 Research Article
Research Article
Prognosis Factors in the Treatment of Chronic Subdural Hematomas at the Mamadou Gueye Clinic of Fann Teaching Hospital: Review of 205 Cases
Ecn SY*, CM Mualaba, M Mbaye, M Thioub, Ab Thiam, MC Ba and SB Badiane
Department of Neurosurgical clinic - CHNU Fann Dakar-Senegal
El hadji, Cheikh Ndiaye SY Celebre Mualaba Mualaba, Department of Neurosurgical clinic - CHNU Fann Dakar-Senegal.
Received Date: July 18, 2022; Published Date: July 27, 2022
Introduction: Subdural hematoma is a collection of blood and blood degradation products in the intracranial subdural space that liquefies over
time. The objective of this work is to highlight the prognostic factors of patients with chronic subdural hematomas (cSDH).
Patients and Method: This is a study that concerned 205 patient files collected and followed up in the Neurosurgery Department of the
Mamadou Gueye clinic at Fann Teaching Hospital for the management of chronic subdural hematoma during a period from September 2017 to
September 2021, i.e., 4 years, having benefited from a follow-up of at least three months.
Results: During this study we received 163 men and 42 women (sex ratio: 3.88). The predominant age group was 65 years and over found in
48.8%. Minor head trauma, following a fall, was the most frequent cause (22.9%). The average duration of symptom evolution was greater than
3 weeks in 27.8%. The intracranial hypertension syndrome was constant, complicated by memory disorders in 31.7% of cases, consciousness
disorders in 33.2% of cases, and even a deficit syndrome in 68.3% of cases (n=140). The radiological diagnosis was made with CT in 96.6% of cases,
and with MRI in 3.4% of cases. 174 patients were operated (84.8%). Surgical techniques essentially consisted of making a burr hole in 86.2% of
cases (n=150), and two burr holes in 13.8% of cases (n=24) associated with rinsing of the cavity followed by drainage for 48 hours. Complementary
medical treatment, aimed at promoting postoperative cerebral expansion, was administered. The average follow-up was 6 months with 76.1% of
patients cured. 22.4% of patients had intellectual or motor sequelae. 1.5% of patients died.
Conclusion: The treatment of cSDH is mainly neurosurgical and must be early, to reduce mortality and morbidity. Evacuation of the hematoma
through one or two burr holes is the most common technique.
Keywords:Chronic subdural hematoma; Surgical treatment; Follow-up of at least three months; Fann teaching hospital
Introduction
Chronic subdural hematoma (cSDH) is a very common pathology in neurosurgery. It is the consequence of a hemorrhage of venous origin in the subdural space, most often following a minor head trauma [1]. Its occurrence is closely related to advanced age and/ or coagulopathy (iatrogenic and/or hepatic). Its treatment is most often surgical and in some cases medical treatment is sufficient to obtain a good cure; However, certain therapies are associated with the surgical procedure with the aim of improving the postoperative course [1]. The objective of this study is to report our experience on patients followed for chronic subdural hematoma (cSDH) by highlighting the epidemiological, clinico-radiological, therapeutic and prognostic aspects.
Patients and Method
We retrospectively studied 205 files of patients received and followed in the neurosurgery department of the Mamadou Gueye clinic at Fann hospital for the management of chronic subdural hematoma during a period from September 2017 to September 2021, having benefited from a follow-up of at least three months. We had highlighted the epidemiological, clinical, radiological parameters as well as the type of treatment and the results of our patients.
Results
During our study period, we collected 205 patients, including 163 men and 42 women, i.e., sex ratio: 3.88. Age was proportional to frequency and the predominant age group was 65 years and over found in 48.8% as shown in (Table 1). The mode of admission was mainly transfer in 55% of cases against 30% received directly in the emergency room and 15% through the external consultation channel (Table 1).
