 Review Article
Review Article
Right-Hemispheric Disinhibition as a Neural Basis of Acquired Savantism and Foreign Accent Syndrome
Fred H Previc*
Department of Psychology, University of Texas at San Antonio, San Antonio, Texas USA
Fred H Previc, Department of Psychology, University of Texas at San Antonio, 1 UTSA Circle, San Antonio TX USA.
Received Date:Febraury 10, 2024; Published Date:March 04, 2024
Abstract
A fundamental feature of the human brain is the substantial inhibition it normally experiences from different sources. One of the most important of these is callosal inhibition, most prominently manifested in the inhibition of the right hemisphere by the left one. Such inhibition may be especially prevalent during the preparation and execution of speech and other motor behavior, which preferentially activate the dopamine-rich left hemisphere of most humans. Release from left-hemispheric inhibition due to damage to the left hemisphere has been shown to lead to compensation by the right-hemisphere in speech, but paradoxical facilitation of the right hemisphere may also occur in many other instances. This review will discuss the basis for the left-hemispheric inhibition and show how disinhibition of the right hemisphere may underlie the emergence of previously latent prosodic and artistic/creative skills in two rare and enigmatic brain syndromes—acquired savantism and foreign accent syndrome.
Keywords:Foreign accent; Savantism; Acquired; Lateralization; Inhibition
Introduction
Remarkable progress has been made in our understanding of the human brain. Prior to 1950, little was known of the major visual pathways of the brain, the origins and nature of cerebral lateralization, the major motivational pathways in the brain, the hippocampal network for creation of new memories, the roles of key neurochemicals such as dopamine, the neural basis of most clinical disorders, etc. But despite all these impressive discoveries, many rare and fascinating syndromes remain mysteries in terms of their neural origins. Two such syndromes—acquired savantism (AS) and foreign accent syndrome (FAS)—will be reviewed in this paper with the purpose of illustrating the importance of callosal inhibition in brain function.
In his famous 1965 paper entitled “Disconnexion Syndromes in Animals and Humans”, Geschwind (1965) illustrated the importance of connecting tracts in the cortex by demonstrating how relatively uncommon and dissimilar disorders such as conduction aphasia, facial apraxia, and alexia without agraphia could be understood in terms of lesions to callosal and other connections between cortical regions. Similarly, AS and FAS were chosen in this paper to highlight the importance of left-hemispheric disinhibition and its release, not because of their overlapping symptoms but because of the evidence that left-hemispheric damage may be associated with the newfound abilities in both syndromes (artistic creativity in AS and unusual prosodic capabilities in FAS). Acquired savantism can occur either suddenly due to traumatic brain injury or more gradually in certain dementias, both of which result in the emergence of latent artistic and creative skills. As will be discussed later, when it is due to unilateral brain damage, typically the left-hemisphere is impaired. Acquired savantism is not to be confused with congenital savantism (CS), which exhibits a different demographic and symptom profile. Foreign accent syndrome, the adoption of a quasi- foreign accent in otherwise normal individuals, is more common than AS and, when it is neurogenic in origin, has been much more convincingly shown to be the product of left-hemispheric (mainly left-frontal) damage.
This review is a narrative rather than a systematic one, for three main reasons. First, the role of left-hemispheric damage in FAS has been the subject of several excellent prior reviews each with over a dozen cases, all consistent with theory presented here. By contrast, aside from a few case reports and a lone statistical study of AS in fronto-temporal dementia (Miller et al., 2000), a search of the literature using a variety of different terms such as “acquired savantism” along with “brain damage”, “brain imaging”, “lateralization”, etc., yielded no other research that could justify a systematic review of left-hemispheric dysfunction and AS. Finally, this narrative review proposes a more comprehensive theory than any to date concerning the mechanisms as to why the left hemisphere exhibits inhibitory superiority and how this inhibition contributes to FAS and AS.
This review will consist of two main parts. First, the nature of left-hemispheric inhibition will be described, focusing on two main mechanisms—callosal inhibition and motoric inhibition—both of which have been studied for decades. Callosal inhibition involves suppressing activity in homologous areas of the opposite hemisphere and has been described by Cook (1984), with some justification, as the “key to the brain code”. Motoric inhibition involves the suppression of competing motor circuitry and the control over sensory processes during the planning and execution of actions. Motoric inhibition can be intra-hemispheric as well as interhemispheric, with the latter being a crucial element of the overall predominance of the left hemisphere in motor behavior (see later section). The review will then proceed to describe some of the essential features of FAS and AS and how they both are consequent to left-hemispheric damage. The original intent of this review was to include another rare brain syndrome known as heautoscopy (the experience of seeing and feeling an image of one’s own body in near-corporeal space), which has often been attributed to left-hemispheric damage and disinhibition of the right-hemisphere’s stored egocentric bodily representations (Anzelotti et al., 2011; Blanke & Mohr, 2005). But a recent review suggests heautoscopy may be more dependent on bilateral damage than either AS or FAS (Blondieux et al., 2021), so it will not be reviewed here.
The Origins and Nature of Left-Hemispheric Inhibition
Neural Inhibition
One of the most remarkable features of the human brain is its efficiency. Despite having massive connectivity and processing on a par with the best supercomputers and possessing capabilities in most intellectual areas superior to the latter, the human brain uses less than the equivalent power of a refrigerator light bulb (10-15W, ~20% of overall body consumption) (Jorgensen, 2022). The reason for this is that most neurons are stimulated only when activated, firing on average only once per second despite a theoretical capability over 100 times more (Lennie, 2003). A large percentage of that energy use comes from excitatory action potentials (Attwell & Iadecola, 2002; Lennie, 2003). Based on estimates derived from single-neuronal sampling, as little as one percent of the human cortex is active at any moment in time, giving rise to such terms as “brain dark matter” and the “dormant brain” (Ovsepian, 2019; Shoham et al., 2006). While some neurons such as vestibular ones may exhibit high baseline firing rates up to 100 Hz (Gittis et al., 2010), as many as 90% may rarely fire at all (Ovsepian, 2019). Even much of the active neuronal outputs may lie outside the realm of conscious awareness, with one widely cited estimate suggesting that as little as 5% of experience is consciously perceived and acted upon (Zaltman, 2003), a view consistent with the latent artistic skills and foreign accents eventually unleashed in AS and FAS.
A major reason why the brain is so silent is because of inhibitory mechanisms. Inhibition has long been viewed as a fundamental feature of the brain (Bari & Robbins, 2013). The balance between excitation and inhibition is largely maintained by neurons using excitatory glutamate (the most common neurotransmitter) and inhibitory gamma aminobutyric acid (GABA, the second-most common neurotransmitter, synthesized from glutamate) (Petroff, 2002). Although glutamatergic neurons outnumber those of GABA, just as the number of excitatory synapses overall exceeds the number of inhibitory ones (especially in the cerebral cortex), the latter play a critical role in key structures such as the basal ganglia and hippocampus (Garret et al., 2018). Well-known neurotransmitters such as dopamine and norepinephrine additionally provide important inhibitory influences in various cortical and subcortical systems (Bari & Robbins, 2003; Cooper et al., 2003; Lorenz et al., 2015).
The predominant form of intra-hemispheric inhibition is arguably that emanating from the prefrontal cortex and basal ganglia (caudate nucleus, putamen, and globus pallidus), all rich in dopamine. Underactivity in the former region may underlie increased impulsivity and disorganized cognitive control in disorders such as attention-deficit/hyperactivity disorder and schizophrenia (Bari & Robbins, 2003). Generally, dorsolateral prefrontal regions—the site of “executive intelligence”—provide the inhibition essential to motor and cognitive control while ventromedial regions appear to be more important in emotional regulation (Demakis, 2003; Dillon & Pizzagalli, 2007). Examples of the dorsolateral prefrontal influence are the inability to inhibit saccades in the anti-saccade task and the inability to switch strategies and avoid perseveration in the Wisconsin Card-Sorting Task after dorsolateral frontal damage (Demakis, 2003). The striatum (caudate and putamen) houses both cognitive and motor functions as part of its role in the +larger cortico- striatal networks and is involved in both initiating and guiding smooth motor responses via inhibitory circuits (Garret et al., 2018). GABA neurons play an important role in inhibiting output of the striatum, as attested to by the aberrant motor and cognitive behavior found in Huntington’s Disease (Garret et al., 2018), but dopaminergic circuits also play an important and largely inhibitory role in the output of the nigrostriatal pathways (Bari & Robbins, 2013; Cooper et al., 2003). By contrast, norepinephrine provides inhibition for sensory and cognitive filtering in prefrontal and other regions of the brain (Bari & Robbins, 2013).
