 Review Article
Review Article
Systematic Research and Case Report: A 60-Year-Old Caucasian Male with Mycotic Aneurysm in the Right Middle Cerebral Artery Stemming from Infective Endocarditis
Swapnil Ahuja1*, Mariam Natsvlishvili1, Maha Prathiksha Arul Essakkiraj1, Tinatin Chkhartishvili1,Rufyat Mohsin Patel1, Harshal Praveen Singh1, Aditi Pandey1, Koka Gogichashvili2 and Mirza Khinikadze2
1New Vision University, Georgia
2Caucasus Medical Centre, Tbilisi, Georgia; New Vision University, Tbilisi, Georgia
Swapnil Ahuja, New Vision University, Tbilisi, Georgia.
Received Date:March 18, 2024; Published Date:May 07, 2024
Abstract
Our study combines a systematic review with a detailed case report to provide a comprehensive overview of Mycotic Aneurysms in the context of Infective Endocarditis. Mycotic Aneurysms, though infrequent (2%-4% incidence), are most commonly associated with Streptococcus viridans and Staphylococcus aureus in cases of acute infective endocarditis. Notably, mycotic aneurysms tend to develop at proximal arterial branches, with a predilection for the middle cerebral artery (57.4%) and cerebellar posterior artery (17.6%) or their proximal branches. Age and gender appear to play no specifc role in pathological predisposition. Globally, Staphylococcus aureus remains the predominant pathogen, while neurological complications, including intracranial hemorrhage, are common and often contribute to higher mortality rates.
Diagnostic criteria encompass clinical presentation, radiological imaging techniques (e.g., CT, MRI), blood cultures, and surgical evaluation. Blood culture results indicate a shift from Streptococcus to Staphylococcus aureus as the predominant pathogen. This trend aligns with an evolving demographic, with infective endocarditis increasingly affecting older patients with comorbidities and no known structural heart diseases. Complications, particularly neurological, are prevalent in left-sided native valve infective endocarditis, leading to challenges in diagnosis and management. Echocardiography plays a pivotal role in monitoring complications and valvular dysfunction. Treatment strategies involve extended courses of intravenous antibiotics (≥6 weeks), endovascular or surgical interventions, and withholding anticoagulation in patients with hemorrhagic neurological complications.
Prognosis varies widely, emphasizing the need for a multidisciplinary approach in managing mycotic aneurysms within the context of infective endocarditis. Our case report exhibits a prolonged and complicated course of treatment. Despite initial interventions, our patient’s condition continued to deteriorate, with fluctuating hemodynamics and an increasing need for vasopressors. Notably, on 17 April, 2023, our patient suffered a bradycardia episode, which transitioned to asystole. Although cardiopulmonary resuscitation was initiated promptly, and despite continuous efforts, the patient eventually succumbed to biological death. These additional case data emphasize the complex and difficult nature of managing mycotic aneurysms in the setting of infective endocarditis, emphasizing the need for more study and better treatment approaches in situations with comparable complications, like the one involving our patient. To manage mycotic aneurysms in the context of infective endocarditis, it is crucial to comprehend age and gender correlations, diagnostic criteria, causative organisms, comorbidities, treatment methods, and prognosis. This combined systematic review and case report highlights the importance of above mentioned. Understanding age and gender relationships, risk factors, clinical manifestations, and treatment outcomes should be a priority in future research to improve the prevention, identification, and management of these complex disorders. Long intravenous antibiotic courses (6 weeks) are part of treatment plans, coupled with possible endovascular or surgical procedures. The type of microorganism and patient-specific characteristics are just two examples of the many variables that can afect prognosis.
To manage mycotic aneurysms in the context of infective endocarditis, it is crucial to comprehend age and gender correlations, diagnostic criteria, causative organisms, comorbidities, treatment methods, and prognosis. This combined systematic review and case report highlights the importance of this.
Keywords:Mycotic aneurysm; Intracranial mycotic aneurysm; Infectious aneurysm; Infective Endocarditis
Introduction
Infective endocarditis is an infection of the endothelium of the heart and is a potentially life-threatening condition. It is predisposed to occur in some individuals with multiple cardiac valve conditions, with an annual incidence of 3–10 per 100,000 people [1]. It is characterized by vegetation within the heart that is composed of infectious agents, platelets, and fibrin. These vegetations can give rise to various symptoms, including fever, edema, Janeway lesions, and Osler’s nodes [2]. The disease is categorized as either acute or subacute, depending on the speed of progression of the illness, before a diagnosis is established. Acute infective endocarditis (IE) is characterized by rapid development over a span of days to weeks, often accompanied by pronounced toxicity in affected patients. On the other hand, subacute IE follows a more gradual and indolent course, progressing slowly over several weeks or months [3]. The diagnostic criteria for IE are called the Duke criteria, which provide a structured approach considering various clinical, imaging, and microbiological factors to make an accurate assessment (Table 1 & 2).
Table 1:Modified Dukes’ Criterion.
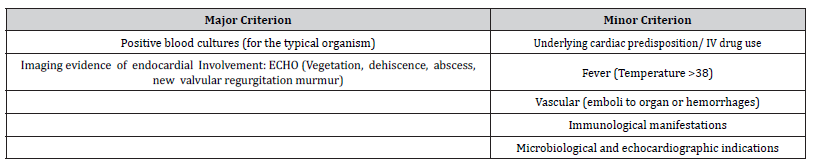
Table 2:Conclusion Of Infective Endocarditis.

The clinical diagnosis of defnite infective endocarditis (Table 2) is confirmed when both major criteria are simultaneously present. Additionally, a diagnosis of definite IE can be established through the following: the presence of one major criterion along with three minor criteria, or the fulfillment of five minor criteria [4]. The most common and severe extracardiac manifestations of infective endocarditis, as we move from the area of the disease to its complex repercussions, are neurological problems. According to the studies, about 25% of patients with IE experience at least one neurological event [5].
