 Research Article
Research Article
Prior Head Trauma Impacts Onset and Severity of Parkinson’s Disease
Sachin Vallamkonda1,2*, Jacob Brik2 and Anna DePold Hohler1,2,3
1Tufts University School of Medicine, Boston, MA, USA
2Department of Neurology, St. Elizabeth’s Medical Center, Boston, MA, USA
3Boston University School of Medicine, Boston, MA, USA
Sachin Vallamkonda, Department of Neurology, St. Elizabeth’s Medical Center 736 Cambridge St, Boston, MA 02135 USA.
Received Date:July 18, 2024; Published Date:August 07, 2024
Abstract
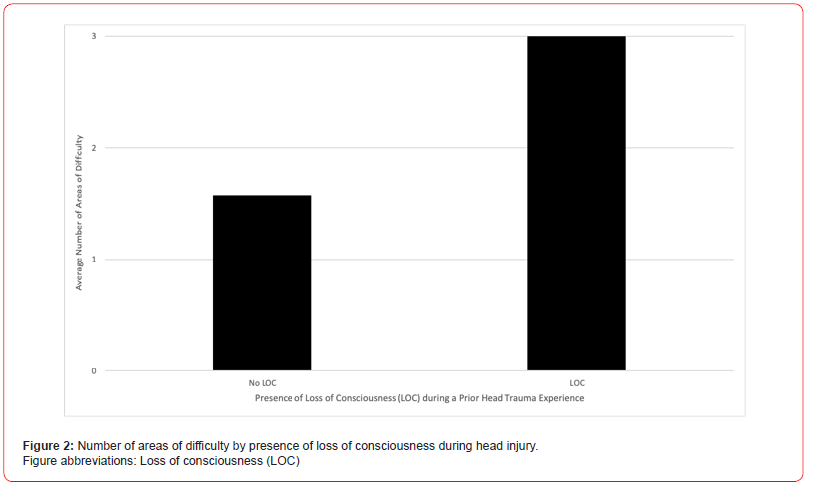
Previous head trauma is correlated with detrimental neuropsychological outcomes and has even been shown to increase risk of Parkinson’s disease (PD). However, there still exists a dearth of literature characterizing how prior head trauma shapes patients’ experience of PD. We conducted a cross-sectional study involving PD patients with and without prior head trauma experiences within a large health system. Our study’s anonymous questionnaire encompassed demographical, health history, and descriptive data. Discussed findings include both quantitative and qualitative data analyses. Twenty-five patients successfully completed the questionnaire; fifteen had at least one prior head trauma experience. The cohort’s average age is 69 years-old and includes both men and women. Patients with prior head trauma experiences on average were diagnosed with PD at an earlier age (60.9 vs 62.4 years-old), report greater number of areas of difficulty (2.3 vs 1.9), and hold more concurrent medical diagnoses (0.9 vs 0.5) than those with no prior head traumas. These trends are also observed when segregating the cohort by sex. When only observing patients without relatives with PD, these trends continue to stand, and PD diagnosis age is even earlier (51.4 vs 65.2 years-old) amongst those with prior head trauma (Figure 1). Patients with prior head trauma also continue to have more areas of difficulty (2.2 vs 1.3) than those without head trauma when only looking at PD patients without any concurrent medical diagnoses. Moreover, amongst patients with prior head trauma, those that experienced loss of consciousness (LOC) during at least one of their head trauma events report a significantly greater number of areas of difficulty than those with injuries that did not include LOC (p=0.012) (Figure 2). While there are variations in PD treatment regimen throughout the cohort, both patients with and without head trauma utilize similar medical therapies. Furthermore, most patients with prior head traumas sought out medical attention after their injury (13 of 15); the majority of care received when seeking out medical attention consisted mainly of head imaging (10 of 13).