Table 1:Distribution of patients according to age group
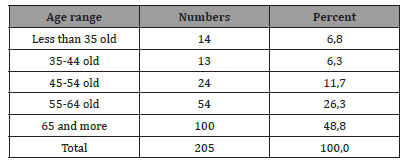
The average duration of symptom development was greater than 3 weeks in 44.9% of cases, against 35.1% between 1-3 weeks and 20% at a duration of ‹1 week. Regarding the history of our patients, hypertension was found in 39.5%, we found a notion of TCE by Fall in 22.9% of cases, by AVP in 36.6% of cases each. Among the 205 patients, 23.9% of patients were on anticoagulants. The concept of diabetes was found in 9.8% and alcoholism 2.5%. Clinically, the intracranial hypertension syndrome was constant, dominated by headaches found in 55.6% of cases, these headaches were associated with vomiting en 22.9% of cases. The intracranial hypertension syndrome was complicated, with impaired consciousness in 33.2% of cases. Memory disorders were found in 31.7% of cases and motor deficit syndrome en 68.3% of cases (n=140. The radiological diagnosis was made with CT in 96.6% of cases, making it possible to visualize the chronic subdural hematoma in the form of a hypodense or isodense hemispherical image with sometimes signs of rebleeding (Figure 1), and with the MRI in 3.4% of cases. The radiological assessment revealed 78% of unilateral sub-dural hematomas versus 22% of bilateral sub-durals. Fifty-six percent of hematomas were between 15mm and 20mm thick (Table 2).
Table 2:Distribution of patients according to hematoma thickness on brain scan.
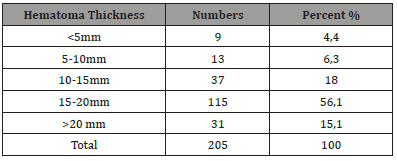
Figure 1:Brain CT A : right hemispheric hypodensity evoking a right hemispheric subdural hematom. B : bilateral hemispherical hypodensity evoking a bilateral hemispherical subdural hematoma predominant on the left with a density of different age HAS.

On the therapeutic level, 15.1% of the patients (n=31) had benefited from medical treatment which consisted of the oral administration of prednisolone at a dose of 1mg/kg per day for a period of 4 weeks; Surgery was the most common mode of treatment, 84.8% (n=174) and consisted of general anesthesia in 62% of cases (n=108) or local anesthesia in 38% of cases (n=66), making it possible to make a burr hole in 74.4% (n=130) or 2 burr holes on the same side in 22.9% (n=44) to evacuate the hematoma. Complementary medical treatment, aimed at promoting postoperative cerebral expansion, was administered and consisted of parenteral and oral hydration and the establishment of corticosteroid therapy in the absence of contre-indications in the presence of a residual of hematoma on a scannographic control which is carried out on D2, M1 and M3 postoperatively (figures 2 and 3). Recurrence was rare at 7.3% (n=15) and concerned patients with 80 old 53.33% (n=8) and over, After a follow-up of at least 6 months and an average duration of follow-up of 3 months, we found complete recovery in 75.1%, 22.4% of patients presented with intellectual or motor sequelae. We recorded 1.5% (n=3) of deaths of which we present a case for illustrative purposes.
This was a 63-year-old hypertensive patient known to be followed with the notion of taking vitamin K antagonists, received through the emergency department for management of rapidly progressive onset of consciousness disorders 3 days before the consultation. The admission examination essentially found a patient in a coma with a Glasgow coma scale of 5/15 (E1V1M3), pupils with poorly reactive mydriasis, with no motor reactions to nociception; with pleuropulmonary examination of bilateral bronchial obstruction rales; medical imaging by brain scan revealed a hypodense right hemispheric lesion with significant mass effect on the median structures. The biological assessment essentially found a TP at 70%, TCK at 18.3. respiratory distress with Sa O2: 80%, and a Glasgow score of 3 (E1V1M1), bilateral areactive mydriasis, with a disturbed biological assessment finding a CRP at 300 mg/l. Resuscitation attempts were made but without success and irreversible cardiorespiratory arrest was observed. Patient died on one postoperative day whose factors having precipitated his death would be the comorbidity including high blood pressure, the notion of taking anticoagulant, the pulmonary infection as well as his neurological state at the start (patient admitted with a Glasgow score at 5/15 and pupils in not very reactive bilateral mydriasis) (Figure 1 to 3).
Figure 2:Brain CT: A: right hemispheric hypodensity evoking a preoperative right hemispheric subdural hematoma. B: Postoperative image on postoperative day 2 of a right hemispherical subdural hematoma evacuated by a single burr hole in HAS B VS.