Infectious intracranial aneurysms, also called mycotic aneurysms, are localized arterial dilatations that arise as a consequence of septic emboli stemming from the complications of infective endocarditis and are reported to constitute a range of 0.5% to 6.5% of all aneurysmal cases [6]. The term “mycotic aneurysm” was first introduced by William Osler back in 1885 when he described where he described a man with multiple aortic mycotic aneurysms in a patient with valve vegetations, which resembled the appearance of a fishy fungus, It does not refer to fungal etiology, as the majority of infected aneurysms are caused by bacterial pathogens. Therefore, the correct term of these aneurysms can be an infected aneurysm. Infectious aortitis refers to vessel infection without aneurysmal dilation. An infected aneurysm develops in the setting of an antecedent systemic infections with bacteremia or through the direct local invasion of the vessel wall (e.g., IV drug users) in the pre-existing aneurysm or atheromatous plaques [7]. It was firstly by Osler described abnormal dilatation of the aortic arch vessel wall in a patient with subacute infective endocarditis [8]. Predominantly, these aneurysms tend to localize themselves along the peripheral branches of the middle cerebral artery (fg. 1) [5]. Infammatory processes trigger the influx of neutrophils that infiltrate the affected area. Subsequent stages encompass the breakdown of the arterial media and adventitia, along with the fragmentation of the internal elastic lamina. The weakened vessel wall, in combination with the pulsatile pressure in the vasculature, leads to aneurysm formation and consequential growth [9]. The mortality linked to the rupture of intracranial mycotic aneurysms, precipitating either subarachnoid hemorrhage or intracerebral hemorrhage, is documented to reach staggering levels, with reported rates as high as 80% [10] (Figure 1).
In this systematic review, we aim to carefully examine all the information about mycotic aneurysms in connection with infective endocarditis that already exists. This research seeks to provide doctors, researchers, and healthcare providers with a clear grasp of this intricate interplay. It aims to simplify the underlying pathogenic processes, analyze the clinical consequences, and assess alternative therapeutic approaches [11]. Moreover, the review aims to focus on areas in need of further research, gaps in current knowledge necessitating deeper investigation, and aid in the development of evidence-based strategies for the prevention, diagnosis, and treatment of mycotic aneurysms in patients with infective endocarditis.
This review aims to carefully examine existing literature, in order to comprehend the intricate relationship between infective endocarditis and mycotic aneurysms. By doing so, it aims to enhance awareness of these critical clinical challenges. Thus, it is believed that this systematic study would eventually aid in enhancing clinical decision-making, improving patient care, and shaping the next research initiatives in this dynamic Feld.
Case Presentation
Mr. David Kordzadze, a 60-year-old caucasian male, presented to the emergency department on February 2, 2023 at 19:50 with the chief complaint of fever. His vital signs at presentation were as follows: respiratory rate (RR) 17/min, blood pressure (T/A) 118/54 mmHg, body temperature (T°C) 38°C, oxygen saturation (O2) 96%, and heart rate (HR) 60/min. His Glasgow Coma Scale (GCS) assessment revealed an eye-opening score of 4 (spontaneous), a verbal response score of 5 (oriented), and a motor response score of 6 (obeying commands), resulting in a total GCS score of 15. Notably, his blood tests revealed negative results for HIV, anti-TP (Treponema pallidum), HBV (hepatitis B virus) surface antigen (HBsAg), and anti-HCV (hepatitis C virus) antibodies. His blood group was identified as B, with a positive rhesus factor (Rh+).
Coagulation studies showed a prothrombin index of 91.20%, a prothrombin time of 13.10 seconds, an INR of 1.05, an aPTT of 57.4 seconds, and a fibrinogen level of 3.70 g/L. Serum creatinine levels were found within the normal range at 96.00 μmol/L, with an estimated glomerular filtration rate (eGFR) of 78.00 mL/min. His complete blood count (CBC) revealed decreased levels of erythrocytes (RBC), hemoglobin (HGB), and hematocrit (HCT) at 3.64 10^12/l, 98.00 g/l, and 29.40%, respectively. Other CBC parameters, including mean corpuscular volume (MCV), red cell distribution width (RDW), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC), were found within or slightly below the normal range.
The platelet count was 98.00 10^9/l, with platelet large cell count (P-LCC) 27.00 10^9/l and platelet large cell ratio (P-LCR) 27.10%, respectively. Additionally, his white blood cell (WBC) count was 5.49 10^9/l, with a neutrophil count of 3.52 10^9/l and a percentage of 64.20%. Lymphocytes, monocytes, eosinophils, and basophils were within the reference ranges. Immature granulocyte levels were also within normal limits. This comprehensive assessment of Mr. Kordzadze’s clinical and laboratory findings provides valuable insights into his current health status, aiding in appropriate diagnosis and management.
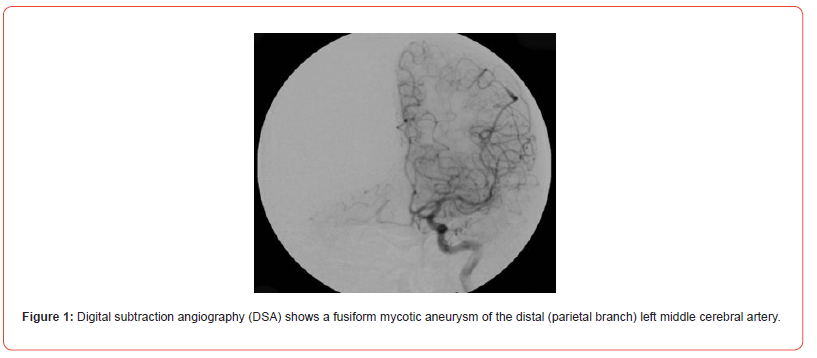
A CT scan conducted on 3rd of March 2023 revealed several notable findings. In the left frontotemporal lobe area, minimal subarachnoid hemorrhage was detected. Additionally, the images showed a metallic implant (coil) fixed in the right middle cerebral artery, along with areas of hyper density and early signs of cerebral edema. Moreover, calcification was observed in the choroid plexuses of the lateral ventricles. The optic nerves were visualized with evidence of optic nerve atrophy due to cerebral damage. Paranasal sinuses were clear, and the cranium (skull’s bony part) did not exhibit any damage. Cerebral structures in the left hemisphere appeared normal, while the right hemisphere showed hyperdense subarachnoid spaces and gyral deformation. The cerebellar tonsils were descended (Figure 2).
The patient’s treatment plan included intravenous administration of 500 ml NaCl 0.9% for hydration, 1000 mg/100 ml Infulgan for pain, and 1g of ceftriaxone for infection control. Oral medications include 30 mg of Nimodec and occasionally 20 mg of Omez for gastrointestinal concerns. IV infusions of 500 ml Ringer’s solution and 1000 mg Vancomycin are administered, with 500 mg/5 ml Tranedex taken orally and subcutaneous lidocaine used as needed. There was occasional use of oral Epixx, IV propofol, fentanyl citrate, and IV midazolam. A one-time IV dose of Rocuronium bromide was planned, and as required, IV Infulgan and Analgin were given for pain relief. The main treatment approach involved ceftriaxone and Sinogal (Sol Ampicillin Sulbactam).
