 Case Report
Case Report
Posterior Reversible Encephalopathy Syndrome: Clinical Case and Literature Review
Fiori Patrizia1, Miele Antonella2, Capaldo Guglielmo1, Del Medico Francesco2, Landolfi Carlo2, Monsellas Jacob2, Spriveri Corrado2, Pelosi Chiara3, Martino Alberigo4, Muccio Carmine Franco4, Pace Erminio5, Petrone Giovanni5, Monaco Antonio1, Volpe Filomena4, Bellizzi Annamaria3 and Stanco Domenico2
1Neurological Unit, S. Ottone Frangipane Hospital, ASL AV, Ariano irpino (AV), Italy
2Obstetric and Gynaecological Unit, S. Ottone Frangipane Hospital, ASL AV, Ariano irpino (AV), Italy
3Internal Medicine, S. Ottone Frangipane Hospital, ASL AV, Ariano irpino (AV), Italy
4Radiology, S. Ottone Frangipane Hospital, ASL AV, Ariano irpino (AV), Italy
5Intensive Care, S. Ottone Frangipane Hospital, ASL AV, Ariano irpino (AV), Italy
Dr. Fiori Patrizia S. Ottone Frangipane Hospital, ASL AV, 83031 Ariano irpino (AV), v. Maddalena snc, Italy.
Received Date:June 27, 2024; Published Date:July 15, 2024
Abstract
Posterior Reversible Encephalopathy Syndrome (PRES) is a rare clinical-neuroradiological condition caused by bilateral vasogenic subcortical white matter oedema, typically in the posterior occipital and parietal lobes. It is clinically characterized by neurological symptoms, such as headache, visual disturbances, nausea, vomiting, altered consciousness and generalized seizures. The clinical picture generally remits within a couple of weeks without outcomes. Prognosis is strictly linked to the timeliness of diagnosis and therapy. A delay might result in irreversible neurological consequences and death.
We report a case of PRES, during the puerperium of a young woman, after a physiological pregnancy. The sudden onset and the early diagnosis, supported by the neuroradiological picture allowed to set up the most appropriate therapies for complete resolution of the clinical picture. Our goal is to raise awareness among staff about the importance of health education, accurate medical history to recognize possible risk factors, and continuous monitoring of vital signs before and after childbirth. Immediate recognition of symptoms and warning clinical signs allows for prompt multidisciplinary decision-making on treatment to avoid short- and long-term complications and outcomes.
Keywords:Posterior Reversible Encephalopathy Syndrome; Early diagnosis: Treatments, health education
Introduction
Posterior Reversible Encephalopathy Syndrome, known as PRES, was first described by Hinchey J. et al. in 1996 [1]. However, some authors questioned its reversibility. Narbone M.C. et al. [2] suggested to define this condition as a potential Reversible Encephalopathy Syndrome (RES), to emphasize two aspects: - the posterior localization of the oedema, even if constant, could represent the most relevant finding of a diffuse oedema; - the reversibility is not spontaneous, but the result of adequate treatment. It is commonly observed in young or middle-aged adults, but it may occur at any age. A female predominance is reported [3,4]. The characteristic clinical signs of PRES begin abruptly and are rapidly evolving. Acute neurological manifestations develop over few hours, but also with a latency of days, even weeks [5]. The main symptoms and signs are headache, visual disturbances, from hemianopsia to cerebral blindness, other focal neurological deficit. Encephalopathy with confusion, altered consciousness and seizures, with possible focal onset and secondary generalization, are pathognomonic. Memory impairments may be observed. Fischer M., Schmutzhard E. [6] reported epileptic seizures in 70-74%, disorder of consciousness in 67-90%, high arterial pressure or fluctuations in arterial pressure in 61-80%, encephalopathy in 28-92%, visual disturbances in 20- 67%, headache in 26-53%, other focal neurological deficit in 5-15%. The rate of epileptic seizure may be even higher, up to 81% of PRES cases [7]. Three to 17% of epileptic seizures evolve to status epilepticus [8-10]. At onset PRES is a rather non-specific clinical picture, which must be differentiated from other conditions with different pathogenesis, requiring specific therapeutic approaches, as infectious encephalitis, autoimmune and paraneoplastic encephalitis, central nervous system vasculitis, primary and secondary neoplasms, progressive multifocal leukoencephalopathy, osmotic demyelinating syndromes, other demyelinating encephalopathies, toxic encephalopathies. An algorithm including acute onset of neurological disorders, neuroimaging abnormality and reversibility of clinical and radiological findings are highly suggestive of PRES (5). A warning score system consider risk factors, clinical features and EEG findings (> 10 = likely PRES) [11].
