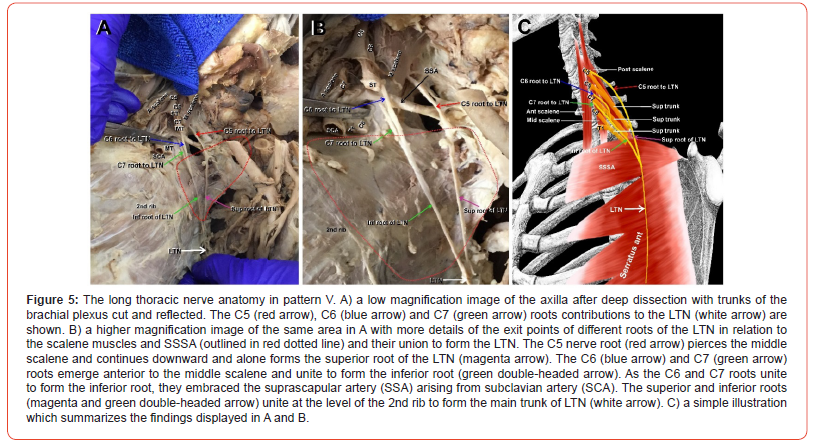
(Figure 5) Pattern V was also rare, appearing only once. In this pattern, the C5 root (Figure 5A-B, red arrows) emerged as an individual root from the belly of the MS and coursed through the axilla forming alone the superior root of the LTN (Figure 5 A-B, magenta arrows) without joining the C6 root. In contrast, the C6 root (Figure 5 A, B, blue arrows) emerged from the posterior surface of the C6 anterior primary ramus between the AS and MS. The C7 root (Figure 5A-B, green arrows) arose from C7 anterior primary ramus, and unlike all other patterns, the C6 and C7 roots (Figure 5A-B, blue and green arrow) joined at the upper border of SSSA (Figure 5 A-B, red dotted line) to form the inferior root of the LTN (Figure 5A-B, green double-headed arrow). Interestingly, before the C6 and C7 roots united, they passed on either side of the suprascapular artery (SSA) (Figure 5A-B, black arrows). The superior and inferior roots of the LTN (Figure 5A-B, magenta and green double-headed arrows) joined at the lower border of SSSA at the level of the second rib to form the main trunk of the LTN (Figure 5A-B, white arrows). A simple illustration of pattern V is depicted in Figure 5C.
Pattern VI
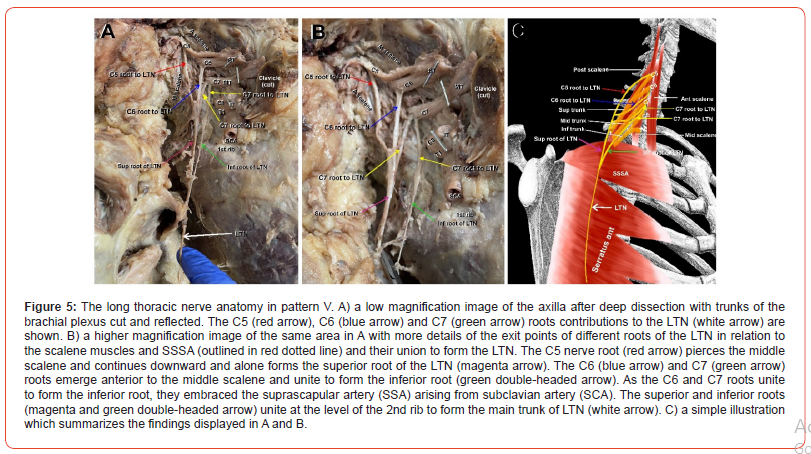
(Figure 6) Pattern VI also appeared only once. Here, the C5 root (Figure 6A-B, red arrow) arose from the inferior surface of the anterior primary ramus of C5 to the superior trunk (ST) deeply within the MS, requiring removal of the superficial part of the muscle to expose the nerve root. The C6 root of the LTN (Figure 6A-B, blue arrow) arose from the deep surface of the C6 root of the superior trunk of the brachial plexus. In contrast to the C5 root, the C6 root emerged between the AS and MS rather than within the muscle belly. The C5 and C6 roots were widely separated over the breadth of the MS, uniting lateral to the lateral border of the MS (Figure 6A-C). Immediately after the C5 and C6 roots converged, they were joined by an accessory root from C7 (Figure 6A-B, yellow arrows) that arose from the C7 anterior primary ramus. The combined C5, C6 and C7 roots (Figure 6A-B, red, blue and yellow arrows) joined to form the superior root of the LTN (Figure 6A-B, magenta arrows). The second C7 root (Figure 6A-B, yellow double-headed arrow) arose from the anterior ramus of C7 and ran down the surface of the SSSA muscle to form alone the inferior root of the LTN (Figure 6A-B, green arrow). The superior and inferior roots of the LTN joined at the lower border of the second intercostal space to form the main trunk of the LTN (Figure 6A, white arrow). In summary, C7 has contributed two roots to the LTN in this pattern; one joining the superior root and the other alone forming the inferior root. A simple illustration of pattern VI is depicted in Figure 6C.