Subsequent chest X-rays revealed the placement of the intubation tube above the tracheal bifurcation and the presence of a central venous catheter shadow towards the right atrium. Imaging also indicated light lung felds and a normal diaphragm size. The heart, aortic arch, and tracheal location were within normal limits. The CT scan depicted no bony skull damage, a slight subarachnoid hemorrhage, and a metallic implant in the right middle cerebral artery. Areas of cerebral edema and localized hypodensity were observed in the posterior cerebral artery, particularly in the medial parts (ASPECTS: 3).
A further comprehensive analysis revealed notable values in the correlogram. The prothrombin index (80.40%) and the prothrombin time (12.80 seconds) were slightly increased. INR was around 1.12, within the acceptable range. aPTT (25.2 seconds) and thrombin time (16.50 seconds) were both within normal limits, and fbrinogen levels were 3.30 g/L and showed a slight elevation.
The CBC+Dif and ESR results revealed erythrocyte count-RBC (3.30 x 10^12/l), hemoglobin (89.00 g/l), and hematocrit (27.60%) were decreased. Mean corpuscular volume (83.70 fL) and mean corpuscular hemoglobin (27.00 pg) were within normal limits. Red cell distribution width (20.20%) was increased. Platelet count (109.00 x 10^9/l) was decreased, while platelet large cell count (24.00 x 10^9/l) and platelet large cell ratio (21.10%) were within normal limits. Thrombocrit (0.10%), mean platelet volume (9.10 fL), and platelet distribution width (16.10%) were within normal limits. Leukocyte count:WBC (5.57 x 10^9/l), neutrophils (3.96 x 10^9/l), lymphocytes (1.22 x 10^9/l), monocytes (0.24 x 10^9/l), eosinophils (0.07 x 10^9/l), basophils (0.07 x 10^9/l), and immature granulocytes (0.20 x 10^9/l) were within normal limits. Notably, medication adjustments include the administration of various medications such as Infulgan, Analgin, Epixx, Vancomycin, Midazolam, Nimodec, Propofol, Fentanyl Citrate, Manitec, and Ceftriaxone. Liver function tests revealed normal ALT (9 U/L), AST (22 U/L), GGT (29 U/L), and ALP (117 U/L) levels, with total bilirubin (T-BIL) at 6.40 μmol/L and direct bilirubin (D-BIL) at 2.40 μmol/L.
On March 7, 2023, adjustments to the medication regimen included Epixx administration, Vancomycin, Midazolam, Nimodec, Propofol, Fentanyl Citrate, Manitec, and Ceftriaxone. The patient’s PCT (procalcitonin) level was 0.060 ng/mL. In terms of normal ranges, the PCT result places the patient within the low-risk category for sepsis and septic shock (< 0.5 ng/mL). The laboratory fndings also encompass the CBC+Dif and ESR outcomes, demonstrating decreased erythrocyte count-RBC (3.32 x 10^12/l), hemoglobin (90.00 g/l), and hematocrit (28.20%). Mean corpuscular volume (85.20 fL) and mean corpuscular hemoglobin (27.10 pg) were within normal limits, with an increased red cell distribution width (21.00%). Decreased platelet count (94.00 x 10^9/l). Platelet large cell count (23.00 x 10^9/l), platelet large cell ratio (24.20%), thrombocrit (0.09%), and mean platelet volume (9.40 fL) are within normal limits. Furthermore, the leukocyte count-WBC (7.57 x 10^9/l ) and neutrophils count (5.62 x 10^9/l) increased. Lymphocytes (1.42 x 10^9/l), monocytes (0.42 x 10^9/l), eosinophils (0.05 x 10^9/l), basophils (0.06 x 10^9/l), and immature granulocytes (0.20 x 10^9/l) were within normal limits. This comprehensive update enhances the understanding of the patient’s evolving condition and ongoing medical interventions (Table 3).
Table 3:Patient’s medical progress report.
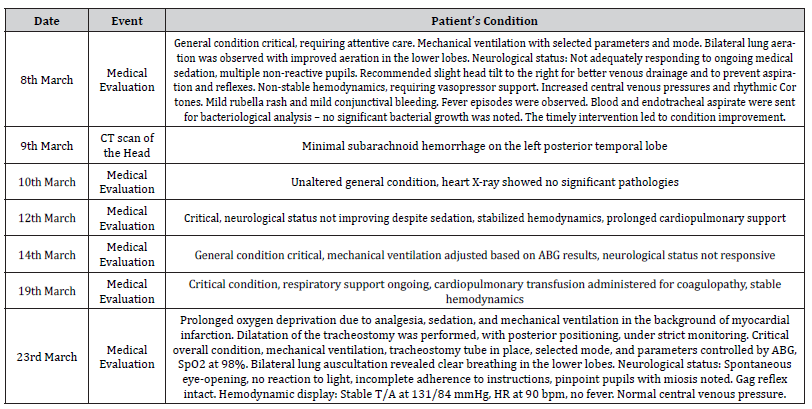
Later in the left lung, a small-size tube was observed in the bronchial act during deep suctioning, while mild resistance was noted. A third-degree decubitus ulcer was identifed on the left heel and managed daily and aseptically. Hemodynamics remained stable, with regular sinus rhythm on the EKG, normal heart sounds, and no murmurs. The abdomen was soft and nontender, with a palpable spleen tip. The neurological status remained unchanged. Blood tests showed a hemoglobin level of 9 g/dL. Due to persistent high fever, endotracheal aspirate was sent for bacterial culture, and guided by the results, ceftriaxone was initiated. Despite treatment, the patient’s condition deteriorated, with an increasing severity of infection and recurrent episodes of fever.
On March 30, 2023, an esophagogastroduodenoscopy was performed, revealing multiple bleeding ulcers in the antrum. Hemostasis was achieved, and gastroprotection was initiated. Hemostasis remained successful, and hemoglobin levels stabilized. However, on April 16, 2023, the patient’s condition deteriorated critically, with fuctuating hemodynamics and an increasing need for vasopressors. Attempts at stabilizing hemodynamics were unsuccessful. On April 17, 2023, the patient sufered a bradycardia episode, which transitioned to asystole. Cardiopulmonary resuscitation was initiated, but the patient could not be revived. Despite continuous eforts, the patient eventually succumbed to biological death.