Regression of the clinical picture may occur rapidly following the administration of drugs, although cerebral oedema may persist over time, especially when the possible causes of PRES are not identified. Rarely, it may lead to disabling outcomes due to epileptic status, ischemic and/or hemorrhagic cerebral stroke, coma that require hospitalization in intensive care [9]. Complete recovery is reported in 75-90%, neurological sequelae in 10-20% [12], in up to 42% [13], poor neurological deficit with a Modified Rankin Scale of 2-6 in 36% [14], mortality in up to 36% of the patients [15].
The gold standard in diagnosis is represented by Nuclear Magnetic Resonance (MRI), particularly the sequences obtained in relaxation time 2 (T2) and Fluid Attenuated Inversion Recovery (FLAIR). The most characteristic imaging pattern is the presence of oedema in the white matter of the posterior regions of both cerebral hemispheres, typically in the parieto-occipital regions. Timely diagnosis and multidisciplinary decision-making regarding the most appropriate therapy are essential to avoid complications and short- and long-term outcomes. We report the case of PRES during puerperium in a young woman, with no risk factors and predisposing conditions.
Case Report
A 33-year-old woman (G.S.), in her second pregnancy was admitted at 39 weeks of gestation for delivery by elective cesarean section, for previous cesarean section for post-term delivery in the first pregnancy. At case history neither previous risk factors for PRES nor diseases were reported. The course of the pregnancy was physiological, although an excessive weight gain (about 20 kg) was reported.
At admission, general condition was good. The following parameters were measured: arterial blood pressure (BP) 100/70 mmHg, heart rate 62 beats/minute, body temperature 36.5°C, Oxygen Saturation 97%. Pre-operative haematological parameters were within normal limits. At cardiology examination, no abnormality was present, electrocardiogram and echocardiogram were normal. The patient underwent cesarean section under spinal anesthesia. Vital signs and diuresis during surgery and in the following two hours postpartum were normal. At day 2, the patient began to complain headache in orthostatism. In the suspicion of headache after lumbar puncture, she was hydrated, kept in bed in lateral-prone or supine recumbency. Betamethasone 4 mg 1 vial, bid, im, and Paracetamol 1000 mg 1 vial, bid, iv., were administered.
At day 5, due to the persistence of headache and the appearance of mild mental confusion, a neurological evaluation was requested, which showed the presence of hyperelicitable patellar deep tendon reflexes. At day 6, after the routine morning check of the vital parameters, which were normal, the patient complained malaise, followed by loss of consciousness for few seconds, with fall to the ground. When consciousness resumed, the general and neurological physical examination was normal. During the same morning, the patient experienced two more episodes of loss of consciousness with tonic stiffening, followed by generalized tonic-clonic seizures, treated with bolus of diazepam 10 mg, twice, iv. Moreover, magnesium sulphate 1 vial in 100 ml 0,9% sodium chloride solution and levetiracetam 500 mg in 500 ml 0,9% sodium chloride solution, 20 ml/h, iv, were added to therapy, already after the first seizure, and increased at 1000 mg/daily after 12 hours (Figure 1 and 2).