Pattern VII
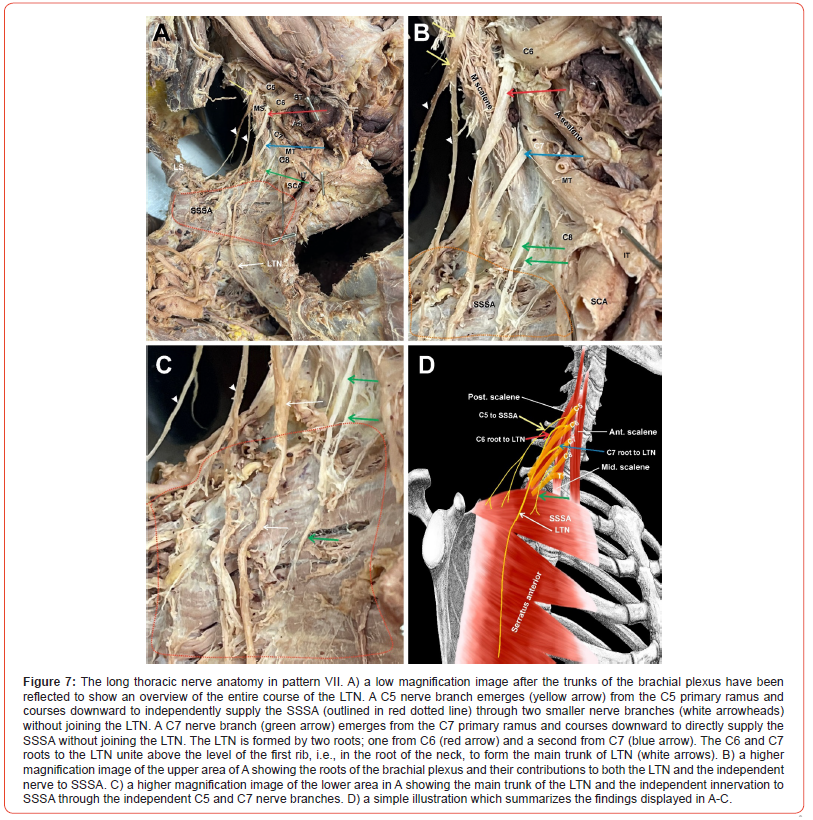
(Figure 7) Pattern VII was also seen only once. The C5 nerve root (Figure 7A-C, yellow arrow) emerged from the C5 anterior primary ramus, piercing the MS and running over its lateral border toward the center of the posterior triangle, where it divided into two smaller branches (Figure 7A-C, arrowheads) both terminating in the SSSA independent of the LTN (Figure 7A-C, SSSA outlined in red dotted line). The C6 root emerged from the anterior primary ramus of C6 (Figure 7A-B, red arrows) and alone formed the superior root of the LTN nerve. The C7 root emerged from the anterior primary ramus of C7 (Figure 7A-C, blue arrows) to form the inferior root of the LTN. The superior and inferior roots (Figure 7A-C, red and blue arrows) united high up in the axilla above the level of the first rib to form the main trunk of the LTN. Furthermore, two more nerve roots emerged from the C7 anterior primary ramus to supply the SSSA independent of the LTN (Figure 7A-C, green arrows). In summary, the traditionally known LTN receiving contributions from C5-7 did not exist in this pattern, but rather the LTN was formed only from contributions of C6 and C7 roots, while the SSSA received innervation from C5 and C7 independent of LTN. A simple illustration of pattern VII is depicted in Figure 7D.