Methodology
In May 2023, the PRISMA 2020 (Figure 1) and Preferred Reporting Items for Overviews of Reviews (PRIOR) criteria were incorporated to be used as the guiding framework for this study’s objectives. The strict adherence to these rules upheld the ideals of transparency, scientifc rigor, and the possibility of reproducibility inherent to this systematic review. The central focus of this systematic review is to elucidate nuanced insights within an area of paramount complexity, one that has regrettably remained understudied. Specifcally, the study endeavors to comprehensively analyze the intricate pathology of mycotic aneurysm in the context of infectious endocarditis, thereby contributing to a more profound comprehension of this multifaceted domain.
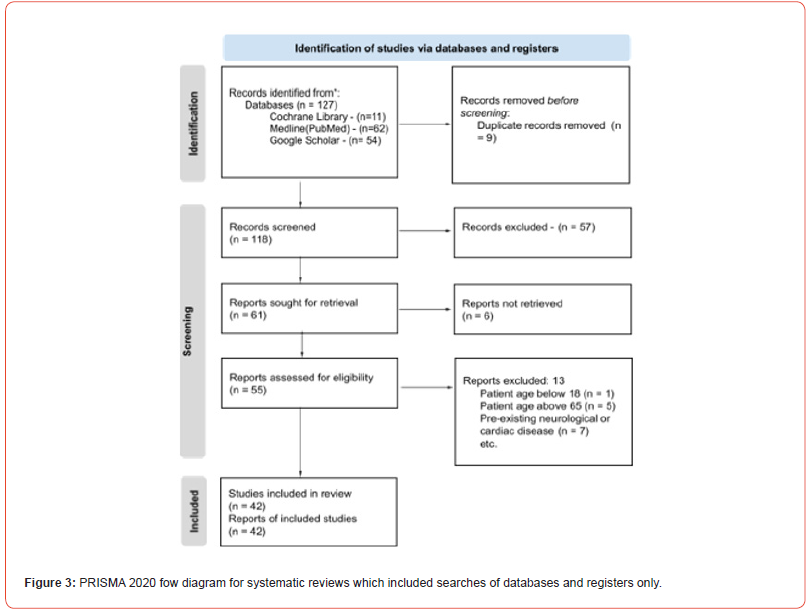
Inclusion Criteria
This study project’s methodical and thorough approach to the identifcation of relevant papers provided insight into the complex phenomenon of mycotic aneurysms in infective endocarditis patients. We carefully searched the well-known academic databases on PubMed, NCBI, Google Scholar, and Medline using precise search phrases such as “mycotic aneurysm, intracranial mycotic aneurysm, infectious aneurysm, and infective endocarditis.”
The study’s demographic scope spans adults aged 18-69, encompassing both genders for equitable representation. Individuals who exhibited a confrmed clinical diagnosis of mycotic aneurysm concomitant with infective endocarditis were incorporated within the inclusion criteria of this research. Each individual profle encompassed essential clinical particulars, imaging outcomes, laboratory fndings, and comprehensive neurological and cardiac assessments.
The scope of admissible study designs encompassed a range of methodologies, which include retrospective investigations, observational inquiries, cohort analyses, and randomized clinical trials (RCTs). This deliberate inclusivity mirrored the multifaceted nature of the research subject and aimed to capture insights from various research paradigms. This study has focused on research contributions that covered the time period from 2018 to 2023, which makes it both current and relevant.
Adequate follow-up data should have also been supplied, with either a minimum follow-up length or a set number of follow-up consultations, in order to examine the long-term neurological and cardiac outcomes and issues related to mycotic aneurysm and infective endocarditis.
This study encompassed a diverse spectrum of individuals who were aficted by mycotic aneurysms caused by a variety of underlying etiologies, which can be broadly categorized into infectious (bacteria Streptococcus viridans, fungal Aspergillus fumigatus, and potentially viral triggers) and non-infectious causes (familial predisposition, pertinent medical histories, pharmacological infuences, environmental factors, and other determinants).
Exclusion Criteria
To maintain study focus and minimize variability, individuals both below the age of 18 and above the age of 65 were deliberately excluded from this systematic review. Depending on the scope of the review, studies published before 2015 are excluded to ensure the inclusion of more recent evidence.
Furthermore, patients presenting with pre-existing neurological or cardiac conditions not directly pertinent to mycotic aneurysms or infective endocarditis were purposefully left out of the analysis. Studies that centered on non-infectious etiologies of endocarditis, such as rheumatic or degenerative origins, were also exclusively eliminated. Also, individuals with conditions such as severe trauma or states of immunosuppression were conscientiously omitted from the study’s purview.
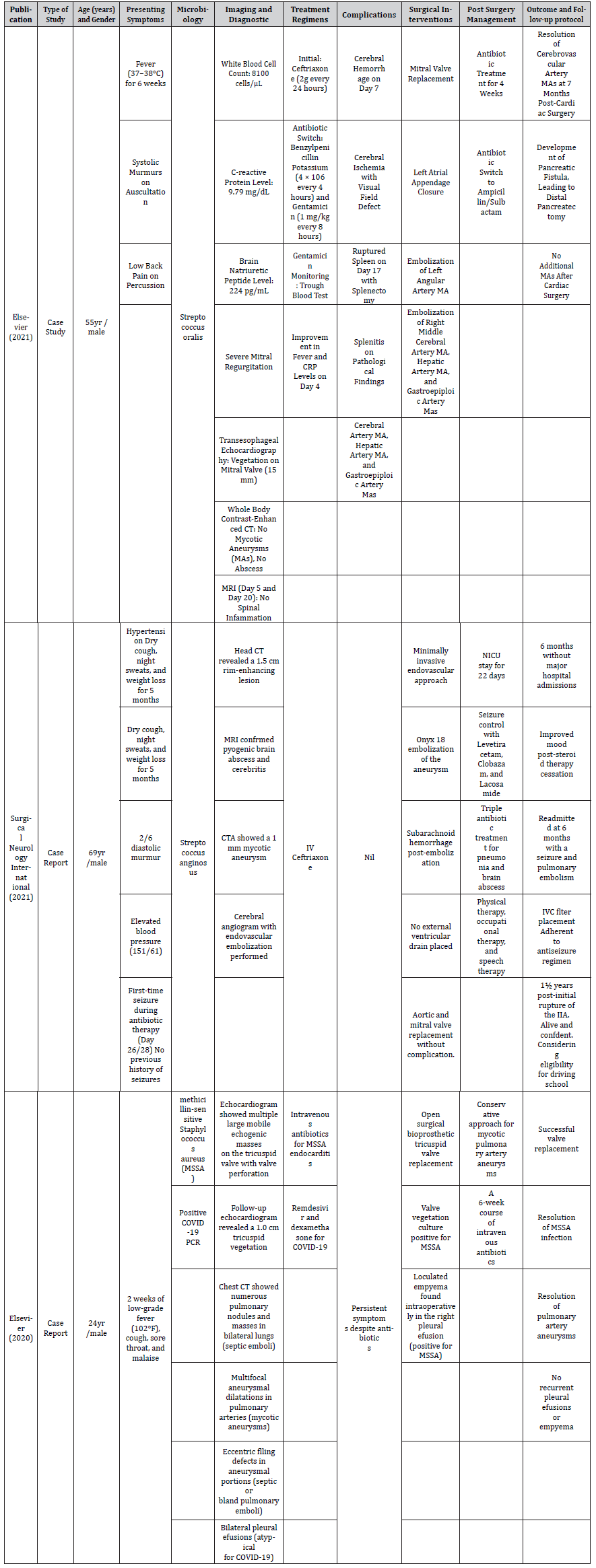
Table 4:Variables identified across the studies collated for the analysis of this systematic review on the mycotic aneurysm in infective endocarditis patient.