Prior head trauma increases risk for earlier PD diagnosis, increased areas of difficulties, and more concurrent medical diagnoses amongst PD patients. LOC during head injuries is strongly predictive for a greater number of areas of difficulty. PD treatment did not differ greatly between patients with and without prior head trauma experiences. Further study of post-injury care experiences and treatment impact on health outcomes is necessary.
Keywords:Parkinson’s disease; Traumatic brain injury; Neurodegenerative disorders
Abbreviations:PD: Parkinson’s Disease; TBI: Traumatic Brain Injury; LOC: Loss of Consciousness; PHT: Prior Head Trauma (PHT)
Introduction
The etiologies behind Parkinson’s disease (PD) are a thoroughly investigated field of study. Currently, clinical and research sciences’ existing understanding contends that the neurodegenerative condition is influenced strongly by genetic, age-related, and environmental factors [1,2]. Among the nongenetic influences, such as chemical toxins and microorganism infection, the role of previous traumatic brain injury (TBI) is a lightly studied topic that has been reported on but sparsely described or explained in detail. Inconsistent findings about the correlation, implications of studied patients’ recall bias, and a lack of a clear causal pathway all contribute to this potential risk factor’s ill-defined impact. Throughout the past three decades, multiple studies and relevant meta-analyses have reported that prior head injury history is correlated with a statistically significant increase in odds of later PD’s diagnosis [3-8]. Further supporting this relationship, several studies have discussed how TBI severity may further increase this impact; for instance, one study noted a significantly stronger odds ratio when stratifying by injuries that required hospitalization [8]. Moreover, many studies do specifically report a correlation between TBI that included loss of consciousness (LOC) and increased odds of later PD [3, 6-8]. There are few studies that do report not finding a statistically significant relationship between severe head injury and PD [9]; however, the majority of literature contends differently.
While increased odds of PD have been tied with TBI and also with TBI that included LOC, there still exists a dearth of literature further detailing how TBI history impacts patients’ experiences with PD. The following research article reports findings about this correlation and additionally about how this history may impact patients’ experience of their disease. The implications of a greater understanding of TBI and PD’s relationship is significant and relevant as it may influence bolstered surveillance of occupational injuries, allow for further study of secondary and tertiary prevention techniques,and aid in our understanding of PD’s etiology itself.
Methods
The study utilized a cross-sectional design. All participants included in the study had diagnoses of PD or were receiving treatment for parkinsonian symptoms and were patients within a large health system. A study questionnaire was constructed and included questions asking about participants’ PD diagnosis date, disease-related experiences and symptoms, comorbidities, demographics, incidences of prior TBI, description of each event, presence of LOC, and possible medical workup and treatment post-injury. Specific areas of difficulty participants were queried about included: memory, sleep, depression/anxiety, orthostatic hypotension, and hallucinations. Participants were also able to include non-listed areas of difficulty. Questionnaire was completed voluntarily and anonymously. Quantitative and qualitative analyses were utilized.
Results
The study included twenty-five participants who were able to successfully complete the questionnaire. The study cohort held an average age of 69 years-old and seven females. Twenty-three patients reported having been diagnosed with PD, and three reported receiving treatment for parkinsonian symptoms. Twenty of the twenty-five participants reported use of a dopaminergic medication for PD. The most common noted areas of difficulties were memory (n=12), sleep (n=10), and depression/anxiety (n=10). The most common comorbid diagnoses included atrial fibrillation (n=3), type II diabetes (n=3), and REM sleep disorder (n=3). Of the twenty-five total participants, fifteen reported at least one incidence of prior head trauma, and ten did not disclose any head injury experiences.