Anatomical remarks on the serratus anterior muscle
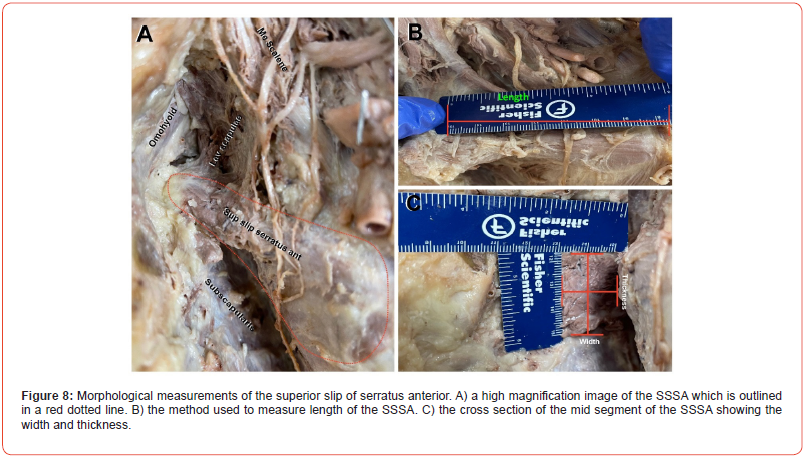
(Figure 8) The first slip of the serratus anterior arises from the first and second ribs, just lateral to the costochondral junction, with a more significant contribution from the second rib than from the first rib. This slip is known to insert into the anterior surface of the medial border of the scapula at the angle.[13] However, in all cases examined in this study, the slip was found to insert into the superior border of the scapula between the origin of the inferior belly of the omohyoid and the insertion of the levator scapulae scapula (Figure 8A). The first slip was triangular in shape with an average length from origin to insertion of approximately 7cm (Figure 8B), making it the shortest slip. The second slip averaged 13cm in length, the fifth slip averaged 16cm in length, and the eighth (last) slip was the longest, measuring an average of 20.2cm in length. We also noted that the first slip was more massive than any other slip in the entire muscle, averaging 3cm in width and 2.2cm in thickness (Figure 8C); the remaining slips measured 0.2-0.3cm in thickness (not shown).
Branching pattern of the LTN and innervation of the serratus anterior muscle
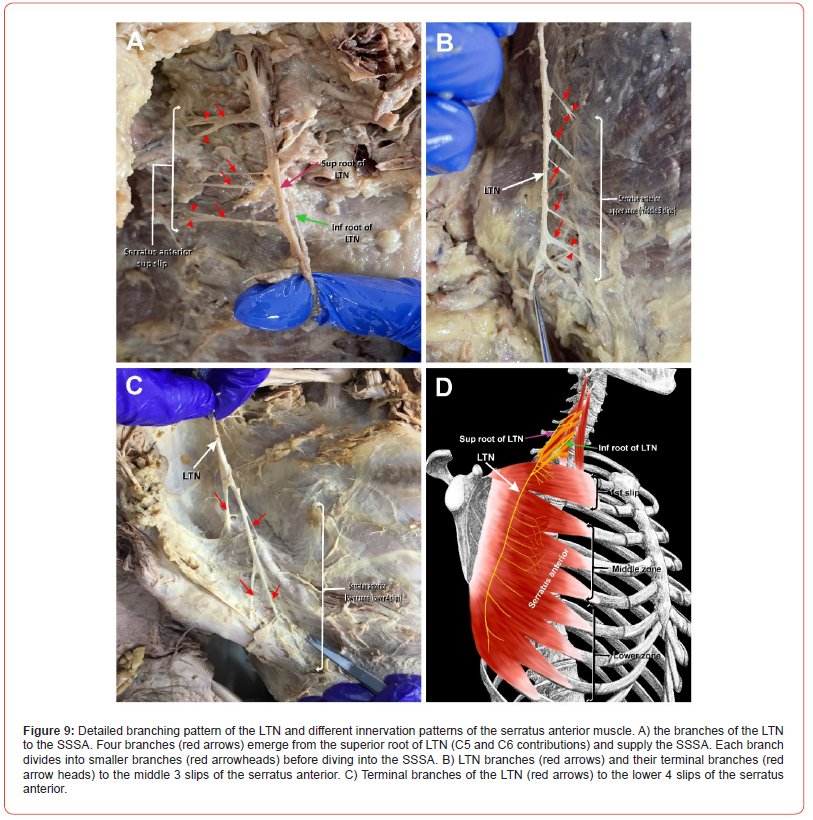
(Figure 8) The distal branching pattern of the LTN was categorized based on the three zones of the serratus anterior (upper, middle, and lower) that were consistently innervated by distinct branches or groups of branches. The upper zone consisted of the first slip of the serratus anterior, which was fed by 3-4 sizable branches from the LTN (Figure 9A, red arrows). These branches almost exclusively arose from the superior root of the LTN, usually from the C5 root. As each branch neared the muscle, it bifurcated into two smaller branches which then further divided into the muscle (Figure 8A; arrowheads). The middle zone branches fed the second, third, and fourth slips. These 5-6 branches were very thin branches (Figure 9B; red arrows), each bifurcating into thinner upper and lower ‘twigs’ (Figure 8B; red arrowheads); the upper twig appeared to supply the lower border of the muscle slip above it, and the lower twig appeared to supply the upper border of the muscle slip below. The lower zone of the serratus anterior consisted of the lower four slips (Figure 8C). In this zone, the main trunk of the LTN came to an end and splayed into four to five sizable branches (Figure 9C; arrows), which diverged away from the main trunk of the LTN and entered the remaining four slips at different points with consistent pattern. A simple illustration of the branching pattern of the LTN and innervation of the different zones of serratus anterior is depicted in Figure 9D.