A related concept to neural inhibition is “paradoxical facilitation” (Kapur, 1996). This refers to the increased excitability and output of a region following removal of an area that is presumed to be inhibiting it. Examples of the varied conditions that lead to paradoxical facilitation include the enhanced activity in certain visual regions (e.g., visual cortex) when other structures (e.g., the superior colliculus) are damaged (Sprague, 1966), the greater striatal output and impulsivity during frontal underactivity (Bari & Robbins, 2013), and the unleashing of dopaminergic motor circuits in the basal ganglia after degeneration of GABA spiny neurons (Gar ret et al., 2018). Paradoxical facilitation frequently involves release from interhemispheric inhibition, as during recovery of speech function following unilateral damage (usually left-hemispheric) and improvement of contralateral neglect caused by right-parietal lesions when the left frontal cortex is subsequently damaged (Kapur, 1996). As mentioned previously, paradoxical facilitation in the form of right-hemispheric disinhibition is theorized to underlie the sudden appearance of unusual abilities and accents in AS and FAS, respectively.
Callosal Inhibition
The human brain of most individuals is distinguished by its large degree of hemispheric specialization. Among the many functions differentially represented in the two hemispheres are language, music, mathematics, and emotional behavior (Banic, 2009). In most of these functions, each hemisphere contributes unique specializations (e.g., grammar and speech in left hemisphere; pragmatic aspects of language in right). Of the various anatomical differences between the hemispheres in the adult human brain, those in the parietal-temporal area housing the primary auditory and vestibular cortical representations are among the most salient (Kuo & Massoud, 2022). In the neonatal brain, the most prominent neuroanatomical differences are located in the posterior brain, while frontal speech areas in the inferior frontal lobe are hardly lateralized (Williams et al., 2023; see also Simonds & Scheibel, 1982). The inhibitory actions of the massive body of callosal axons have been viewed as crucial for the establishment of hemispheric differences (Cook, 1984). Electrophysiological evidence has verified the existence of interhemispheric inhibition in human motor cortex following magnetic stimulation of one hemisphere (Ferbert et al., 1992), and such inhibition is reduced after infarcts of the callosum (Li et al., 2013). White-matter imaging techniques such as diffusion-tensor imaging have shown that the volume of the corpus callosum axons in the parietal-temporal region joining together the auditory and vestibular cortical processing centers predicts the amount of language lateralization (Karpychev et al., 2022) and handedness (Kurth et al., 2013).
According to Previc (1991), primordial vestibular and auditory differences at birth cascade into major functional differences in adulthood through inhibitory processes mediated by the corpus callosum. This theory is supported not only by the greater anatomical asymmetries in the posterior neonatal brain but also by the inability to establish normal speech lateralization if deafness is prolonged past the critical period of early childhood (Marcotte & Morere, 1990) and by the relationship between vestibular cortical asymmetry and handedness (Kirsch et al., 2018; Previc, 1991).
The most important evidence for the role of left-hemispheric inhibition in the establishment of cerebral lateralization is provided by studies of callosal agenesis and the right-hemispheric compensation during recovery of linguistic functions following left-hemispheric damage to the adult brain. About one in 4000 individuals are born with complete or partial absence of the corpus callosum, in a condition known as “callosal agenesis” (Brown & Paul, 2019). This condition is caused by neurodevelopmental disruption during the late-embryonic/early-fetal period. There is a general consensus that callosal agenesis leads to reduced interhemispheric inhibition and more bilateral control of speech production as well as other motor behaviors (Bartha-Doering et al., 2021; Hinkley et al., 2012; Komaba et al., 1998; Pelletier et al., 2011), although primordial speech perceptual lateralization already present at birth may be more normal (Lassonde et al., 1998; Pelletier et al., 2011). Callosal agenesis is also associated with overall deficits in speech fluency and fine motor control, as might be expected from the reduced interhemispheric cooperation, but speech receptive skills housed in the posterior-temporal cortex are largely intact (Lassonde et al., 1998; Pelletier et al., 2011). Socio-emotional skills, normally mediated more by the right hemisphere but now crowded out by the enhanced right-hemispheric language processing, are also impaired (Anderson et al., 2017; Brown & Paul, 2019). Even in those born with a normal corpus callosum, the inhibition of the right hemisphere’s speech faculties seems to be more prominent in its motor components, as the right hemisphere continues to maintain a substantial receptive language competence (Gainotti, 1993; Zaidel, 1976), especially as regards the pragmatic features of language (e.g., prosody) (Gajardo-Vidal et al., 2018; Sidtis & Sidtis, 2018; Yi et al., 2019). The inhibition of the right hemisphere increases over time, with its functional capabilities permanently altered after early childhood. Left-sided hemispherectomies to control seizures cause more speech impairment than right ones at any age, but the expressive speech deficits are progressively more pronounced and less easily compensated for with age (Boatman et al., 1999; Gainotti, 1993; Nahum & Liegeois, 2020), suggesting a critical period for the establishment of speech lateralization. However, with more restricted left-hemispheric lesions caused by strokes, the right hemisphere can help compensate for the destruction to the left-hemispheric speech areas even into adulthood (Hartwigsen et al., 2013; Kourtidou et al., 2021; Lukic et al., 2017), despite its largely “mute” contribution to speech in the intact brain (Zaidel, 1976). This enhanced role of the right hemisphere has been shown in the case of naming (Skipper-Kallal et al., 2017) and semantic priming (Smith-Conway et al., 2012)1.
The right hemisphere also inhibits the left hemisphere in certain areas (Kowatari et al., 2009), but its inhibition is not equivalent to that of the left. Most of the more salient symptoms following right-hemispheric lesions are in the form of visuospatial and attentional deficits (anosognosia, neglect, prosopagnosia, etc.) or socio-emotional ones (e.g., proverb interpretation) (Hier et al., 1983; Yi et al., 2019). Right-sided lesions do not appear to unleash dormant visuospatial skills in the left hemisphere nor bestow upon it competent socio-emotional skills; indeed, callosal agenesis severely impairs overall social and emotional perception and expression, with estimates of 18-30% comorbidity with autism in the agenesis population (Anderson et al., 2017). Perilesional activation contributes most of the recovery following right-hemispheric damage, although left-hemispheric compensatory mechanisms are also involved (Hier et al., 1983; Yi et al., 2019). And, while right-hemispheric lesions can unleash various delusions and hallucinations (Cummings, 1997; Gurin & Blum, 2017; Previc, 2006), including out-of-body and other corporeal disturbances, these may be due simply to the reduced right-hemispheric inputs that provide the left hemisphere with veridical information concerning bodily sensations and movements (Gurin & Blum, 2017) rather than a release from interhemispheric inhibition per se. It must be conceded that the extent of right-hemispheric inhibition may be underestimated because of less research into it, given that dramatic symptoms such as aphasia are unlikely to follow right-hemispheric damage. But the fact that the right hemisphere is far less prone to hyper-excitability in the form of seizures (Dean et al., 1997; Previc, 1996; Varoglu, 2022) is a strong indication that, for whatever reason, it is ordinarily under much greater tonic inhibition than its left-hemispheric counterpart.
Why left-hemispheric inhibition may be more powerful than right-hemispheric inhibition may be due to a second major neural feature—the “motor bottleneck”—that is believed to critically involve the left hemisphere and its frontal regions.
Motoric Inhibition
Sensory and motor processing are closely integrated in the human brain. Sensory inputs are critical in modulating the circuitry involved in the execution of motor responses, for providing closedloop feedback following motor actions, and in recovery of function following brain injury (Bolognini et al., 2016; Patel et al., 2014). Motor responses also must constrain the processing of sensory information to allow for optimal motor execution, as will be discussed next. The general influence of motor preparation and responses on sensory processing has been variously termed “motor efference”, “motor expectancies”, “efference copy”, and “reafference” (Brooks & Cullen, 2019). Motor planning and execution are intensely computational (Wolpert and Ghahramani, 2000), which is why dual-task performance suffers from what has been termed the “motor bottleneck” (Bratzke et al., 2009; Jung et al., 2021). Movements must both predict and be confirmed by changes in the sensory environment, so our actions must both filter and incorporate subsequent sensory feedback. Constraints upon sensory information occur during motor actions in a variety of systems, with the higher-brain areas treating sensory information from actively generated movements differently than that from passive movements or when no movements are made (Brooks & Cullen, 2019). Two such examples are in the integration of sensory signals during eye movements and during speech.
When we move our eyes, the world does not appear to blur or shift. The lack of blurring during rapid eye movements is attributable to “saccadic suppression”, the elevation of visual thresholds during a saccade (Matin, 1974). Another blur-prevention mechanism, possibly centrally mediated, is the limited duration of the raw visual image known as the icon, which approximates the duration of each average dwell time (250-300 msec) while scanning the visual environment (Fabius et al., 2019), thereby preventing “double exposures”. The lack of perceived shifting of the visual world during active vs. passive eye movements is related to the concept of “corollary discharge” or reafference (von Holst & Middlestadt, 1950), in which a neural signal is sent to the visual system to move its coordinate frame to the intended location of the eye movement and thereby negate the ensuing movement of the visual world. Many studies have demonstrated corollary discharge in neurons in the occipital-parietal (dorsal) visual stream (Golomb & Mazur, 2021), whose receptive fields shift in concert with the intended saccadic endpoint. The process of reafference also involves shifts in visual attention, whereby information at the once-fixated location is filtered out. Although humans can attend without moving our eyes, attentional shifts are almost invariably coupled to the direction of an intended eye movement (Souto & Kerzel, 2011).