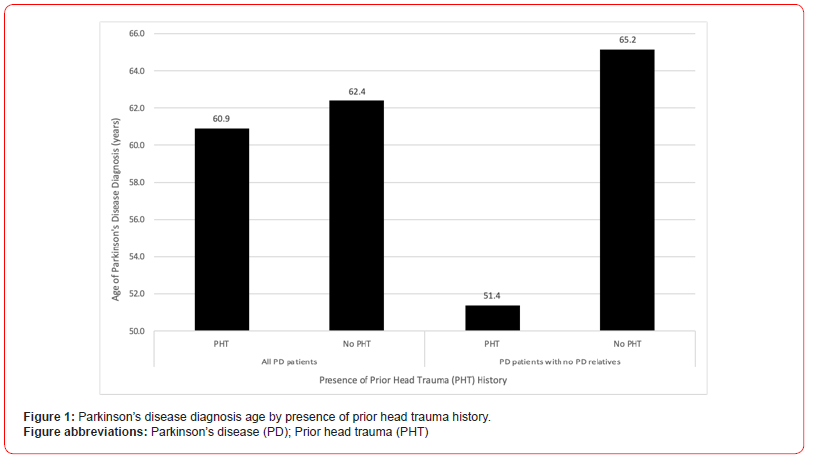
Participants that reported at-least one prior head trauma experience on average were diagnosed with PD at an earlier age, with a mean of 60.9 years-old, compared to those without TBI history, with a mean of 62.4 years-old. When only looking at participants without relatives with PD, those who have TBI history have an even earlier mean PD diagnosis age of 51.4 years-old compared to those with no TBI history with a mean of 65.2 years old (Figure 1).
Compared to patients without any TBI history, those with reported TBI experiences also reported a greater number of listed areas of difficulty (2.3 versus 1.9 areas). Participants with TBI history also held more concurrent medical diagnosis than those without any previous TBIs (0.9 versus 0.5 diagnoses). These trends remained consistent when segregating the cohort by sex as well. Furthermore, when including only participants without any other reported concurrent medical diagnoses, patients with TBI history continue to have a greater average number of areas of difficulty than those without TBI history (2.2 versus 1.3 areas).
Patients’ reported TBI events occurred at a diverse range of years before their PD diagnoses. Of the fifteen participants that had TBI histories, eight had experienced at-least one incidence of loss of consciousness (LOC) during at-least one of their TBI events. When examining only those with TBI histories, participants that had experienced LOC during a head trauma event on average reported a significantly greater number of areas of difficulties than participants who had experienced TBI without LOC p=0.012, CL 95% [0.37 to 2.49] (Figure 2).
Thirteen of the fifteen participants who reported TBI histories also reported seeking out medical attention after at-least one of their TBI events. The most common reported care received consisted of head imaging (n=10). Five participants noted receiving surgical interventions or inpatient hospitalization.
Discussion
Influence of Prior TBI History
The present study illustrates trends detailing earlier PD diagnosis age, greater number of comorbid diagnoses, and greater number of areas of difficulty for participants with prior TBI histories compared to those without reported TBI experiences. Prior reports and reviews have discussed similar trends favoring the correlation between PD diagnosis and TBI history [3-8]. Such studies have showcased this relationship through risk determinations involving odds ratios (OR) regarding PD diagnosis and the presence of TBI history [4-6]. ORs between 1.5 and 2.6 regarding PD and prior TBI history have been recorded, and a 2006 study examining 93 twin pairs even determined an OR of 3.8 between PD and history of TBI with LOC [3-6]. These reported correlations between TBI history and later PD diagnosis are concordant with this study’s determination of TBI’s potential influence on earlier PD neurodegeneration and diagnosis. However, the present study contributes information not only about TBI’s correlation with PD but about TBI’s impact on PD experience. As detailed within the aforementioned results, PD-related areas of difficulties and age of PD diagnosis are also influenced by TBI history. The severity of one’s PD experience is found to be connected, at-minimum in part, to patients’ TBI histories.