Discussion
The serratus anterior muscle arises from eight or nine digitations of the upper eight or nine ribs. These eight digitations join to form a continuous sheet, which runs over the lateral thoracic cage and inserts into the ventral aspect of the entire medial border of the scapula [1,13]. The serratus anterior is supplied by the LTN and functions as: a powerful protractor of scapula to move the arm anteriorly; an upward rotator of the glenoid cavity to allow raising of the arm above the head; and an anchor to keep the scapula tightly attached to the chest wall [1,13]. The serratus anterior is an essential element to the integrity of the entire shoulder complex, providing support to the shoulder joint, sternoclavicular joint and functional scapulothoracic joint [14]. Dysfunction of the serratus anterior due to either direct muscular injury or LTN insult can cause pain and loss of function, leading to severe limitations in movements at the shoulder complex and resulting in significant disability. Since the serratus anterior is supplied solely by the LTN, isolated serratus anterior weakness or palsy is typically approached primarily as an LTN insult, with differential diagnoses including nerve entrapment, direct nerve injury, and other neuropathies. This study was undertaken to provide a thorough analysis of the anatomy of the LTN from its roots to its termination in the serratus anterior muscle, hopefully serving as an anatomical foundation for a better understanding of the etiology of LTN lesions and thus better prevention and treatment of serratus anterior dysfunction.
The course of the LTN is of particular importance to physicians and surgeons planning interventions at the chest wall. The distal LTN typically lies superficial to the serratus anterior as it courses caudally along the lateral chest wall, placing it in danger of iatrogenic injury during thoracotomy, mastectomy, and other invasive procedures. Proximally, the LTN may be seen running in one of several locations: in the interscalene triangle between the anterior and middle scalene, within the middle scalene, or between the middle and posterior scalene, and typically courses caudally deep to the roots, trunks, and division of the brachial plexus, as described in this and other studies [1]. A more informed understanding of these variable anatomical relationships allows for better surgical planning, avoidance of harmful patient positioning during operations or imaging, and an overall improvement in patient safety as it relates to iatrogenic LTN injury.
The LTN’s course has been studied to identify potential sites of entrapment or traction that could explain isolated LTN palsy seen in patients without a known history of thoracic trauma [8,15,16]. The LTN’s long anatomical course through the crowded and highly mobile regions of the root of the neck and apex of the axilla may render it susceptible to compression injury by nearby muscle hypertrophy and traction injury during excessive stretching or manipulation of the head and neck. The diagnosis of atraumatic isolated LTN palsy due to entrapment at the scalene muscles remains controversial; a 2017 retrospective MRI analysis study by Maldonado et al. [17] identified peripheral nerve or skeletal muscle changes beyond the LTN and its target muscles in all 7 patients in the study population that had been diagnosed with atraumatic isolated LTN palsy, suggesting a regional inflammatory etiology rather than a true focal entrapment neuropathy. In contrast, there have been several case reports published that describe participants of activities with exaggerated abduction and external rotation of the shoulder - like archers, swimmers, people working with their hands overhead for a prolonged time, and some weightlifters - that have developed shoulder weakness and scapular winging associated with LTN palsy [8,16,18–21]. This overuse of the scalene muscles has been linked to LTN entrapment and subsequent serratus anterior paralysis, which responds well to surgical release of the LTN from surrounding tissue, implying mechanical compression as an etiology of the neuropathy [22].