Speech is a complex cybernetic system that requires precise timing of the sensory feedback. During speech preparation, feed-forward signals are relayed that alter responses to auditory stimuli (Mock, et al., 2015; Toyomura et al., 2020). Violations of the expected auditory feedback produces dysfluency in normal speakers, as exemplified by the effects of delayed auditory feedback. At delays of approximately 100-200 ms, speech errors arise that are accompanied by a slowed speech rate (Stuart et al., 2002; Toyomura et al., 2020). A similar increase in musical errors occurs while performing musical sequences with altered pitch feedback (Pfordresher & Beasley, 2014). A disrupted speech cybernetic system has been hypothesized to partly underlie the disorder of stuttering, in which overcompensation and blocks can occur for various reasons, including psychological stress. Feed-forward mechanisms may be altered to some extent since those who stutter show more of a negative shift in the N200 component of the auditory event-related potential during speech preparation (Chang et al., 2019; Mock et al., 2015). The normal left-hemispheric dominance in speech is reduced in both anterior and posterior speech areas in stutterers (Chang et al., 2019; Fox et al., 1996; Liotti et al, 2010; Robb et al., 2013), and the normally greater volume of the left planum temporal containing the receptive centers for speech is less pronounced in this population (Foundas et al., 2004). This indicates that the inability to inhibit speech preparation and processing in the right hemisphere may contribute to the dysfluent speech. In contrast to normal speakers, stutterers actually benefit from delays and other alterations in the timing and pitch of the vocal feedback (Howell, 2004). Interestingly, singing—more likely to be housed in the right hemisphere of most individuals—may be preserved in stutterers (Glover et al., 1996).
The stuttering findings as well as those from callosal agenesis and lesion studies suggest that the regulation of both sensory feedback as well as competing motor responses may be important consequences of the left hemisphere’s inhibitory prowess. The greater inhibitory strength of the left hemisphere may be partly related to its well-documented greater dopamine content (Glick et al., 1982; Larisch et al., 1998; Previc, 1996), since dopamine is critically involved in motor control and is mostly inhibitory in its actions in the nigrostriatal/cortical system, as discussed previously (Cooper et al., 2003). This is particularly true during speech, where dopaminergic circuits drive left-hemispheric activity while speaking (Fuertinger et al., 2018). The greater dopamine content is correlated with overall dominance of the left hemisphere in most voluntary motor actions, as first described by Leipmann in the early 1900’s (Janssen et al., 2011; Kimura & Archibald, 1974). Besides the greater role of the left hemisphere in speech, the left hemisphere has been long recognized for its lead in the programming and execution of manual actions (Janssen et al., 2011; Hodson & Hudson, 2018; Mutha et al., 2012), with lesions to the left parietal lobe in particular producing various types of apraxias (Goldenberg, 2009; Janssen et al., 2011; Kimura & Archibald, 1974). Although speech and handedness are not highly correlated (Mazoyer et al., 2014), they both require elaborate motor-sequencing skills (Hodson & Hudson, 2018) and motor learning, which most reliably activate the left dorsal prefrontal cortex (Hardwick et al., 2013) and also depend on dopaminergic neuronal activity in the basal ganglia (Alm, 2021). It is less likely that GABA underlies the greater left-hemispheric inhibition, since it is much less lateralized than dopamine (Grewal et al., 2016). And acetylcholine, which like dopamine is involved in motor control and has a higher concentration in the left hemisphere (Glick et al., 1982), seems unlikely to mediate left-hemispheric inhibition of the right hemisphere because its actions in the CNS are mostly excitatory (Wang et al., 2021).
One brain imaging study has localized the source of the motor bottleneck during dual-task performance to the posterior lateral prefrontal cortex of the left hemisphere (Dux et al., 2006), with inhibition of this area by means of transcranial direct (cathodal) stimulation resulting in improved dual-task performance (Filmer et al., 2013). Lesion findings also support an important but nonexclusive role of the left hemisphere in inhibiting responses in the “no-go” task (Swick et al., 2008). Evidence for how the removal of left-frontal regions involved in motor control and inhibition may unshackle latent abilities in AS and FAS will be described in the next sections.
Two Rare Brain Syndromes Associated with Left Hemispheric Damage
Acquired savantism and FAS have four major features in common: 1) their presumed rarity (<50 cases of AS; 150-200 documented FAS cases); 2) they feature the emergence of a previously latent talent or ability; 3) they may be caused by both psychogenic and neurogenic factors, with the latter including migraine, epilepsy, or lesions to certain brain areas; and 4) when lesions are causal, they are predominantly found in the left hemisphere. Although similar frontal structures may be involved in both syndromes, there does not appear to be a case of co-occurrence of them, as will be discussed later. Because outside of linguistic functions the natural ratio of left-right (right-left) specializations has been hypothesized as closer to ~2:1 (Previc, 1991), evidence of right-hemispheric facilitation in the emergence of AS is somewhat more tenuous than in the case of FAS.
Acquired Savantism
Acquired savantism due to deterioration of the brain in frontotemporal dementia (FTD) or due to trauma is a condition typically developing in adulthood that involves the unleashing of previously suppressed creative abilities, usually artistic or musical (Snyder, 2009; Treffert, 2009). Not all cases of AS involve actual brain damage, as some may be linked to a psychogenic origin (termed “sudden savantism” by Treffert & Tries, 2021) or may even be drug-induced (Smith, 2015). Acquired savantism differs from normal genius in that the latter is not associated with any known neuroanatomical pathology, and it also differs from congenital savantism (CS) in several important ways, despite their frequent association (e.g., Chung & Son, 2023; Onin et al., 2023; Snyder, et al., 2006; Treffert, 2009). Unlike AS, CS is linked to autism in 50-75% of cases and has a similarly high male prevalence of 5-6:1 (Treffert, 2009; Treffert & Rebedew, 2015). (By contrast, the prevalence of CS in the much more common disorder of autism is probably in the range of 1-10% (Treffert, 2009).) It is also much more common than AS, with over 300 cases listed in the Wisconsin Medical Society registry as of 2015, with some studies suggesting a much higher number (Treffert & Rebedew, 2015). As with high-functioning autism, CS suggests a predominance of “left” over right” hemispheric abilities, including hyper-focusing, predominance of “local” perception, deficient pragmatic use of language, and overall impairment of socio-emotional skills (Dolata et al., 2022; Gunter et al., 2002; Sabbagh, 1999; Volden et al., 2009). Congenital savants can possess prodigious sensory memories, extraordinary calculating skills often dependent on such memories, and impressive abilities to recognize and reproduce patterns (Mottron et al., 2009). But while a few congenital savants, particularly higher-functioning ones, evidence clear artistic creativity (e.g., Chung & Son, 2023), in most cases artistic output in CS is constrained and distinguishable from that of actual artists (Pring et al., 2012). The scans of many CS brains have been studied, but the neuroanatomical profiles are generally inconsistent (Corrigan et al., 2012), and some show no neural abnormalities at all (Corrigan et al., 2012; Cowan & Frith, 2009). Calendrical calculating skills, a noted feature of many savants, appears to show a mostly left-hemispheric or bilateral parietal activation (Boddaert et al., 2005; Crown & Frith, 2009). The generally inconclusive neuroanatomical findings in CS mirror the large body of neuroanatomical findings in comorbid autism, which has proven inconsistent both overall and in terms of lateralization (Amaral et al., 2008; Jumah et al., 2016). There has consequently been growing interest in neurochemical imbalances in autism, most prominently involving dopamine (Mandic- Maravic et al., 2021; Paval, 2017; Previc, 2007), and any neurochemical conclusions eventually reached in autism can presumably be applied to highly comorbid CS. In contrast to CS, the acquired syndrome is much rarer (Treffert & Treis, 2021) and apparently unrelated to pre-morbid intellectual impairment, autistic symptoms, or biological sex. And whereas most CS traits and abilities suggest greater activation of the left hemisphere, the newfound talents in AS are more attributable to left-hemispheric damage (Hughes, 2010; Onin et al., 2023; Snyder, 2009). For example, the unleashed talents in AS, whether resulting from brain trauma or FTD, are mostly “right-hemispheric” creative ones, involving artistic, musical, or constructive skills (Miller, 2000; Treffert, 2014)2. However, as of 2015 there were only approximately 30 known cases of AS (Treffert & Rebedew, 2015), and virtually no brain imaging of trauma- linked AS has ever been detailed in the scientific case literature.