In the post-critical phase, the patient appeared in a mild state of confusion with amnesia of the event. Vital signs continuously monitored from the first episode were normal, except for a BP measurement of 155/90 mmHg. The patient underwent neuroimaging. Cranial computerized tomography showed a swelling of the genienal soft tissues on the left, with tenuously hyperdense tissue, related to mild extravasation of blood, because of the trauma. T2 FLAIR MRI showed alterations in the cortico-subcortical signal, not only in bilateral parieto-occipital regions, but also in frontal lobes and at the vertex (Fig.1 A). Clinical picture, supported by MRI findings, confirmed the diagnosis of PRES. Anti-inflammatory, analgesic, anti- epileptic, anti-hypertensive and anti-oedema therapy was set up according to the following scheme: Perfalgan 1000 mg 1 vial, bid, iv; Decadron 4 mg 1 vial x 3, iv; Levetiracetam 500 mg/5 ml 2 vials in 500 ml 0,9% sodium chloride solution in 24 hours, Nimodipine 5 drops x 3, after BP control; osmotic therapy was suggested in case of symptoms and signs of intracranial hypertension. During post-critical observation, vital signs were normal, except for a BP value of 152/94 mmHg. The hydroelectrolyte balance and blood chemistry tests were normal. The condition of the patient improved rapidly. At day 9, the patient was discharged wit maintenance therapy and an indication of follow-up at one month. The last EEG and MRI check, performed at month IV, showed a complete regression of the clinical picture (Figure. 1 B).
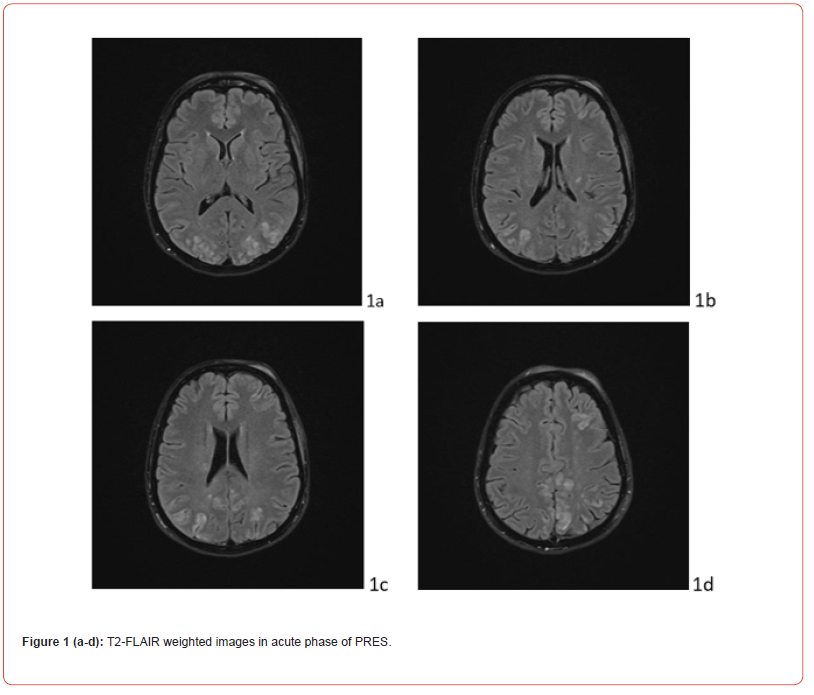
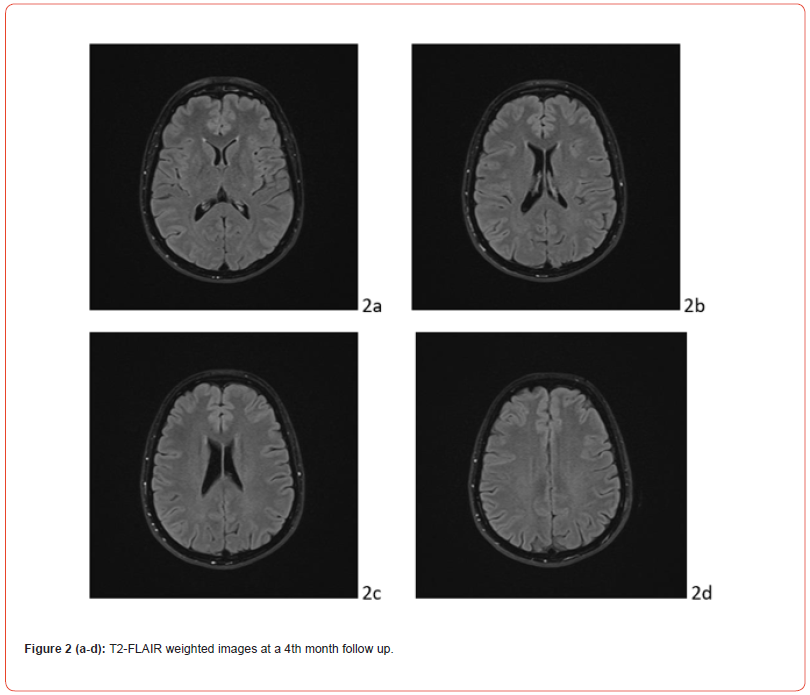
Discussion
PRES is a rare neuroradiological clinical condition, whose pathogenesis is not fully elucidated, yet. Physiological cerebral perfusion pressure is 50-150 mmHg. This allows a constant cerebral blood flow, regulated through a fine innervation of the tunica media of the cerebral arterioles, that modifies their caliber in response to stimuli of various kinds and perturbations of the homeostatic balance, such as transmural pressure, partial pressure of CO2, levels of catecholamines. The most accredited hypothesis sustains an alteration of these mechanisms of autoregulation of the cerebral circulation in PRES, caused by multiple factors. This dysfunction may account for development of focal vasogenic oedema. The poor sympathetic innervation of the posterior cerebral circulation and reduced opposition to parasympathetic reflex vasodilatation might explain the increased sensitivity of the parieto-occipital areas to changes in blood pressure and parenchymal perfusion [16]. Usually, acute peaks of arterial hypertension, pre clampsia/eclampsia, haemolysis syndrome, elevated liver enzymes, low platelets (HELLP) in pregnancy, puerperium, infections, sepsis, hypercalcemia, other systemic conditions, autoimmune and neoplastic diseases, renal failure, organ transplantation, administration of drugs (including immunosuppressants, chemotherapy, etc.) are the most common comorbidities. However, severe cases of PRES have also been described