Another explanation for isolated LTN injury in these patients is traction to the nerve during prolonged or exaggerated shoulder motions that stretch the nerve between two relatively fixed attachment points, such as its emergence from the MS and its destination in the SSSA [23]. Makela et al. described isolated LTN palsy in Finnish military recruits carrying heavy backpacks during field work, and hypothesized that prolonged shoulder retraction with heavy load carriage may cause traction injury to the nerve [24]. Interestingly, the results of EMG studies in patients with isolated LTN palsy do not appear to correlate with either clinical recovery over the natural course of the disease or with intraoperative LTN excitability during decompression and/or nerve transfer surgeries, further clouding the explanation [25]. The complex origins, course, and branching patterns seen in this study demonstrate the presence of significant variability of the LTN between individuals, and also indicate a potential consequence of that variability as it introduces sites of possible entrapment and traction by nearby structures. We have reported seven different patterns of LTN nerve root emergence points as well as different locations where the primary roots (C5, C6, C7) converge into their respective secondary roots. Another source of variation in the LTN anatomy is where the secondary roots – the superior (C5 and C6) and the inferior (C7) roots – join, ranging from the root of the neck above the first rib to the 3rd intercostal space. Such variations in the site of union of the primary roots and secondary roots can introduce significant variation to the length of the LTN between individuals, and therefore influence the nerve’s tolerance to stretch with movement. For example, patients with a similar LTN course to Pattern V in this study may be relatively more prone to irritation or anchoring of the nerve by the suprascapular artery, predisposing them to LTN pathology. Similarly, Patterns I, IV, and V all involved nerves or nerve roots piercing scalene muscle bellies, which are also potential sites of entrapment.
Among our sample set, we have also noted more than 10 delicate branches emerging from the LTN to supply different zones of the serratus anterior as the nerve courses superficially to the muscle, causing the nerve to appear as if pinned down to the muscle from the first intercostal space to its termination (Figure 9). This unique branching pattern may limit the nerves tolerance to movement regardless of the length of the nerve. Constant movement of the shoulder seen in sports mentioned above may either put a strain on the relatively fixed nerve or may place constant tension on these tiny branches, potentially causing microtears in the nerve fibers which may not be evident in any imaging modality. Regardless of pattern, the superficial nature of the LTN as it relates to the serratus anterior almost certainly contributes to its vulnerability to trauma and mechanical entrapment. While most major motor nerves in the body are protected in deep neurovascular bundles and shielded from external damage by muscle tissue and fascia, the LTN rests superficially on the serratus anterior just below the skin and subcutaneous tissue. Though the full development of the serratus anterior muscle is not entirely clear, the prevailing theory is that the serratus anterior’s embryological origin in the cervical myotomes may indicate that it attaches to the trunk before extending into the limb bud, which would occur before full LTN development and would thus leave the muscle deep to the nerve [1,13,26,27].
Another curious anatomical observation we noted was the size disparity between the first slip and the remaining slips of serratus anterior. The superior was the thickest slip at 22mm compared to all other slips at ~2mm. Similarly, the width of the superior slip was roughly double that of any other slip. However, the superior slip’s length (7cm) was less than half the length of the 4th slip (16 cm) and less than one-third of the length of the 8th slip (22cm) (Figure 8). Although more massive in breadth and thickness, the short span of this slip decreases the arm of force of the 1st slip in the lever system and significantly decreases the moving torque of this slip. It is possible that the superior slip is not as significant in terms of movement produced by serratus anterior, but rather may serve as an extensible, dynamic check ligament that that stabilizes scapula and preserves the integrity of the shoulder complex by preventing excessive elevation, retraction and rotation of the scapula. Therefore, sports that requires excessive retraction, elevation, and extension of the shoulder may result in a traumatic myopathy from repeated stretching of this slip; the winging of the scapulae observed in select cases of these sports may be a pseudoparalysis from superior slip weakness similar to that seen rotator cuff pathology, rather than an LTN injury. We are currently testing this hypothesis by selectively dividing the superior slip of serratus anterior and testing scapular movement and winging in a surgical-reality embalmed cadaver, and plan to present this data in a later manuscript.