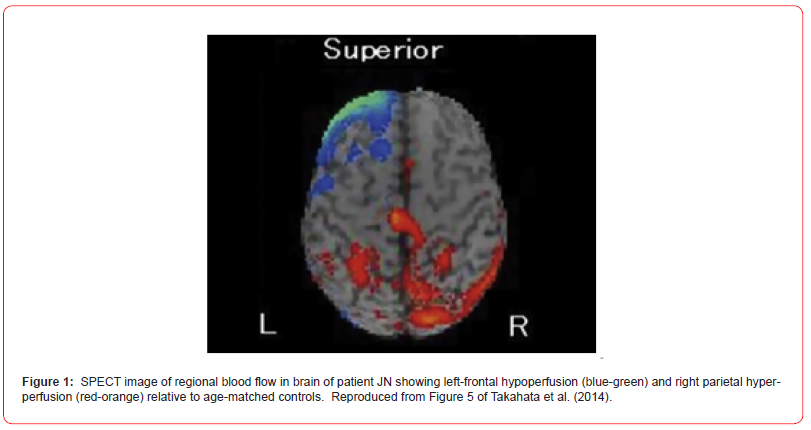
Some of the most famous cases of AS caused by brain trauma in childhood or adulthood, such as Jason Padgett (artistic skills), Derek Amato (musical skills), and Alonzo Clements (sculpting skills), have evidently not had their neuroanatomical damage reported in the scientific literature. There are at least three trauma-related AS cases with confirmed lateralized brain damage, all involving the left hemisphere. One of these was a “Mr. Z” (Brink, 1980), who suffered a bullet wound that entered through his left temple at age nine. His injury led to speech deficits and a right hemiparesis lasting several years along with impressive new mechanical and inventive skills, particularly regarding bicycles (Brink, 1980). A second case was of a 64-year-old right-handed Japanese man (JN) (Takahata et al., 2014), who suffered an infarction of the left frontal lobe that led to mostly verbal deficits. Although JN had sparsely painted ten years prior to his stroke, the ones he created afterwards were much more vivid and full of detail and color. What is relevant about JN’s case is that the frontal damage was accompanied by a hyper-perfusion of his posterior (sensory) right hemisphere (Figure 1), in line with both the paradoxical facilitation and motor inhibition concepts and evidence that left-frontal lesions can boost right parietal function in alleviating contralateral neglect (Kapur, 1966). A third case studied by Dorman (1991) was an 18-year-old man (RD) who had a left-hemispherectomy at age eight to control seizures. This individual had a low-normal verbal and performance IQ but showed an impressive post-surgery calendar-calculating ability, ordinarily more associated with left-hemispheric function. Because his handedness was not specified and there was presumed neurological damage prior to his hemispherectomy, his case is difficult to interpret within the context of the right-hemispheric disinhibition model (Figure 1).
The most convincing evidence for reduced left-hemispheric function as a cause of AS comes from studies of those with frontotemporal dementia (FTD) (Erkkinen et al. 2018; Midorikawa et al., 2008; Miller et al., 2000). Frontotemporal dementia accounts for 10-20% of all dementias and often begins its progression with left-hemispheric deterioration (Jeong et al., 2005), with aphasic symptoms being common. Miller et al (2000) studied 57 patients with FTD and isolated 12 who showed previously unrevealed artistic, musical, or inventive prowess during the course of their dementia. Nine of the 12 with AS were shown to have greater left-hemispheric deterioration, especially in the anterior temporal regions; one showed bilateral atrophy and two had greater right frontotemporal deterioration (although one of these was left-handed, along with three patients with left-sided brain loss). Two FTD cases described by Midorikawa et al., (2008) also showed newfound artistic proclivities associated with primarily left-temporal deterioration, while Erkkinen et al. (2018) described another FTD case with AS after right frontotemporal deterioration, but this individual was left-handed. A famous example of enhanced creativity following left FTD with concomitant aphasia is that of the composer Maurice Ravel, who suffered from what currently might be diagnosed as progressive aphasia and FTD, progressively affecting his left hemisphere (Amaducci et al., 2002). It was during this final period of mental decline that he wrote his arguably most famous and creative piece, Bolero. Ironically, Seeley et al. (2008) described a left FTD patient who developed a painting talent that included visual representations of Bolero. The importance of the left hemisphere in inhibiting certain types of creativity such as divergent thinking and originality is reinforced by findings of creativity changes following lateralized frontal-parietal lesions (Shamay-Tsoory et al. 2011). Whereas lesions of the right medial-frontal cortex in that study reduced originality, lesions of the left parietal lobe resulted in enhanced originality scores (Shamay-Tsoory et al. 2011). It is uncertain, however, how closely the measures of originality in this study simulated the unlocked artistic creativity in AS. There have been multiple attempts to produce various savant-type abilities using noninvasive left-hemispheric procedures that inhibit activity in the underling cortex. Snyder et al. (2006) showed significant improvements in numerosity estimations in most of their 12 participants after transcranial magnetic stimulation (TMS), and Snyder et al. (2003) showed significant improvement in a range of skills, including proof-reading, in a separate population of 11 participants. But these studies did not use an active control stimulation site, either over the corresponding right hemisphere or a neutral location, and only a few of the participants showed improvements in skills analogous to the artistic creativity most characteristic of AS in FTD patients. Young et al. (2004) showed mostly nonsignificant improvements in savant-type skills with TMS at a left frontotemporal site; only five of 17 participants showed improvements overall, and there were few differences between the effects of TMS applied to the frontotemporal and control sites. Using transcranial direct current stimulation with both cathodal (inhibitory) and anodal (excitatory) procedures, Chi et al. (2010) demonstrated that inhibition of the left hemisphere (or excitation of the right) considerably improved visual memory and visual numerosity judgments, whereas the reverse stimulation pattern had no effect on visual memory. Again, however, the significance of this finding is unclear, since the emergence of superior visual memory is not one of the prominent abilities normally associated with AS, although enhanced visual numerosity was described in Takahata’s artistic patient following his left-frontal stroke.
Brain imaging studies in normals also shed light on creativity in the two hemispheres. Creativity is a very multi-faceted construct, given that it involves different types of products (e.g., finding solutions, creating novel outputs) and different modalities (e.g., verbal vs. visual). A consistent finding of these studies is that creative processes are generally bilateral, with verbal measures of creativity such as verbal fluency more likely to recruit left hemispheric areas and figural/artistic creativity more likely to involve the right hemisphere (Huang et al., 2013; Mayseless et al., 2014; Pidgeon et al., 2016; but see Mihov et al., 2010). Many creativity-linked activations have been identified within each hemisphere, most consistently in the middle and inferior frontal gyri. One noteworthy finding pertinent to the AS syndrome is that artists may show more of a right-hemispheric predominance in figural creativity than novices, with left-hemispheric inhibition contributing to the reduced right-hemispheric involvement in the latter group (Huang et al., 2013; Kowatari et al. 2009). That interhemispheric inhibition is linked to reduced creativity in humans is demonstrated by the significant negative correlation of callosal thickness and creativity, particularly in the most posterior section joining together the sensory cortical areas (Moore et al., 2009). This relationship is opposite to the positive correlations between posterior callosal thickness and left-hemispheric speech (Karpychev et al., 2022) and motoric (Kurth et al., 201) dominance, which as noted earlier are other manifestations of the left hemisphere’s inhibition of the right one.
The unleashing of artistic and musical talents in AS exemplifies motoric as well as creative disinhibition, since a facilitation of motoric proficiency as well as sensory/perceptual processing occurs in AS. Some of emergent motor skills such as painting and instrument playing may rarely if ever have been practiced by the person in the past; hence, one must assume that they developed covertly, given that passive auditory and other sensory experiences can help establish motor circuitry even in the absence of actual practice (Froese & Gonzalez-Grandon, 2020).
In summary, AS appears to be a distinct form of savantism, apart from CS. Outside of its occurrence in FTD, however, much less is known about AS since neuroimaging of AS brains is lacking. Analogue studies using transcranial and direct current studies in normal humans have produced somewhat inconclusive results, using measures that may not be highly analogous to the newfound artistic skills in AS. But evidence that right-hemispheric artistic creativity may be inhibited in normals is consistent with the FTD findings and merits further research.
Foreign Accent Syndrome
Foreign accent syndrome is a condition in which an individual may suddenly start speaking his or her native language with an accent that resembles, at least superficially, that of a nonnative speaker. It is much more common in females (>5:1) and can arise from both neurological damage as well as psychogenic sources, with the latter estimated at 15-25% (Mariën et al., 2019; McWhiter et al., 2019). Alterations in the prosodic and rhythmic elements of speech are the key features that give rise to the new accents. However, FAS is very complicated in that it can be transient or intermittent and can be intermixed with a variety of dysarthric and aphasic speech disturbances related to phoneme production, grammar, and programming (Lee et al., 2016; McWhirter et al., 2019). The accents of those presenting with FAS tend to be judged as intermediate between those of native and true foreign speakers (Verhoeven et al., 2013; but see Jose et al., 2016) and can even be perceived as more than one accent (Blumstein & Kurowski, 2006). It should be noted that foreign-sounding accents can occur in other conditions, such as developmental speech and hearing impairment (Marien et al., 2009); indeed, certain features of accents in FAS are intermediate between true foreign accents and accents associated with language impairment (Jose et al., 2016).