Additionally, studies lacking the provision of relevant results on mycotic aneurysms within the context of infective endocarditis had been excluded from the review process. Also, studies that were not published in the review team's primary language(s) or those without available translation resources were deliberately excluded. This stringent approach ensures alignment with the review's primary investigative objectives, mitigates the infuence of linguistic bias, and upholds both the review's viability and methodological integrity.
The thoroughness of the data was carefully considered, leading to the exclusion of studies marked by insufcient followup information or data pertinent to mycotic aneurysms in the population of patients with infective endocarditis. Consequently, studies that were duplicates or that manifested as multiple iterations of the same scientifc endeavor were disregarded.
Information Sources and Search Strategies
A methodical approach was meticulously employed throughout the course of this systematic review. The initial step involved formulating precise key search terms, guided by meticulous inclusion and exclusion criteria. To ensure the utmost relevance of the identifed studies, exclusion criteria keywords played a pivotal role in fne-tuning the search process.
In the pursuit of reliable and comprehensive research materials, prominent databases, including but not limited to PubMed (Medline), Cochrane Review, and the National Library of Medicine databases, underwent rigorous and extensive study in the hunt for trustworthy and exhaustive research materials. Extensive screening of several clinical trials was carried out from 2016 to 2023. This thorough search technique carefully selected a current and extensive reservoir of applicable papers, which laid the foundation for the subsequent phases of the review. The conscientious and comprehensive nature of this methodology facilitated a robust and rigorous evaluation of mycotic aneurysms in the context of infective endocarditis patients.
Selection and Data Collection Process
Review selection: The process of selection was meticulously executed to ensure relevance and rigor. Initial database searches on platforms such as PubMed, NCBI, Google Scholar, and Medline were guided by specifc search terms, including "mycotic aneurysm, intracranial mycotic aneurysm, infectious aneurysm, and infective endocarditis." The demographic scope encompassed adults aged 18-69 with confrmed mycotic aneurysms concomitant with infective endocarditis. A range of study designs were considered, aiming to capture insights from diverse research paradigms.
Data extraction: Data extraction was conducted systematically from selected studies to ensure robustness and consistency. Essential clinical particulars, imaging outcomes, laboratory fndings, and neurological and cardiac assessments were extracted from individual profles. The focus on research contributions from 2018 to 2023 ensured the study's relevance and current applicability. Long-term outcomes were assessed using adequate follow-up data. This meticulous data extraction process laid the foundation for subsequent analysis and synthesis.
Primary outcome: The primary outcome of this systematic review was to comprehensively assess the diferent types and frequencies of mycotic aneurysms in patients with infective endocarditis. This encompassed both desirable and undesirable outcomes, allowing for a comprehensive assessment of all the outcomes of the pathology. The favorable outcomes included successful blood culture results, which indicated that Streptococcus was a more prevalent causative agent as compared to Staphylococcus and improved patient survival. Whereas the unfavorable outcomes included complications such as rupture and hemorrhage of aneurysm, septic embolism, invasive infection spread, persistent endocarditis, delayed diagnosis, long-term morbidity, and mortality.
By understanding the primary outcome and focusing on infective endocarditis complications, this systematic review aims to provide valuable insights into the overall efect of mycotic aneurysms in patients with infective endocarditis, thereby informing clinical decision-making and improving patient care in this specialized population.
Secondary outcome: This systematic review examined secondary variables, including patient age, gender distribution, total subjects in the studies, optimal mycotic aneurysm treatments in infective endocarditis, causative microorganisms, prognosis with concurrent mycotic aneurysm and infective endocarditis, treatment protocols for complications, and follow-up procedures.
The secondary goal was to assess diagnostic methods for detecting mycotic aneurysms in infective endocarditis. This involved evaluating CT angiography, MRI, and echocardiography to ensure sensitivity, specifcity, and accuracy. The review also aimed to link mycotic aneurysm detection with its clinical outcomes, such as complications, treatment successes, and patient prognosis.
Preparation for synthesis: Data was gathered from various databases such as Medline (Pubmed), NCBI, and the Cochrane Library using specifc keywords like "mycotic aneurysm, intracranial mycotic aneurysm, infectious aneurysm, and infective endocarditis" for patients with mycotic aneurysm in the context of infective endocarditis. Clinical trials were sorted and presented in a table based on their year and category to streamline the data analysis process. This summary table, created using Microsoft Excel, played a pivotal role in categorizing and organizing the information from the studies, forming the basis of the systematic review's outcomes.
Tabulation and graphical methods: To ensure transparency and compliance with inclusion and exclusion criteria, a PRISMA fow chart was generated in Microsoft Word. Data analysis encompassed descriptive, bivariate statistics, and numeric outcome predictions using SPSS software, enabling a comprehensive exploration of data and potential variable associations. Notably, diversity among subgroup variables in the studies emphasized the need for meticulous result synthesis and interpretation.
Methods to explore heterogeneity: Heterogeneity within the systematic review was assessed by tabulating the collected data. Diverse clinical trial types emerged from the fndings, yet uniformity in outcomes regarding mycotic aneurysms in infective endocarditis patients was evident across all trial categories.
Assessment of bias risk: To boost the systematic review's reliability and minimize bias, we employed strict inclusion criteria, particularly focusing on clinical trials. Additionally, we utilized specialized tools like the Agency for Healthcare Research and Quality (AHRQ) bias assessment to systematically identify and rectify bias possibilities in the selected papers. This approach signifcantly enhanced the review's credibility and validity.