Possible non-mechanical etiologies of atraumatic isolated LTN palsy include neuralgic amyotrophy, also known as Parsonage- Turner syndrome, which is believed to be immune-mediated and is strongly associated with viral infections [28,29]. Neuralgic amyotrophy is a poorly understood peripheral nerve dysfunction that usually affects the nerves of the brachial plexus - most often the LTN - and presents with shoulder pain and sustained scapular elevation, followed by muscle weakness, paralysis, and atrophy in the ensuing weeks and months [28]. Though it has been shown to have associations with viral illnesses, a definitive cause of neuralgic amyotrophy has not been established, and thus it is not always differentiable from entrapment neuropathy of the LTN.
The most obvious limitation of this cadaver study is the lack of any clinical correlation between LTN pattern and presence of LTN entrapment symptoms or palsy. While our sample size of 42 LTNs on 21 cadavers is not terribly underpowered, the fact that several patterns were seen only once would indicate that other LTN variations are likely present in the general population that we did not see in our sample. Future studies with larger sample size and potential clinical correlation with living medical history would be beneficial for a better understanding of LTN course variation and its relationship to serratus anterior dysfunction. Furthermore, computer- assisted modeling of the LTN’s dynamic relationships to thoracic structures and measurement of subsequent tension or compression forces on the nerve during shoulder motions may be an interesting addition to future research if this software is available.
Conclusions
Long thoracic nerve palsy resulting in dysfunction of the serratus anterior muscle can have multiple etiologies, including both traumatic and atraumatic causes. Atraumatic isolated LTN palsy due to entrapment or traction is not well understood and may be more prevalent with certain anatomical variants of LTN nerve course as well as with certain activities. This cadaver study demonstrated seven different LTN branching patterns in a sample of 42 LTNs from 21 donor patients, some of which may predispose the nerve to mechanical entrapment with muscle overuse or traction during movement. A better understanding of the LTN’s most common course variations will not only enable clinicians to better avoid iatrogenic LTN trauma during procedures, but also aid in workup for serratus anterior dysfunction by identifying potential mechanical etiologies for nerve palsy.
Acknowledgement
None.
Conflict of Interest
No Conflict of interest.
References
- K Lung, F Lui (2022) Anatomy, Thorax, Long Thoracic Nerve, In: StatPearls, StatPearls Publishing, Treasure Island (FL).
- F Yazar, C Kilic, Hi Acar, N Candir, A Comert (2009) The long thoracic nerve: Its origin, branches, and relationship to the middle scalene muscle, Clin Anat 22: 476-480.
- F Yazar, A Comert (2009) Origin types of the long thoracic nerve, Surg. Radiol Anat 31: 737-737.
- M Emamhadi, SY Chabok, F Samini, B Alijani, H Behzadnia, et al. (2016) Anatomical Variations of Brachial Plexus in Adult Cadavers; A Descriptive Study, Arch. Bone Jt. Surg. 4: 253-258.
- JO H Kwon, T Cho, S Won, H Yang (2021) Analysis of the positional relationship of the long thoracic nerve considering clinical treatment, Clin Anat 34: 617-623.
- LE Ballesteros, LM Ramirez (2007) Variations of the origin of collateral branches emerging from the posterior aspect of the brachial plexus, J. Brachial Plex. Peripher. Nerve Inj 02: e22-e27.
- NM Ormsby, DH Hawkes, CY Ng (2022) Variation of surgical anatomy of the thoracic portion of the long thoracic nerve, Clin Anat 35: 442-446.
- Nawa (2015) Scapular Winging Secondary to Apparent Long Thoracic Nerve Palsy in a Young Female Swimmer, J. Brachial Plex. Peripher. Nerve Inj 10: e57-e61.
- Y Vincelet, P Journeau, D Popkov, T Haumont, P Lascombes (2013) The anatomical basis for anterior interosseous nerve palsy secondary to supracondylar humerus fractures in children, Orthop. Traumatol. Surg. Res. OTSR 99: 543-547.
- FPFM Ricci, RI Barbosa, VMC Elui, CH Barbieri, N Mazzer, et al. (2015) Fonseca, Radial nerve injury associated with humeral shaft fracture: a retrospective study, Acta Ortop. Bras. 23: 19-21.
- JW Brubacher, FJ Leversedge (2017) Ulnar Neuropathy in Cyclists, Hand Clin 33: 199-205.