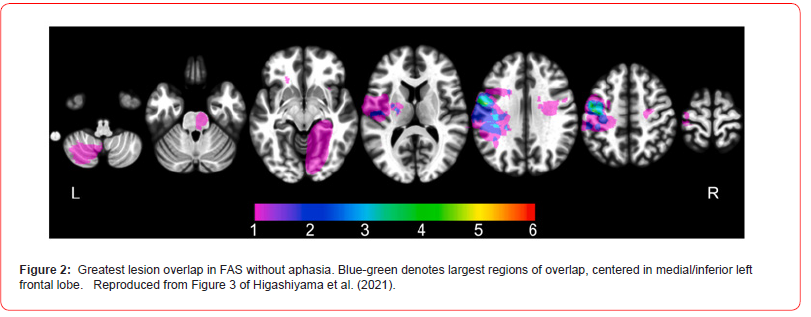
It is widely accepted that the neural basis of FAS mainly involves damage to supratentorial structures of the left hemisphere (Blumstein & Kurowski, 2006; Di Stefano et al., 2019; Jonkers et al., Higashiyama et al., 2021; Marien et al., 2019). Of the 70% or more of FAS lesions localized to the left hemisphere, the majority were in the frontal cortex and basal ganglia, both linked to the motoric aspects of speech (Marien et al., 2019). Although the handedness of most of these cases was rarely reported, Mariens et al. (2019) did record handedness in their population of 72 FAS cases and found 96% to be right-handed, suggesting most had the typical pattern of cerebral lateralization prior to the onset of their FAS. While aphasia and dysarthria can accompany FAS acutely, FAS it is distinct from aphasia per se (Blumstein & Kurowski, 2006). Indeed, Higashiyama et al. (2021) reviewed 25 cases of FAS without aphasia, of which 18 involved lesions to the left hemisphere, three to the right one, and four in subcortical areas. The greatest overlap of lesions causing FAS was located in the middle and lower left precentral gyrus in the left hemisphere (see Figure 2). This region is involved in laryngeal control and phonation, which are especially relevant to FAS given that the fundamental vocal frequency is an important cue to both prosody (Laures & Bunton, 2003), speaker accent (Hasegawa & Hata, 1992), and linguistic discrimination in general (Arvanti & Rodriguez, 2013). In a more recent study, Dadario et al. (2023) showed that, even when anatomically intact, the middle prefrontal area on the left side may be one of the most likely to have anomalous connectivity with other language and brain areas (Figure 2).
Although the right hemisphere is considered more crucial in the generation of prosody in normal speakers (Stockbridge, 2022), its role is in this regard is likely constrained by the overall predominance of the left hemisphere in speech. Hence, one likely explanation of FAS is that left-hemispheric damage disinhibits latent prosodic circuits in homologous regions of the right hemisphere. Because FAS usually involves speech that is somewhat familiar to speakers through everyday listening or electronic media, the foreign speech sounds may have in many cases been absorbed during passive listening but not reproduced previously due to the left-hemispheric inhibition of the right hemisphere’s spoken repertoire. For example, Verhoeven et al. (2013) showed that the accents mainly produced in their Belgian Flemish-speaking FAS patients were French (one of the official languages of Belgium) and Moroccan (which is heard quite frequently in parts of Belgium). Seliger et al. (1992) studied a New Yorker who developed an Irish brogue following a left-hemispheric stroke, but the patient had previous experience listening to Irish accents. By contrast, less frequently heard Asian and African accents were rarely produced in Verhoeven et al.’s 2013 study. That we can passively process and store much more than we can produce is consonant with the general concept of the motor bottleneck described earlier. It is also consistent with the large sex difference in FAS, because females tend generally to be more receptive auditorily, whether it be learning new melodic patterns (Miles et al., 2016) or second languages (van der Slik et al., 2015). By contrast, theories that the FAS may be tied to a failure to transfer planning of utterances into proper articulation (e.g., Marien et al., 2019) do not explain the large sex difference nor the relationship between frequently heard foreign accents and the quasi-replicated foreign accents. Of further potential relevance is that singing—a function already predominant in the right hemisphere—may not exhibit the same foreign accent alteration as speech in FAS (Di Stefano et al., 2019).
Storing auditory sounds and sequences is not the same as being able to produce them. But producing as well as perceiving vocal inflections during speech and singing is a specialization of the right hemisphere, which would be facilitated after left-hemispheric damage. As noted with AS, right-hemispheric sensory facilitation, even by means of passive listening, can in turn activate dormant ipsilateral motor circuitry (Froese & Gonzales-Grandon, 2020). It might be predicted that FAS and AS should be present together given their apparent common neural locus, at least in some cases, in the medial frontal lobe. But even though aphasia, which also partly originates in the left inferior frontal lobe, accompanies AS in FTD, FAS frequently occurs even in the absence of aphasia. Of course, the rarity of each of these syndromes alone makes in highly unlikely they would present together. However, it is interesting that Mr. Z not only developed AS after his bullet injury to the left hemisphere but also apparently spoke in a different dialect (Brink, 1980).
Conclusion
The evidence presented in this review confirms that AS and FAS are both manifestations of a disinhibition of right-hemispheric processing following damage to or deterioration of certain regions of the left hemisphere. Following left-hemispheric damage, facilitation of the right hemisphere and its creative and receptive abilities may occur. The evidence for such a facilitation is stronger in the case of FAS, which is linked more to the highly lateralized language system, but it is also to be found in AS, with the most consistent findings coming from patients suffering from FTD. Linking these syndromes to well-established callosal inhibitory mechanisms in the human brain both highlights the importance of such inhibition to overall brain function (Cook, 1984) and helps to propel these syndromes towards a better scientific understanding. It must be reiterated that the postulation of left-hemispheric inhibition of the right can only be applied to those individuals with a typical pattern of hemispheric specialization, more likely to be strongly right-handed with expressive language presiding in the left hemisphere. That is why, in the future, all studies of lateralization in AS must measure handedness in their patients. Most cases of AS and FAS have not only occurred following left-hemispheric impairment but more typically involve disturbances to the frontal lobe, particularly the middle-inferior prefrontal region. Evidence exists that this region may be a potential source of the “motor bottleneck” and the callosal fibers inhibiting the right hemisphere in AS and FAS. But, unlike in FAS, temporal lobe damage is also prevalent in AS, especially in FTD. While left-hemispheric inhibition has been metaphorically decried as a “tyranny” by some researchers (Hughes, 2010; Onin et al., 2023), preventing the emergence of right-hemispheric talents, its advantages in terms of streamlined motor control and specialization of function must also be recognized. And the larger neural inhibitory apparatus that includes callosal and motoric inhibition is critical for the human brain to conduct its massive operations using but a tiny percentage of its total potential energy at a given moment.
Declaration
The author has no competing interests to declare that are relevant to the content of this article. No funding was received to assist with the preparation of this manuscript.
Acknowledgement
The author acknowledges the important lifelong contributions of Dr. Darold Treffert to the understanding of savantism, especially acquired savantism.
Conflict of Interest
No Conflict of interest.
References
- Alm PA (2021) The dopamine system and automatization of movement sequences: A review with relevance for speech and stuttering. Frontiers in Human Neuroscience 15: 661880.
- Amaducci L, Grassi E, Boller F (2002) Maurice Ravel and right-hemisphere musical creativity: Influence of disease on his last musical works. European Journal of Neurology 9(1): 75-82.
- Amaral DG, Schumann CM, Nordahl CW (2008) Neuroanatomy of autism. Trends in Neurosciences 31(3): 137-145.
- Anderson LB, Paul LK, Brown WS (2017) Emotional intelligence in agenesis of the corpus callosum. Archives of Clinical Neuropsychology: The Official Journal of the National Academy of Neuropsychologists 32(3): 267-279.
- Anzellotti F, Onofrj V, Maruotti V, Ricciardi L, Franciotti R, et al. (2011) Autoscopic phenomena: Case report and review of literature. Behavioral and Brain functions: BBF 7(1): 2.
- Arvaniti A, Rodriquez T (2013) The role of rhythm class, speaking rate, and F0 in language discrimination. Laboratory Physiology 4(1): 7-38.
- Attwell D, Iadecola C (2002) The neural basis of functional brain imaging signals. Trends in Neurosciences 25(12): 621-625.
- Banich MT (2009) Hemispheric specialization and cognition. Encyclopedia of Neuroscience 1081-1086.
- Bari A, Robbins TW (2013) Inhibition and impulsivity: Behavioural and neural basis of response control. Progress in Neurobiology 108: 44-79.
- Bartha Doering L, Schwartz E, Kollndorfer K, Fisch meister FPS, Novak A, et al. (2021) Effect of corpus callosum agenesis on the language network in children and adolescents. Brain Structure & Function 226(3): 701-713.
- Blanke O, Mohr C (2005) Out-of-body experience, hepatoscopy, and autoscopic hallucination of neurological origin Implications for neurocognitive mechanisms of corporeal awareness and self-consciousness. Brain Research. Brain Research Reviews 50(1): 184-199.
- Blondiaux E, Heydrich L, Blanke O (2021) Common and distinct brain networks of autoscopic phenomena. Neuroimage Clinical 30:102612.
- Blumstein SE, Kurowski K (2006) The foreign accent syndrome: A perspective. Journal of Neurolinguistics 19(5): 346-355.
- Boatman D, Freeman J, Vining E, Pulsifer M, Miglioretti D, et al. (1999) Language recovery after left hemispherectomy in children with late-onset seizures. Annals of Neurology 46(4): 579-586.