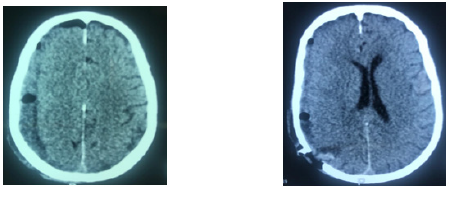
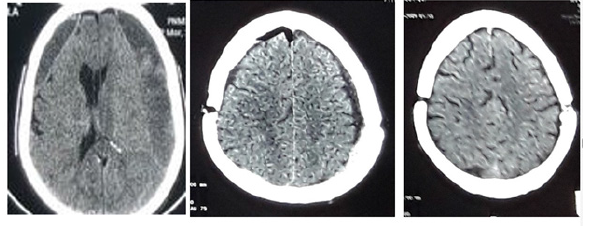
Figure 3:Brain CT A: bilateral hemispherical hypodensity evoking a bilateral hemispherical subdural hematoma predominant on the left with a density of different age. B: Postoperative image on D2 postoperative of a bilateral subdural hemispherical hematoma evacuated by a single burr hole with a small postoperative pneumoencephaly. C: Postoperative image at 1 month postoperatively of a bilateral hemispherical subdural hematoma evacuated by a single hole showing the total disappearance of the hematoma.
Discussion
Subdural hematoma (SDH) is a collection of blood between the dura and the arachnoid meningeal layers surrounding the brain. Acute subdural hematoma (aSDH) develops rapidly, often resulting in symptoms within 72 hours and requiring surgery, while chronic subdural hematoma develops over 3 weeks or more, in this series only chronic subdural hematomas have attracted our attention in their therapeutic aspects. [1,6]. The annual incidence of chronic subdural hematoma is 5.3 cases per 100,000 individuals, reaching even such notable figures as Ronald Reagan and Richard Feynman [2,3]. Chronic subdural hematoma has a 3:1 male predilection for females, occurring at the mean age of 77 years, something also found in our series where the sex ratio was 3.8 in favor men with a dominance of the age group beyond 65 years, this because of age-related cortical atrophy widening the subdural space. Historically, head trauma was considered the most common cause of chronic subdural hematoma. However, with the increased use of anticoagulants and antiplatelets, chronic subdural hematoma frequently occurs in the absence of any identifiable traum [5,7,8]. In our experience we found the same reality as the literature, minor head trauma predominated in 36% with sometimes an association of risk factors in some patients (hypertension, anticoagulant intake, and repetitive falls). The average duration of symptom evolution in our study was greater than 3 weeks in 27.8%, Patients with chronic subdural hematoma often present with cognitive impairment, gait disturbance, limb weakness, or headache.In our series the syndrome of intracranial hypertension was constant, complicated by memory disorders in cases, consciousness disorder in 33.2% of cases, even a deficit syndrome in 68.3% of cases (n=140). R. Jomli found in 2011 in his series a predominance of a dementia syndrome, mental confusion, convulsions or psychiatric manifestations (frontal seat). Diagnosis is currently revolutionized by the advent of cranial imaging. In our study cerebral CT was used in 96.6% of cases versus MRI in 3.4% to confirm the diagnosis of chronic subdural hematomas. Several factors enter into the formation of the volume of the subdural hematoma among which the high concentrations of interleukin-6, vascular endothelial growth factor and growth factor of fibroblasts in the subdural liquid against an increased level of fibrinogen degradation products in chronic subdural hematoma help clot reabsorption and chronic hemorrhage; [5] In our series the hematomas were thicker than 15mm, being in 78% unilateral hematomas against 22% bilateral sub durals. Asymptomatic chronic subdural hematoma, without evidence of cerebral compression, is usually managed conservatively with a period of observation and/or medical management, including monitoring of intracranial pressure, reversal anticoagulation and serial examinations. The decision to pursue surgery is frequently based on the presence of neurological symptoms associated with clinical or radiographic signs of cerebral compression (eg, maximum thickness > 1 cm), in patients undergoing surgery. Associated severe headaches or other symptoms clearly attributable to the injury may also be an indication for surgery. As for medical treatment, all drugs used so far share a potential role on the inflammatory processes underlying