- MR Thatte, KA Mansukhani (2011) Compressive neuropathy in the upper limb, Indian J. Plast. Surg. Off. Publ. Assoc Plast Surg India 44: 283-297.
- K Lung, K St Lucia, F Lui (2022) Anatomy, Thorax, Serratus Anterior Muscles, in: StatPearls, StatPearls Publishing, Treasure Island (FL): 2022.
- DA Neumann, PR Camargo (2019) Kinesiologic considerations for targeting activation of scapulothoracic muscles - part 1: serratus anterior, Braz. J. Phys. Ther. 23: 459-466.
- NA Ebraheim, J Lu, B Porshinsky, BE Heck, RA Yeasting (1998) Vulnerability of long thoracic nerve: An anatomic study, J. Shoulder Elbow Surg 7: 458-461.
- J Shimizu, K Nishiyama, K Takeda, T Ichiba, M Sakuta (1990) [A case of long thoracic nerve palsy, with winged scapula, as a result of prolonged exertion on practicing archery], Rinsho Shinkeigaku. 30: 873-876.
- AA Maldonado, SL Zuckerman, BM Howe, ML Mauermann, RJ Spinner (2017) “Isolated long thoracic nerve palsy”: More than meets the eye, J Plast Reconstr Aesthet Surg 70: 1272-1279.
- MJ Oakes, DL Sherwood (2004) An Isolated Long Thoracic Nerve Injury in a Navy Airman, Mil. Med. 169: 713-715.
- M Vastamäki, LI Kauppila (1993) Etiologic factors in isolated paralysis of the serratus anterior muscle: A report of 197 cases, J Shoulder Elbow Surg 2: 240-243.
- JR Gregg, D Labosky, M Harty, P Lotke, M Ecker, et al. (1979) Serratus anterior paralysis in the young athlete, J. Bone Joint Surg Am 61: 825-832.
- La m Elders, Fga Van der Meché, A Burdorf (2001) Serratus anterior paralysis as an occupational injury in scaffolders: Two case reports, Am J Ind Med 40: 710-713.
- LR Le Nail, G Bacle, E Marteau, P Corcia, L Favard, et al. (2014) Isolated paralysis of the serratus anterior muscle: surgical release of the distal segment of the long thoracic nerve in 52 patients, Orthop Traumatol Surg Res OTSR 100: S243-248.
- P. Lorei, E.B. Hershman (1993) Peripheral Nerve Injuries in Athletes, Sports Med 16: 130-147.
- JP Mäkelä, R Ramstad, V Mattila, H Pihlajamäki (2006) Brachial Plexus Lesions after Backpack Carriage in Young Adults, Clin Orthop Relat Res 452: 205.
- SS Noland, EM Krauss, JM Felder, SE Mackinnon (2018) Surgical and Clinical Decision Making in Isolated Long Thoracic Nerve Palsy, Hand N. Y. N. 13: 689-694.
- BW Bakkum, N Miller, Back Muscles (2016) in: Bergmans Compr. Encycl. Hum. Anat. Var., John Wiley & Sons, Ltd: pp. 262-288.
- Q Pu, R Huang, B Brand-Saberi (2016) Development of the shoulder girdle musculature, Dev Dyn 245: 342-350.
- RB Monteiro dos Santos, SM dos Santos, FJC Carneiro Leal, OG Lins, C Magalhães, et al. (2015) Fittipaldi, Parsonage-Turner syndrome, Rev Bras Ortop 50: 336-341.
- E Fortanier, T Le Corroller, M Hocquart, E Delmont, S Attarian (2022) Shoulder palsy following SARS‐CoV‐2 infection: two cases of typical Parsonage-Turner syndrome. Eur J Neurol 29: 2548-2550.
-
Adam Pasquinelly, Megan Busch, Patrick Frank, David Giovannucci, Hamoun Delaviz and Adel Maklad*. The Anatomy of the Long Thoracic Nerve Origin, Course, And Branching Pattern: Implications for Long Thoracic Nerve Palsy and Serratus Anterior Dysfunction. Arch Neurol & Neurosci. 16(3): 2024. ANN.MS.ID.000889.
-
Acute Polyradiculoneuritis, Neurology, Guillain-barre; Fann teaching hospital, neuropathy, cranial nerves, immunotherapy, Neuroscience, neurogenic syndrome, epidemiological, Dysphonia.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