- Boddaert N, Barthelemy C, Poline JB, Samson Y, Brunelle F, et al. (2005) Autism: Functional brain mapping of exceptional calendar capacity. The British Journal of Psychiatry 187: 83-86.
- Bolognini N, Russo C, Edwards DJ (2016) The sensory side of post-stroke motor rehabilitation. Restorative Neurology and Neuroscience 34(4): 571-586.
- Bratzke D, Rolke B, Ulrich R (2009) The source of execution-related dual-task interference: Motor bottleneck or response monitoring. Journal of Experimental Psychology Human Perception and Performance 35(5): 1413-1426.
- Brink TL (1980) Idiot savant with unusual mechanical ability: An organic explanation. The American journal of psychiatry 137(2): 250-251.
- Brooks JX, Cullen KE (2019) Predictive sensing: The role of motor signals in sensory processing. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging 4(9): 842-850.
- Brown WS, Paul LK (2019) The neuropsychological syndrome of agenesis of the corpus callosum. Journal of the International Neuropsychological Society: JINS 25(3): 324-330.
- Chang SE, Garnett EO, Etchell A, Chow HM (2019) Functional and neuroanatomical bases of developmental stuttering: Current insights. The Neuroscientist: A Review journal Bringing Neurobiology Neurology and Psychiatry 25(6): 566-582.
- Chi RP, Fregni F, Snyder AW (2010) Visual memory improved by non-invasive brain stimulation. Brain Research 1353: 168-175.
- Chung S, Son JW (2023) How well do we understand autistic savant artists: A review of various hypotheses and research findings to date. Journal of Child & Adolescent Psychiatry 34(2): 93-111.
- Cook ND (1984) Callosal inhibition: The key to the brain code. Behavioural Science 29: 98-110.
- Cooper JR, Bloom FE, Roth RH (2003) The biochemical basis of neuropharmacology (8th Ed.). New York: Oxford.
- Corrigan NM, Richards TL, Treffert DA, Dager SR (2012) Toward a better understanding of the savant brain. Comprehensive Psychiatry 53(6): 706-717.
- Cowan R, Frith C (2009) Do calendrical savants use calculation to answer date questions? A functional magnetic resonance imaging study. Philosophical transactions of the Royal Society of London. Series B Biological sciences 364(1522): 1417-1424.
- Cummings JL (1997) Neuropsychiatric manifestations of right hemisphere lesions. Brain and Language 57:22-37
- Dadario NB, Piper K, Young IM, Sherman JH, Sughrue ME (2023) Functional connectivity reveals different brain networks underlying the idiopathic foreign accent syndrome. Neurological Sciences 44(9): 3087-3097.
- Dean AC, Solomon G, Harden C, Papakostas G, Labar DR (1997) Left hemispheric dominance of epileptiform discharges. Epilepsia 38(4): 503-505
- Demakis GJ (2003) A meta-analytic review of the sensitivity of the Wisconsin Card Sorting Test to frontal and lateralized frontal brain damage. Neuropsychology 17(2): 255-264.
- Di Stefano V, De Novellis AMP, Dono F, Onofrj M, De Angelis MV (2019) "Accent issue": Foreign accent syndrome following ischemic stroke. Neurological Sciences: Official Journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 40(11): 2391-2397.
- Dillon DG, Pizzagalli DA (2007) Inhibition of action, thought, and emotion: A selective neurobiological review. Applied & Preventive Psychology: Journal of the American Association of Applied and Preventive Psychology 12(3):99-114.
- Dolata JK, Suarez S, Calame B, Fombonne E (2022) Pragmatic language markers of autism diagnosis and severity. Research in Autism Spectrum Disorders 94: 101970.
- Dorman C (1991) Exceptional calendar calculation ability after early left hemispherectomy. Brain and cognition 15(1): 26-36.
- Dux PE, Ivanoff J, Asplund CL, Marois R (2006) Isolation of a central bottleneck of information processing with time-resolved FMRI. Neuron 52(6): 1109-1120.
- Erkkinen MG, Zuniga RG, Pardo CC, Miller BL, Miller ZA (2018) Artistic renaissance in frontotemporal dementia. JAMA 319(13): 1304-1306.
- Fabius JH, Fracasso A, Nijboer TCW, Van der Stigchel S (2019) Time course of spatiotopic updating across saccades. Proceedings of the National Academy of Sciences of the United States of America 116(6): 2027-2032.
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, et al. (1992) Interhemispheric inhibition of the human motor cortex. The Journal of physiology 453: 525-546.
- Filmer HL, Mattingley JB, Dux PE (2013) Improved multitasking following prefrontal tDCS. Cortex; A Journal Devoted to the Study of the Nervous System and Behaviour 49(10): 2845-2852.
- Foundas AL, Bollich AM, Feldman J, Corey DM, Hurley M, et al. (2004) Aberrant auditory processing and atypical planum temporal in developmental stuttering. Neurology 63(9): 1640-1646.
- Fox PT, Ingham RJ, Ingham JC, Hirsch TB, Downs JH, et al. (1996) A PET study of the neural systems of stuttering. Nature 382(6587):158-161.
- Froese T, Gonzalez Grandon XH (2020) How passive is passive listening. Phenomenology and the Cognitive Sciences 19:619-651.
- Fuertinger S, Zinn JC, SharanAD, Hamzei Sichani F, Simonyan K (2018) Dopamine drives left-hemispheric lateralization of neural networks during human speech. The Journal of Comparative Neurology 526(5): 920-931
- Gainotti G (1993) The riddle of the right hemisphere's contribution to the recovery of language. European Journal of Disorders of Communication 28(3): 227-246.
- Gajardo Vidal A, Lorca Puls DL, Hope TMH, Parker Jones O, Seghier ML, et al. (2018). How right hemisphere damage after stroke can impair speech comprehension. Brain: A Journal of Neurology 141(12): 3389-3404.
- Garret M, Du Z, Chazalon M, Cho YH, Baufreton J (2018) Alteration of Gabaergic neurotransmission in Huntington's disease. CNS Neuroscience & Therapeutics 24(4): 292-300.
- Geschwind N (1965) Di connexion syndromes in animals and man. I Brain 88(2): 237-294.
- Gittis AH, Moghadam SH, Du Lac S (2010) Mechanisms of sustained high firing rates in two classes of vestibular nucleus neurons: differential contributions of resurgent Na, Kv3, and BK currents. Journal of Neurophysiology 104(3): 1625-1634.
- Glick SD, Ross DA, Hough LB (1982) Lateral asymmetry of neurotransmitters in human brain. Brain Research 234(1): 53-63.
- Glover H, Kalinowski J, Rastatter M, Stuart A (1996) Effect of instruction to sing on stuttering frequency at normal and fast rates. Perceptual and Motor Skills 83(2):511-522.
- Goldenberg G (2009) Apraxia and the parietal lobes. Neuropsychologic 47(6): 1449-1459.
- Golomb JD, Mazer JA (2021) Visual remapping. Annual Review of Vision Science 7: 257-277.
- Grewal M, Dabas A, Saharan S, Barker PB, Edden RA, et al. (2016) GABA quantitation using MEGA-PRESS: Regional and hemispheric differences. Journal of Magnetic Resonance Imaging: JMRI 44(6): 1619-1623.
- Gunter HL, Ghaziuddin M, Ellis HD (2002) Asperger syndrome: Tests of right hemisphere functioning and interhemispheric communication. Journal of Autism and Developmental Disorders 32(4): 263-281.
- Gurin L, Blum S (2017) Delusions and the right hemisphere: A review of the case for the right hemisphere as a mediator of reality-based belief. The Journal of Neuropsychiatry and Clinical Neurosciences 29(3): 225-235.
- Hardwick RM, Rottschy C, Miall RC, Eickhoff SB (2013) A quantitative meta-analysis and review of motor learning in the human brain. Neuro Image 67: 283-297.
- Hartwigsen G, Saur D, Price CJ, Ulmer S, Baumgaertner A, et al. (2013) Perturbation of the left inferior frontal gyrus triggers adaptive plasticity in the right homologous area during speech production. Proceedings of the National Academy of Sciences of the United States of America 110(41): 16402-16407.
- Hasegawa Y, Hata K (1992) Fundamental frequency as an acoustic cue to accent perception. Language and Speech 35 (1-2): 87-98.
- Hier DB, Mondlock J, Caplan LR (1983) Recovery of behavioral abnormalities after right hemisphere stroke. Neurology 33(3): 345-350.
- Higashiyama Y, Hamada T, Saito A, Morihara K, Okamoto M, et al. (2021) Neural mechanisms of foreign accent syndrome: Lesion and network analysis. Neuro Image Clinical 31: 102760
- Hinkley LB, Marco EJ, Findlay AM, Honma S, Jeremy RJ, et al. (2012) The role of corpus callosum development in functional connectivity and cognitive processing. PloS One 7(8): e39804.
- Hodgson JC, Hudson JM (2018) Speech lateralization and motor control. Progress in Brain Research 238: 145-178.