the formation of chronic subdural hematoma. There are already studies pointing to moderate benefits of atorvastatin, tranexamic acid, etizolam, and corticosteroids [1,6]. G. Dran et all showed the place of corticosteroid therapy in the management of subdurals, although no pathophysiological mechanism explains their actions, only the inhibition of pro-inflammatory factors would justify their use in the management of hematomas. Under durals [6]. It should be remembered that symptomatic chronic subdural hematoma is a surgical disease and only prompt surgical evacuation can relieve urgent intracranial hypertension. Thereby, in our series, surgery was the most common mode of treatment, i.e., 54.6%. Place of a subdural drain. The drain is usually removed after 3 days, and a first postoperative scan is obtained. In our experience, a trephine hole in 74.4% made it possible to evacuate the hematoma while leaving in most cases a suction redon drain carried out in our series in 44.9% and ablation in 18% of cases. Of this drain was done within 48 hours after evacuation surgery. Outcome after surgical treatment of symptomatic chronic subdural hematoma are generally favorable, with rapid clinical improvement occurring in more than 80% of patients [9]. After a 3- month follow-up, subdural hematomas presented a good prognosis in 75.1% in our series, results already shared by the literature where Ramnarayan Ramachandran and all had reported that most patients (89%) had a very good recovery on the seventh postoperative day of evacuation of a subdural hematoma in his series [9].
Conclusion
Chronic subdural hematoma is a neurosurgical pathology in adults whose diagnosis and treatment must be early in order to reduce mortality and morbidity. Evacuation of the hematoma through one or two burr holes is the most common technique and often gives a good result although there is a small margin of complications.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Dranhas, F Berthierb, D Fontainevs, D Rasenrarijaovs, P Paquisvshas (2007) Effectiveness of adjuvant corticosteroid therapy for chronic subdural hematoma: A retrospective study of 198 cases. Neurochirurgie 53: 477– 482.
- Ronald Sahyouni, Khodayar Goshtasbi, Amin Mahmoodi, Diem K. Tran, Jefferson W. Chen, et al. (2017) Chronic Subdural Hematoma: A Historical and Clinical Perspective. World Neurosurg 108: 948-953.
- James Feghali, Wuyang Yang, Judy Huang, (2020) Updates in Chronic Subdural Hematoma: Epidemiology, Etiology, Pathogenesis, Treatment and Outcome. World Neurosurgery 141: 339-345.
- Vikram Mehta, Stephen C Harward, Eric W Sankey, Gautam Nayar, Patrick J Codd, et al. (2018) Evidence based diagnosis and management of chronic subdural hematoma: A review of the literature. J Clin Neurosci 50: 7-15.
- Wuyang Yang, Judy Huang (2017) Chronic Subdural Hematoma Epidemiology and Natural History. Neurosurg Clin N Am 28(2): 205-210.
- PJ Hutchinson, E Edlmann, D Bulters, A Zolnourian (2020) Trial of Dexamethasone for Chronic Subdural Hematoma. N Engl J Med 383(27): 2616-2627.
- Matheus Fernandes de Oliveira (2019) Chronic Subdural Hematomas and Pursuit of Nonsurgical Treatment Alternatives. World Neurosurg 126: 481-483.
- David Roh, Michael Reznik, Jan Claassen (2017) Chronic Subdural Medical Management. Neurosurg Clin N Am 28: 211–217.
- Ramnarayan Ramachandran, Thimmappa Hegde (2007) Chronic subdural hematomas—causes of morbidity and mortality. Surgical Neurology 67(4): 367–373.
-
Ecn SY, CM Mualaba, M Mbaye, M Thioub, Ab Thiam, et al. Prognosis Factors in the Treatment of Chronic Subdural Hematomas at the Mamadou Gueye Clinic of Fann Teaching Hospital: Review of 205 Cases. Arch Neurol & Neurosci. 12(5): 2022. ANN.MS.ID.000798.
-
Chronic subdural hematoma, surgical treatment, follow-up of at least three months, Fann Teaching Hospital, therapeutic and prognostic aspects, epidemiological
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