The potential capacity to impact PD experience held by TBI history is especially noticeable when examining TBI severity. The presence of LOC in TBI events led to a statistical significant increase in the number of areas of difficulty later amongst PD patients that had reported TBI histories compared to those who experienced TBI without LOC (Figure 2). Similar to the present study’s correlation between TBI severity and PD experience, the literature has connected prior TBI severity and PD diagnosis. A 2013 case-control analysis studying factors associated with PD recorded greater ORs for TBIs with LOC than for TBIs with concussion [3]. In fact, when examining TBI history’s relationship with presence of microinfarct signs on tissue pathology, history of TBI with LOC produced a greater OR compared to TBI without LOC [13]. Evidence showcasing TBI severity producing more significant neurological damage and symptoms may allude to a potential dose-dependent relationship between TBI history and later neurodegenerative effects.
The segregation of the cohort by participants with and without relatives with PD illustrated additional findings about factors that influence PD diagnosis age. Examining only participants without any relatives with PD led to a larger difference in PD diagnosis age between those with and without TBI histories (Figure 1). First, as displayed, when controlling for other PD-relevant factors such as genetic influence represented by the presence of relatives with PD, TBI history is found to have a greater impact on PD development. Moreover, the difference in the magnitude of this connection when altering the presence of relatives with PD showcases how genetic influences also hold significant impact. These observations regarding the presence of possible genetic predisposition and prior TBI history further support a multifactorial etiology behind PD.
TBI and Neurodegeneration
Prior head trauma has been postulated to increase risk for later neurodegeneration. Multiple studies and reviews report positive correlations between reported TBIa history and either neurodegenerative diagnoses or related signs found in neurodegeneration [10-14]. Notably, patients with reported TBIs have been found to hold increased risk of dementias and Alzheimer’s disease [10, 15]. Studies have even found correlations with increased amyloid deposition and with imaging findings concerning for neurodegeneration [11-13].
Multiple mechanisms for how traumatic brain injury increases risk for later neurodegeneration and PD have been theorized. Graham and Sharp discuss how brain injury may lead to earlier presence of pathologic proteins, such as beta amyloid and TDP-43; this starts a cascade that gradually influences neurodegeneration and atrophic changes [16]. This explanation may play a role when considering reported increases in beta amyloid loads and lewy bodies, a feature in parkinsonian diseases, amongst patients with history of traumatic brain injury (TBI) [11, 13, 15]. Furthermore, TBIs may lead to microinfarcts in the cortical and subcortical matter that gradually promote atrophic and neurodegenerative changes. In fact, history of TBI was associated with significantly greater odds of microinfarct pathological findings on brain autopsy [13]. These findings continued to exist when controlling for vascular risk factors [13]. Another pathway consists of neuroinflammatory changes post-TBI that lead to overactivation of glial cells and create a harmful neural environment promoting neurodegeneration [15]. Signs of microglial activation have been recorded up to seventeen years after TBI incidents in patients; this was found to affect specifically the subcortical regions and namely the putamen [15, 17]. Findings of increased glial activation and toxicity to basal ganglia structures are relevant when studying TBI’s potential ability to increase risk for movement disorders such as PD.
Limitations
There are several study limitations to acknowledge. Firstly, recall bias must be considered when conducting analysis that involves retrospective and subjective data collection. Furthermore, smaller sample sizes may have limited the statistical significance of several aforementioned trends. Further investigations utilizing larger cohort sizes to examine these correlations would be valuable.
Conclusion
Overall, this study finds a significant relationship between TBI history and PD. Individuals with TBI histories are at a higher risk for an earlier diagnosis of PD, increased areas of difficulties, and a higher number of concurrent medical diagnoses. Notably, PD patients with TBI history that included LOC experience a greater number of areas of difficulty than those that did not experience LOC. Currently, PD patients with and without a history of TBIs receive similar initial treatment. Further research is needed to corroborate this relationship and investigate potential therapies for reducing risk of neurodegenerative effects post-TBI.
Acknowledgement
There are no relevant additional acknowledgements.
Conflict of Interest
There are no conflicts of interests to disclose.
References
- Chade AR, Kasten M, Tanner CM (2006) Nongenetic causes of Parkinson’s disease. In: Riederer P, Reichmann H, Youdim MBH, Gerlach M, eds. Parkinson’s Disease and Related Disorders. Springer 147-151.