- Howell P (2004) Effects of delayed auditory feedback and frequency-shifted feedback on speech control and some potentials for future development of prosthetic aids for stammering. Stammering Research: An On-line Journal Published by the British Stammering Association 1(1): 31-46.
- Huang P, Qiu L, Shen L, Zhang Y, Song Z, et al. (2013) Evidence for a left-over-right inhibitory mechanism during figural creative thinking in healthy nonartists. Human Brain Mapping 34(10): 2724-2732.
- Hughes JR (2010) A review of Savant Syndrome and its possible relationship to epilepsy. Epilepsy & Behavior: E&B, 17(2): 147-152.
- Janssen L, Meulenbroek RG, Steenbergen B (2011) Behavioral evidence for left-hemisphere specialization of motor planning. Experimental Brain Research 209(1): 65-72.
- Jeong Y, Cho SS, Park JM, Kang SJ, Lee JS, et al. (2005) 18F-FDG PET findings in frontotemporal dementia: An SPM analysis of 29 patients. Journal of Nuclear Medicine: Official Publication Society of Nuclear Medicine 46(2): 233-239.
- Jonkers R, van der Scheer F, Gilbers D (2017) The common denominator in the perception of accents in cases with foreign accent syndrome. Aphasiology 31(9): 1021-1043.
- Jorgensen TJ (2022) Is the human brain a biological computer. Princeton University Press.
- Jose L, Read J, Miller N (2016) Is language a factor in the perception of foreign accent syndrome. Language and Speech 59(2): 219-235.
- Jumah F, Ghannam M, Jaber M, Adeeb N, Tubbs RS (2016) Neuroanatomical variation in autism spectrum disorder: A comprehensive review. Clinical Anatomy (New York, N.Y.) 29(4): 454-465.
- Jung KH, Martin T, Ruthruff E (2021) Electrophysiological examination of response-related interference while dual-tasking: Is it motoric or attentional? Psychological Research 85(2): 660-678.
- Kapur N (1996) Paradoxical functional facilitation in brain-behaviour research. A critical review. Brain: A journal of neurology 119(5): 1775-1790.
- Karpychev V, Bolgina T, Malytina S, Zinchenko V, Ushakov V, et al. (2022) Greater volumes of a callosal sub-region terminating in posterior language-related areas predict a stronger degree of language lateralization: A tractography study. PloS One 17(12): e0276721.
- Kimura D, Archibald Y (1974) Motor functions of the left hemisphere. Brain: A Journal of Neurology 97(2): 337-350.
- Kirsch V, Boegle R, Keeser D, Kierig E, Ertl-Wagner B, et al. (2018) Handedness-dependent functional organizational patterns within the bilateral vestibular cortical network revealed by fMRI connectivity based parcellation. NeuroImage 178: 224-237.
- Komaba Y, Senda M, Ohyama M, Mori T, Ishii K, et al. (1998) Bilateral representation of language function. Agenesis of corpus callosum by Wada and PET activation. Journal of Neuroimaging: Official Journal of the American Society of Neuroimaging, 8(4): 246-249.
- Kourtidou E, Kasselimis D, Angelopoulou G, Karavasilis E, Velonakis G, et al. (2021) The role of the right hemisphere white matter tracts in chronic aphasic patients after damage of the language tracts in the left hemisphere. Frontiers in Human Neuroscience 15: 635750.
- Kowatari Y, Lee SH, Yamamura H, Nagamori Y, Levy P, et al. (2009) Neural networks involved in artistic creativity. Human Brain Mapping 30(5): 1678-1690.
- Kuo F, Massoud TF (2022) Structural asymmetries in normal brain anatomy: A brief overview. Annals of Anatomy 241: 151894.
- Kurth F, Mayer EA, Toga AW, Thompson PM, Luders E, et al. (2013) The right inhibition? Callosal correlates hand performance in healthy children and adolescents callosal correlates hand performance. Human Brain Mapping 34(9): 2259-2265.
- Larisch R, Meyer W, Klimke A, Kehren F, Vosberg H, et al. (1998) Left-right asymmetry of striatal dopamine D2 receptors. Nuclear Medicine Communications, 19(8): 781-787.
- Lassonde M, Bryden MP, Demers P (1990) The corpus callosum and cerebral speech laterality. Brain and Language 38: 195-206.
- Laures JS, Bunton K (2003) Perceptual effects of a flattened fundamental frequency at the sentence level under different listening conditions. Journal of Communication Disorders 36(6): 449-464.
- Lee O, Ludwig L, Davenport R, Stone J (2016) Functional foreign accent syndrome. Practical Neurology 16(5): 409-411.
- Lennie P (2003) The cost of cortical computation. Current Biology: CB 13(6): 493-497.
- Li JY, Lai PH, Chen R (2013) Transcallosal inhibition in patients with callosal infarction. Journal of Neurophysiology 109(3): 659-665.
- Liotti M, Ingham JC, Takai O, Paskos DK, Perez R, et al. (2010) Spatiotemporal dynamics of speech sound perception in chronic developmental stuttering. Brain and Language 115(2): 141-147.
- Lorenz RC, Gleich T, Buchert R, Schlagenhauf F, Kühn S, et al. (2015) Interactions between glutamate, dopamine, and the neuronal signature of response inhibition in the human striatum. Human Brain Mapping 36(10): 4031-4040.
- Lukic S, Barbieri E, Wang X, Caplan D, Kiran S, et al. (2017) Right hemisphere grey matter volume and language functions in stroke aphasia. Neural Plasticity: 5601509.
- Mandic-Maravic V, Grujicic R, Milutinovic L, Munjiza-Jovanovic A, Pejovic-Milovancevic M, et al. (2022) Dopamine in autism spectrum disorders-Focus on D2/D3 partial agonists and their possible use in treatment. Frontiers in Psychiatry 12: 787097.
- Marcotte AC, Morere DA (1990) Speech lateralization in deaf populations: Evidence for a developmental critical period. Brain and Language 39(1): 134-152.
- Mariën P, Keulen S, Verhoeven J (2019) Neurological aspects of foreign accent syndrome in stroke patients. Journal of Communication Disorders 77: 94-113.
- Mariën P, Verhoeven J, Wackenier P, Engelborghs S, De Deyn PP, et al. (2009) Foreign accent syndrome as a developmental motor speech disorder. Cortex 45(7): 870-878.
- Matin E (1974) Saccadic suppression: A review and an analysis. Psychological Bulletin 81(12): 899-917.
- Mayseless N, Aharon-Peretz J, Shamay-Tsoory S (2014) Unleashing creativity: The role of left temporoparietal regions in evaluating and inhibiting the generation of creative ideas. Neuropsychologia 64: 157-168.
- Mazoyer B, Zago L, Jobard G, Crivello F, Joliot M, et al. (2014) Gaussian mixture modeling of hemispheric lateralization for language in a large sample of healthy individuals balanced for handedness. PloS One 9(6): e101165.
- McWhirter L, Miller N, Campbell C, Hoeritzauer I, Lawton A, et al. (2019) Understanding foreign accent syndrome. Journal of Neurology, Neurosurgery, and Psychiatry 90(11): 1265-1269.
- Midorikawa A, Fukutake T, Kawamura M (2008) Dementia and painting in patients from different cultural backgrounds. European Neurology 60(5): 224-229.
- Mihov KM, Denzler M, Förster J (2010) Hemispheric specialization and creative thinking: aAmeta-analytic review of lateralization of creativity. Brain and Cognition 72(3): 442-448.
- Miles SA, Miranda RA, Ullman MT (2016) Sex differences in music: A female advantage at recognizing familiar melodies. Frontiers in Psychology 7: 278.
- Miller BL, Boone K, Cummings JL, Read SL, Mishkin F, et al. (2000) Functional correlates of musical and visual ability in frontotemporal dementia. The British Journal of Psychiatry: The Journal of Mental Science 176: 458-463.
- Mock JR, Foundas AL, Golob EJ (2015) Speech preparation in adults with persistent developmental stuttering. Brain and Language 149: 97-105.
- Moore DW, Bhadelia RA, Billings RL, Fulwiler C, Heilman KM, et al. (2009) Hemispheric connectivity and the visual-spatial divergent-thinking component of creativity. Brain and Cognition 70(3): 267-272.
- Mottron L, Dawson M, Soulières I (2009) Enhanced perception in savant syndrome: Patterns, structure and creativity. Philosophical Transactions of the Royal Society of London. Series B, BiologicalSsciences 364(1522): 1385-1391.
- Mutha PK, Haaland KY, Sainburg RL (2012) The effects of brain lateralization on motor control and adaptation. Journal of Motor Behavior 44(6): 455-469.
- Nahum AS, Liégeois FJ (2020) Language after childhood hemispherectomy: A systematic review. Neurology 95(23): 1043-1056.
- Onin I, Hanoglu L, Yulug B (2023) The savant syndrome: A gift or a disability? A deeper look into metabolic correlates of hidden cognitive capacity. Endocrine, Metabolic & Immune dDsorders Drug Targets 23(2): 250-253.
- Ovsepian SV (2019) The dark matter of the brain. Brain Structure & Function 224(3): 973-983.