Reporting bias: To mitigate bias, we followed predetermined methodology criteria diligently. Objective evidence was substantiated through strong statistical analysis, while any observed inconsistencies were openly acknowledged and resolved in both the results and discussion sections of the systematic review.
Results
MAs are uncommon IE complications, occurring in just 2% to 4% of individuals. Streptococcus viridans and Staphylococcus aureus are the most often associated bacteria in the course of acute IE. MAs have distinct radiological characteristics. They form at a proximal branch rather than a peripheral one. They are found in the MCA (57.4%) and CPA (17.6%), or their proximal branches.
Age of the patient
In individuals with underlying reasons, including valve disease or other cardiac issues, mycotic aneurysms frequently coexist with infectious endocarditis. As a result, mycotic aneurysms and infectious endocarditis are more likely to develop in middle age and the elderly (age spanning from 35 to 80 years). The age range might change depending on a number of variables, including location, lifestyle, healthcare practices, and prevalence in particular communities.
Gender of patient
Patients of any gender can develop mycotic aneurysms linked to infective endocarditis. When it comes to infective endocarditis, there is no particular gender propensity for the growth of mycotic aneurysms. Males and females might both be impacted. And it mostly depends on the underlying risk factors.
Causative Organism of Infective Endocarditis
According to blood culture reports, the common pathogen found is streptococcus. But anyhow, Staphylococcus aureus is routinely cultured around the world, which makes S. aureus the most frequently isolated pathogen. There are also other pathogens like brucella, listeria, S. bovis, coagulase-negative staphylococci, and Coxiella burnetii.
Complications Observed after Infection
The neurological presentation often involves intracranial hemorrhage. The complication involves cardiovascular and pulmonary involvement as well. According to univariate research, several factors contribute to higher fatality rates. These include aneurysm rupture, involvement of the parent vessel, aneurysm progression, and the decision between non-surgical and interventional aneurysm treatment.
Treatment protocol for patients experiencing mycotic aneurysm coexisting with infective endocarditis
Long-term intravenous antibiotic therapy for at least 6 weeks is the medical intervention that is universally advised. Morawetz and Karp discovered in 1984 that unruptured MAs might undergo spontaneous thrombosis, implying that MAs could be entirely resolved with antibiotic therapy alone.
Endovascular therapy has advanced rapidly in terms of efcacy and ability to treat more distant aneurysms. The safety profle of this technique is difcult to assess because it is based mainly on anecdotal and case-report information. Endovascular treatment was signifcantly more likely than open craniotomy with surgical ligation to result in parent artery sacrifce. This afects the treatment of sick veins that supply eloquent brain parenchymas such as language, sensorimotor cortex, visual cortex, hypothalamus, thalamus, cerebral peduncles, and brain stem.
Open craniotomy and aneurysm clipping are reserved for individuals with intraparenchymal bleeding or who require clot evacuation and urgent reversal of rising intracranial pressure. Another signifcant beneft of surgical intervention is the potential for vascular bypass to protect distal blood fow, which is critical when the aneurysm involves eloquent territory.
In the Setting of Intracranial Septic Emboli, Valvular Repair. The goal of cardiac surgery is to eliminate the cause of cerebral emboli and enhance hemodynamics. The timing and order of cardiothoracic and neurosurgery are afected by whether the aneurysm has ruptured. If the MA is not ruptured, cardiac surgery is relatively safe.
Prognosis and follow-up protocol for the patient
Patients with infective endocarditis (IE) and mycotic aneurysms have varying prognoses depending on factors such as the type of microorganism causing the infection, the patient's overall health, the extent of cardiac involvement, the presence of complications, and the timeliness of appropriate treatment. Mycotic aneurysms are a dangerous consequence of infective endocarditis that causes infected outpouching in the arterial wall. Therefore, proper antimicrobial treatment is advised. Early administration of antimicrobial treatment emerges as a pivotal strategy to mitigate the potential catastrophic neurological complications arising from IE. As a recommendation, individuals aficted with both IE and intracranial hemorrhage are advised to undergo vascular imaging, preferably in the form of cerebral angiography. This comprehensive approach aims to enhance understanding and management of IErelated neurological intricacies while minimizing associated risks.
A multidisciplinary team is often involved in the follow-up routine for a patient with infective endocarditis with mycotic aneurysms. It is necessary for the patients to undergo imaging studies to detect any further complications after the treatment. It is also mandatory to assess the cardiac function on a regular basis. Routine blood tests are advisable. The patient's overall clinical status, infection-related symptoms, and any new neurological symptoms that might point to emboli should be frequently monitored. Prior to some medical or dental operations, patients with a history of mycotic aneurysms and infective endocarditis may require antibiotic prophylaxis to prevent recurrence.
Discussion
Age and gender of patients in correlation with infective endocarditis and mycotic aneurysm
It is essential for healthcare providers to understand the connection between age and gender in infective endocarditis and mycotic aneurysm [12]. This understanding helps with evaluating risks, diagnosing them and customizing treatment approaches based on the requirements of various patient groups [13]. Future research should prioritize investigating the factors that contribute to age and gender differences in infectious endocarditis and mycotic aneurysm. By doing so, we may discover approaches for prevention and treatment. Following patients' progress over a period of time through studies can offer further valuable insights. By examining the frequency factors that contribute to risk, how patients present clinically, and the results obtained, we can acquire knowledge that can improve the treatment of patients and direct future research in the feld of medicine. Recognizing these differences among populations is a step towards enhancing the prevention, detection, and handling of these extremely serious conditions.
Diagnostic criteria for mycotic aneurysm and infective endocarditis
Clinical Presentation: Patients may experience symptoms such as fever, chills, localized pain, and indications of an infection. These symptoms can be non-specifc. It may overlap with other medical conditions. Imaging Studies: Radiological imaging techniques like computed tomography (CT) echocardiograms or magnetic resonance imaging (MRI) play a role in making a diagnosis. The fndings may show a widening of an artery accompanied by signs of infection (such as the formation of an abscess, the presence of gas within the artery, or infltration into surrounding soft tissues). Blood Cultures: Obtaining blood cultures is necessary to identify the microorganism responsible for the infection. This step is vital in determining antibiotic therapy. Surgical Evaluation: It is often necessary to consult with a surgeon to evaluate the extent of the infection and determine the course of action. This may involve repair, debridement (removal of tissue), and antibiotic treatment [14].