- Schapira AH, Jenner P (2011) Etiology and pathogenesis of Parkinson’s disease. Mov Disord 26(6):1049-1055.
- Harris MA, Shen H, Marion SA, Tsui JKC, Teschke K (2013) Head injuries and Parkinson’s disease in a case-control study. Occup Environ Med 70(12): 839-844.
- Jafari S, Etminan M, Aminzadeh F, Samii A (2013) Head injury and risk of Parkinson disease: A systematic review and meta-analysis. Mov Disord 28(9):1222-1229.
- Nicoletti A, Vasta R, Mostile G, et al. (2017) Head trauma and Parkinson’s disease: results from an Italian case-control study. Neurol Sci 38(10):1835-1839.
- Goldman SM, Tanner CM, Oakes D, Bhudhikanok GS, Gupta A, Langston JW (2006) Head injury and Parkinson’s disease risk in twins. Ann Neurol 60(1): 65-72.
- Taylor KM, Saint-Hilaire MH, Sudarsky L, et al. (2016) Head injury at early ages is associated with risk of Parkinson’s disease. Parkinsonism Relat Disord 23: 57-61.
- Bower JH, Maraganore DM, Peterson BJ, McDonnell SK, Ahlskog JE, Rocca WA (2003) Head trauma preceding PD. Neurology 60(10): 1610-1615.
- Hofman A, Collette HJA, Bartelds AIM (1989) Incidence and Risk Factors of Parkinson’s Disease in The Neuroepidemiology 8(6): 296-299.
- Li Y, Li Y, Li X, et al. (2017) Head Injury as a Risk Factor for Dementia and Alzheimer’s Disease: A Systematic Review and Meta-Analysis of 32 Observational Studies. PLoS ONE 12(1): e0169650.
- Mielke MM, Savica R, Wiste HJ, et al. (2014) Head trauma and in vivo measures of amyloid and neurodegeneration in a population-based study. Neurology 82(1): 70-76.
- Little DM, Geary EK, Moynihan M, et al. (2014) Imaging chronic traumatic brain injury as a risk factor for neurodegeneration. Alzheimers Dement 10(3S).
- Agrawal S, Leurgans SE, James BD, et al. (2022) Association of Traumatic Brain Injury With and Without Loss of Consciousness With Neuropathologic Outcomes in Community-Dwelling Older Persons. JAMA Netw Open 5(4): e229311.
- Tripodis Y, Alosco ML, Zirogiannis N, et al. (2017) The Effect of Traumatic Brain Injury History with Loss of Consciousness on Rate of Cognitive Decline Among Older Adults with Normal Cognition and Alzheimer’s Disease Dementia. J Alzheimers Dis JAD 59(1): 251-263.
- Brett BL, Gardner RC, Godbout J, Dams-O’Connor K, Keene CD (2022) Traumatic Brain Injury and Risk of Neurodegenerative Disorder. Biol Psychiatry 91(5): 498-507.
- Graham NS, Sharp DJ (2019) Understanding neurodegeneration after traumatic brain injury: from mechanisms to clinical trials in dementia. J Neurol Neurosurg Psychiatry 90(11): 1221-1233.
- Ramlackhansingh AF, Brooks DJ, Greenwood RJ, et al. (2011) Inflammation after trauma: Microglial activation and traumatic brain injury. Ann Neurol 70(3): 374-383.
-
Sachin Vallamkonda, Jacob Brik and Anna DePold Hohler. Prior Head Trauma Impacts Onset and Severity of Parkinson’s Disease. Arch Neurol & Neurosci. 16(5): 2024. ANN.MS.ID.000900.
-
Acute Polyradiculoneuritis, Neurology, Guillain-barre; Fann teaching hospital, neuropathy, cranial nerves, immunotherapy, Neuroscience, neurogenic syndrome, epidemiological, Dysphonia.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