- Patel N, Jankovic J, Hallett M (2014) Sensory aspects of movement disorders. The Lancet. Neurology 13(1): 100-112.
- Pavăl D (2017) A dopamine hypothesis of autism spectrum disorder. Developmental Neuroscience 39(5): 355-360.
- Pelletier I, Paquette N, Lepore F, Rouleau I, Sauerwein CH, et al. (2011) Language lateralization in individuals with callosal agenesis: an fMRI study. Neuropsychologia 49(7): 1987-1995.
- Petroff OA (2002) GABA and glutamate in the human brain. The Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry 8(6): 562-573.
- Pfordresher PQ, Beasley RT (2014) Making and monitoring errors based on altered auditory feedback. Frontiers in Psychology 5: 914.
- Pidgeon LM, Grealy M, Duffy AH, Hay L, McTeague C, et al. (2016) Functional neuroimaging of visual creativity: A systematic review and meta-analysis. Brain and Behavior 6(10): e00540.
- Previc FH (1991) A general theory concerning the prenatal origins of cerebral lateralization in humans. Psychological Review 98(3): 299-334.
- Previc FH (1996) Nonright‐handedness, central nervous system and related pathology, and its lateralization: A reformulation and synthesis. Developmental Neuropsychology 12 (4): 443-515.
- Previc FH (2006) The role of the extra personal brain systems in religious activity. Consciousness and Cognition 15(3): 500-539.
- Previc FH (2007) Prenatal influences on brain dopamine and its relevance to the rising incidence of autism. Medical Hypotheses 68(1): 46-60.
- Pring L, Ryder N, Crane L, Hermelin B (2012) Creativity in savant artists with autism. Autism: The International Journal of Research and Practice 16(1): 45-57.
- Robb MP, Lynn WL, O’Beirne GA (2013) An exploration of dichotic listening among adults who stutter. Clinical Linguistics & Phonetics 27(9): 681-693.
- Sabbagh MA (1999) Communicative intentions and language: evidence from right-hemisphere damage and autism. Brain and Language 70(1): 29-69.
- Seeley WW, Matthews BR, Crawford RK, Gorno-Tempini ML, Foti D, et al. (2008) Unravelling Boléro: Progressive aphasia, transmodal creativity and the right posterior neocortex. Brain: A Journal of Neurology 131(Pt 1): 39-49.
- Seliger GM, Abrams GM, Horton A (1992) Irish brogue after stroke. Stroke 23(11): 1655-1656.
- Shamay-Tsoory SG, Adler N, Aharon-Peretz J, Perry D, Mayseless N, et al. (2011) The origins of originality: the neural bases of creative thinking and originality. Neuropsychologia 49(2): 178-185.
- Shoham S, O'Connor DH, Segev R (2006) How silent is the brain: Is there a "dark matter" problem in neuroscience. Journal of Comparative Physiology Neuroethology, Sensory, Neural, and Behavioral Physiology 192(8): 777-784.
- Sidtis DV L, Sidtis JJ (2018) The affective nature of formulaic language: A Right-hemisphere subcortical process. Frontiers in Neurology 9: 573.
- Simonds RJ, Scheibel AB (1989) The postnatal development of the motor speech area: a preliminary study. Brain and Language 37(1): 42-58.
- Skipper-Kallal LM, Lacey EH, Xing S, Turkeltaub PE (2017) Right hemisphere remapping of naming functions depends on lesion size and location in poststroke aphasia. Neural Plasticity: 8740353.
- Smith I (2015) Psychostimulants and artistic, musical, and literary creativity. International Review of Neurobiology 120: 301-326.
- Smith-Conway ER, Chenery HJ, Angwin AJ, Copland DA (2012) A dual task priming investigation of right hemisphere inhibition for people with left hemisphere lesions. Behavioral and Brain Functions: BBF 8: 14.
- Snyder A (2009) Explaining and inducing savant skills: Privileged access to lower level, less-processed information. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 364(1522): 1399-1405.
- Snyder A, Bahramali H, Hawker T, Mitchell DJ (2006) Savant-like numerosity skills revealed in normal people by magnetic pulses. Perception 35(6): 837-845.
- Snyder AW, Mulcahy E, Taylor JL, Mitchell DJ, Sachdev P, Gandevia SC et al. (2003) Savant-like skills exposed in normal people by suppressing the left fronto-temporal lobe. Journal of Integrative Neuroscience 2(2): 149-158.
- Souto D, Kerzel D (2011) Attentional constraints on target selection for smooth pursuit eye movements. Vision Research 51(1): 13-20.
- Sprague JM (1966) Interaction of cortex and superior colliculus in mediation of visually guided behavior in the cat. Science 153: 1544-1547.
- Stockbridge MD, Sheppard SM, Keator LM, Murray LL, Lehman Blake M, et al. (2022) Right Hemisphere Disorders working group, Evidence-Based Clinical Research Committee, Academy of Neurological Communication Disorders and Sciences. Aprosodia subsequent to right hemisphere brain damage: A systematic review and meta-analysis. Journal of the International Neuropsychological Society: JINS 28(7): 709-735.
- Stuart A, Kalinowski J, Rastatter MP, Lynch K (2002) Effect of delayed auditory feedback on normal speakers at two speech rates. The Journal of the Acoustical Society of America 111(5 Pt 1): 2237-2241.
- Swick D, Ashley V, Turken AU (2008) Left inferior frontal gyrus is critical for response inhibition. BMC Neuroscience 9: 102.
- Takahata K, Saito F, Muramatsu T, Yamada M, Shirahase J, et al. (2014) Emergence of realism: Enhanced visual artistry and high accuracy of visual numerosity representation after left prefrontal damage. Neuropsychologia 57: 38-49.
- Toyomura A, Miyashiro D, Kuriki S, Sowman PF (2020) Speech-induced suppression for delayed auditory feedback in adults who do and do not stutter. Frontiers in Human Neuroscience 14: 150.
- Treffert DA (2009) The savant syndrome: an extraordinary condition. A synopsis: past, present, future. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 364(1522): 1351-1357.
- Treffert DA (2014) Accidental genius. Scientific American 311(2): 52-57.
- Treffert DA, Rebedew DL (2015) The savant syndrome registry: A preliminary report. WMJ: Official Publication of the State Medical Society of Wisconsin 114: 58-62.
- Treffert DA, Tries HJ (2021) The Sudden savant: A new form of extraordinary abilities. WMJ: Official Publication of the State Medical Society of Wisconsin 120(1): 69-73.
- van der Slik FW, van Hout RW, Schepens JJ (2015) The gender gap in second language acquisition: Gender differences in the acquisition of Dutch among immigrants from 88 countries with 49 mother tongues. PloS One 10(11): e0142056.
- Varoglu AO (2022) Is the left hemisphere more prone to epilepsy and poor prognosis than the right hemisphere. The International Journal of Neuroscience: 1-5.
- Verhoeven J, De Pauw G, Pettinato M, Hirson A, Van Borsel J, et al. (2013) Accent attribution in speakers with Foreign Accent Syndrome. Journal of Communication Disorders 46(2): 156-168.
- Volden J, Coolican J, Garon N, White J, Bryson S, et al. (2009) Brief report: Pragmatic language in autism spectrum disorder: Relationships to measures of ability and disability. Journal of Autism and Developmental Disorders 39(2): 388-393.
- von Holst E, Mittelstaedt H (1950) Das reafferenzprinzip. Naturwissenschaften 37: 464-476.
- Wang Y, Tan B, Wang Y, Chen Z (2021) Cholinergic signaling, neural excitability, and epilepsy. Molecules 26(8): 2258.
- Williams LZJ, Fitzgibbon SP, Bozek J, Winkler AM, Dimitrova R, et al. (2023) Structural and functional asymmetry of the neonatal cerebral cortex. Nature Human Behaviour 7(6): 942-955.
- Wolpert DM, Ghahramani Z (2000) Computational principles of movement neuroscience. Nature Neuroscience 3: 1212-1217.
- Yi YG, Kim DY, Shim WH, Oh JY, Kim HS, et al. (2019) Perilesional and homotopic area activation during proverb comprehension after stroke. Brain and Behavior 9: e01202.
- Young RL, Ridding MS, Morrell TL (2004) Switching skills on by turning off part of the brain Neurocase 10(3): 215-222.
- Zaidel E (1976) Auditory vocabulary of the right hemisphere following brain bisection or hemidecortication. Cortex: A Journal Devoted to The Study of The Nervous System and Behavior 12(3): 191-211.
- Zaltman G (2022) Hidden minds. Harvard Business Review: 26-28.
-
Fred H Previc*. Right-Hemispheric Disinhibition as a Neural Basis of Acquired Savantism and Foreign Accent Syndrome. Arch Neurol & Neurosci. 16(4): 2024. ANN.MS.ID.000894.
-
Acute Polyradiculoneuritis, Neurology, Guillain-barre; Fann teaching hospital, neuropathy, cranial nerves, immunotherapy, Neuroscience, neurogenic syndrome, epidemiological, Dysphonia.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