Causative organism of infective endocarditis
Blood culture results indicate that Streptococcus is a more prevalent pathogen compared to Staphylococcus, with Streptococcus being the most prevalent. But globally, Staphylococcus aureus (S. aureus) takes the lead as the most commonly isolated bacteria, followed by coagulase-negative staphylococci, Streptococcus viridans, and enterococci [15]. (S. aureus) is more common in older patients and those with healthcare-associated IE. Infective endocarditis (IE), even though uncommon, causes signifcant morbidity and mortality in both children and adults. The trend of IE has evolved to afect older patients with co-morbidities and no known structural heart disease.
Complications of Infective Endocarditis in Association with Mycotic Aneurysm
neurological complication is a common manifestation of leftsided native valve infective endocarditis. Septic embolization may cause ischaemic stroke, mycotic aneurysm, intracranial hemorrhage, and brain abscess. This is due to the changing epidemiology of the disease, the wide spectrum of presentation extending from the neonate to the elderly, diagnostic difculties, delayed surgical interventions, and embolic complications. Echocardiography plays a crucial role in the diagnosis of IE, monitoring for complications and progression of valvular dysfunction and assessing the outcome. > Heart failure is Commonly associated with valve dysfunction. Surgery is indicated for those with acute decompensated heart failure due to valvular dysfunction. Persistent infection and perivalvular extensions: Monitor for conduction abnormalities, e.g., atrioventricular (AV) block. TEE should be performed to look for perivalvular extensions. Systemic embolism usually occurs in leftsided IE and within the frst 2 weeks of therapy. Common sites are the brain and the spleen [16].
Risk factors for embolism are associated with vegetation size (> 10 mm), mobility, location (anterior mitral valve leafet), and the causative microorganism (S. aureus). Neurological complications: it occurs early in the course of IE (the frst 2 weeks). Common complications are ischaemic or hemorrhagic strokes and mycotic aneurysms. Management should be individualized, and care plans should be decided by a multidisciplinary team that also includes neurologists and neurosurgeons. It is advisable to withhold anticoagulation in mechanical prosthetic valve endocarditis (MPVE) patients who have hemorrhagic neurological complications for at least 2 weeks with close monitoring of the valves and the patient’s clinical condition. The duration of withholding anticoagulation is dependent on the severity of the neurological complication and the patient’s clinical condition [17].
Treatment protocol for a patient with mycotic aneurysm coexisting with infective endocarditis
The management of IE is aimed at eradicating the infection and preventing and treating both intracardiac and extracardiac complications. Patients with complicated IE should preferably be referred to a specialist center. Specialist centers are those with cardiothoracic, cardiac imaging, and specialized cardiology services. The management of infective endocarditis is challenging, as the 1-year mortality rate is approximately 30%. Neurological complications, most commonly cerebral embolism, are seen in 20–40% of the patients and are associated with high morbidity and mortality [18]. The reported incidence of mycotic cerebral aneurysms is 2–3% of all the patients with infective endocarditis. This is possibly underestimated because the majority of the patients remain asymptomatic and the aneurysm may resolve after antibiotic therapy. Mycotic aneurysm is an ominous fnding, with high mortality rates ranging from 30% in untreated unruptured cases to 80% in cases of rupture. MAs are most common in the anterior circulation. The mainstay of treatment is appropriate and adequate antimicrobial therapy. The minimum inhibitory concentration (MIC) should be achieved to ensure optimal antimicrobial therapy.
For penicillin-susceptible (MIC ≤ 0.125 μg/ml) Viridans streptococci, monotherapy with benzyl penicillin, ampicillin, or ceftriaxone is adequate. [IIa/B] » The duration of therapy is 4 weeks for native valve endocarditis (NVE) and 6 weeks for prosthetic valve endocarditis (PVE). Antimicrobial prophylaxis is not routinely recommended for cardiac patients undergoing invasive dental or other medical procedures and should be limited only to cardiac patients associated with the highest risk of adverse outcomes from IE [19]. Those with a high predisposing risk for developing IE should be advised to maintain good oral and skin hygiene.
Prognosis and follow-up protocol for a patient with mycotic aneurysm coexisting with infective endocarditis
Follow-up management: Monitor for relapse and reinfections, review indications for elective cardiac surgery, education on preventive measures, prognosis and patient counseling. Mycotic aneurysms are rare but life-threatening. Infected aortic aneurysms have a high rate of rupture if not treated promptly. Histopathological examination (HPE) of cardiac tissue or vegetation obtained during surgery is of diagnostic value and is recommended. A transthoracic echocardiogram (TTE) should be obtained without delay if the diagnosis of IE is suspected [20]. Echocardiogram fndings should be interpreted in the context of the clinical scenario and repeated if the clinical suspicion of IE persists despite a negative initial echocardiogram. A transoesophageal echocardiogram (TOE/ TEE) should be done if the initial TTE examination is negative, in patients with strong clinical suspicion of IE, in those with prosthetic valves or cardiac material, and in those with high-risk features. Echocardiography plays a crucial role in the diagnosis of IE, monitoring for complications and progression of valvular dysfunction, assessing the outcome of surgical repair, and in the follow-up after completion of antimicrobials (refer to Table: Role of Echocardiography in the Diagnosis and Management of IE, below) [21]. In patients with S. aureus bacteremia from an unknown etiology or persistent bacteraemia despite antimicrobials, echocardiography should be considered. Some newer imaging modalities (multislice computed tomography; MSCT; magnetic resonance imaging; MRI; and nuclear imaging) can assist in diagnosing IE and its complications. The modifed Duke criteria is used to diagnose IE but has limited diagnostic accuracy in the early phase of the disease and in those patients with prosthetic valve or cardiac implantable electronic device (CIED) endocarditis.
According to the virulence of the infecting organism, some patients seek medical attention with a prolonged history of systemic symptoms such as low-grade fever, weight loss, anorexia, or weakness, while others present with typical local symptoms such as aneurysm rupture or sepsis [21-28]. Aorto-enteric or aorta-bronchial fstulas are one of the most disastrous clinical features in patients with infected aortic aneurysm. As a remote symptom of infected aortic aneurysms. we encountered one patient complaining of eye pain and swelling, which rapidly progressed into septic endophthalmitis requiring enucleation [16].
Acknowledgement
None.
Conflict of Interest
Each author attests to the lack of a confict of interest and states that they have no relevant competing fnancial or non-fnancial interests.
References
- Chen H, Zhan Y, Zhang K, Gao Y, Chen L, et al. (2022) The global, regional, and national burden and trends of infective endocarditis from 1990 to 2019: Results from the Global Burden of Disease Study. Frontiers in Medicine 9: 774224.
- Musthafa I, Kandel D, Rajlawot K, Neupane NP, Sitaula A (2022) Infective endocarditis complicated by cerebral abscess and mycotic intracranial aneurysm: A case report. Radiology Case Reports 17(10): 3690-3693.
- Klein M, Wang A (2016) Infective endocarditis. Journal of Intensive Care Medicine 31(3): 151-163.
- Topan A, Carstina D, Slavcovici A, Rancea R, Capalneanu R, et al. (2015) Assessment of the Duke criteria for the diagnosis of infective endocarditis after twenty-years. An analysis of 241 cases. Medicine and Pharmacy Reports 88(3): 321-326.
- Sotero FD, Rosário M, Fonseca AC, Ferro JM (2019) Neurological complications of infective endocarditis. Current Neurology and Neuroscience Reports 19(5).
- Brimley C, Griessenauer CJ, Dalal S, Schirmer CM, Goren O (2019) Acute development and rupture of a basilar tip mycotic aneurysm. World Neurosurgery 124: 373-377.
- Majeed H, Ahmad F Mycotic Aneurysm (2023) In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
- Afshari FT, Al-Lawati K, Chavda S, Billing S, Flint G (2019) Cerebral mycotic aneurysms secondary to Streptococcus Agalactiae induced infective endocarditis. British Journal of Neurosurgery 33(6): 693-695.
- Zanaty M, Chalouhi N, Starke RM, Tjoumakaris S, Gonzalez LF, et al. (2013) Endovascular treatment of cerebral mycotic aneurysm: A review of the literature and single center experience. BioMed Research International 2013: 151643.
- Masuda N, Azuma T, Furukawa H, Uwabe K (2023) Endovascular Aortic Repair for a Symptomatic Mycotic Aneurysm With Listeria monocytogenes: A Case Report. Vasc Endovascular Surg 57(4): 411-413.
- Kuo I, Long T, Nguyen N, Chaudry B, Karp M, et al. (2010) Ruptured intracranial mycotic aneurysm in infective endocarditis: a natural history. Case Rep Med 2010: 168408.
- Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, et al. (2009) ESC Committee for Practice Guidelines. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. Eur Heart J 30(19): 2369-2413.
- Fukuda W, Daitoku K, Minakawa M, Fukui K, Suzuki Y, et al. (2012) Infective endocarditis with cerebrovascular complications: timing of surgical intervention. Interact Cardiovasc Thorac Surg 14(1): 26-30.
- Singla V, Sharma R, Nagamani AC, Manjunath CN (2013) Mycotic aneurysm: a rare and dreaded complication of infective endocarditis. BMJ Case Rep 2013: bcr2013200016.
- Bennett DE (1967) Primary mycotic aneurysms of the aorta. Report of case and review of the literature. Arch Surg 94(6): 758-765.
- Mundth ED, Darling RC, Alvarado RH, Buckley MJ, Linton RR, et al. (1969) Surgical management of mycotic aneurysms and the complications of infection in vascular reconstructive surgery. Am J Surg 117(4): 460-470.
- Verhelst R, Lacroix V, Vraux H, Lavigne JP, Vandamme H, et al (2000) Use of cryopreserved arterial homografts for management of infected prosthetic grafts: a multicentric study. Ann Vasc Surg 14(6):602-607.
- Eishi K, Kawazoe K, Kuriyama Y, Kitoh Y, Kawashima Y, et al. (1995) Surgical management of infective endocarditis associated with cerebral complications. Multi-center retrospective study in Japan. J Thorac Cardiovasc Surg 110(6):1745-1755.
- Horstkotte D, Follath F, Gutschik E, Lengyel M, Oto A, Pavie A, et al. (2004) Task Force Members on Infective Endocarditis of the European Society of Cardiology; ESC Committee for Practice Guidelines (CPG); Document Reviewers. Guidelines on prevention, diagnosis and treatment of infective endocarditis executive summary; the task force on infective endocarditis of the European society of cardiology. Eur Heart J 25(3): 267-276.
- Braverman AC, Schermerhorn MC (2015) Diseases of the aorta. In: Mann DL, Zipes DP, Libby P eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. (11th edn). Philadelphia, PA: Elsevier/Saunders 832.
- Aileen Paula A Chua, Bryan Rene F Toledano, Jose Paolo A Prado (2023) Complicated Mycotic Thoracic Aortic Aneurysm Secondary to Staphylococcus aureus Endocarditis. Journal of Asian Pacifc Society of Cardiology 2: e17.
- Beckerman Z, Martínez-Bravo LE, Johnson G, Holt B, Fraser CD (2020) Rare Presentation of Endocarditis and Mycotic Brain Aneurysm. Ann Thorac Surg 109(3): e179-e181.
- Voruganti D, Gajurel K, Bhama JK, Cotarlan V (2018) Ruptured Intracranial Mycotic Aneurysm in Infective Endocarditis with Left Ventricular Assist Device and Implantable Cardiac Defbrillator Device: A Clinical Course. Transplant Proc 50(10): 4064-4066.
- Diop AM, Mbodj AB, Fall SAA, Niang I (2022) Ischemic Stroke and Ruptured Mycotic Aneurysm, Two Complications of Infective Endocarditis in One Patient. Case Rep Neurol Med 2022: 6275537.
- Mitsui K, Oda R, Lee T, Watanabe K, Nakamura T, et al. (2021) Multiple mycotic aneurysms with infective endocarditis: A case report. J Infect Chemother 27(10): 1513-1516.
- Avallone SV, Levy AS, Starke RM (2021) A rare case of Streptococcus anginosus infectious intracranial aneurysm: Proper management of a poor prognosis. Surg Neurol Int 12: 487.
- Liang C, Bista B (2020) Multiple pulmonary artery mycotic aneurysms and septic emboli in a patient with tricuspid valve vegetation and infective endocarditis. Radiol Case Rep 16(1):128-131.
- Alotaibi AM, Arafat AA, Alotaibi K, Algarni KD, Adam AI (2020) Multiple mycotic arterial aneurysms involving the left anterior descending coronary artery following mitral and aortic valve endocarditis: A case report. J Card Surg 35(7): 1717-1720.
-
Fred H Previc*. Right-Hemispheric Disinhibition as a Neural Basis of Acquired Savantism and Foreign Accent Syndrome. Arch Neurol & Neurosci. 16(4): 2024. ANN.MS.ID.000896.
-
Acute Polyradiculoneuritis, Neurology, Guillain-barre; Fann teaching hospital, neuropathy, cranial nerves, immunotherapy, Neuroscience, neurogenic syndrome, epidemiological, Dysphonia.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