in the absence of elevated blood arterial pressure and/or other predisposing factors. Then, the most reliable critical factor seems to be related to abrupt changes in blood pressure values, leading to cerebral hypo- and hyperperfusion phenomena, which may cause damage to the vascular endothelium with rupture of the blood-brain barrier and possible subsequent extravasation of plasma and macromolecules into the brain parenchyma [17]. According to the “vasogenic theory” the damage is caused by sudden raise in blood arterial pressure, linked to an increased production of vasopressin and catecholamines, activation of the renin-angio tensin system, alteration of the cerebral autoregulatory response, production of endothelins with both vasoconstrictor and vasodilator effects through EBA and EBB receptors, respectively [18,19], subsequent damage of the blood-brain barrier, apparent hyperperfusion with extravasation of plasma and macromolecules, vasogenic oedema, followed by the activation of a cascade of inflammatory events, increased expression of the NF-kB pathway, production of cytokines, such as IL-6.
Thirty percent of PRES patients are normo or hypotensive [6,20]. According to the “neuropeptide theory”, deriving from the observation of the syndrome also in subjects suffering from arterial hypotension, the damage of the blood-brain barrier is caused by hypoperfusion, related to the presence of endogenous factors or exogenous toxins [21] that cause the release of vasoactive molecules, such as bradykinin, histamine, endotelins, nitric oxide, arachidonic acid, thromboxane A2, prostacyclin, oxygen radicals. Phenomena of vasoconstriction and vasodilation, downstream hypoperfusion, modification of the expression of endothelial adhesion molecules, with recruitment and crossing of cells of the immune system through the endothelial wall, cytokine production occur. Possible complement activation, humoral and cell-mediated cytotoxicity may further worsen endothelial and cerebral damage and trigger autoimmune responses. However, a study on cerebrospinal fluid and peripheral blood showed activation of innate immune response, characterized by the presence of intermediate monocytes CD14++/CD16+, predictive of PRES diagnosis and correlated with duration of hospital stay. These cells are potent activators of Th17 cells. They may herald a downstream vascular dysfunction in response to systemic challenges, as infections or other conditions of immunosuppression [22].
These mechanisms are confirmed by studies performed with cerebral angiography and MRI with angio sequences, which showed vessel wall irregularities (“string of beads appearance”), suggestive of vasoconstriction and vasodilation phenomena, in more than 80% of patients with PRES [23].
Endothelial damage and dysfunction of the cerebral arterioles are evident after exposure to agents that damage the blood-brain barrier (cytotoxic drugs, immunosuppressants, endothelial toxins), in pre-eclampsia and hypertensive encephalopathy. The former is a condition related to an imbalance between pro-angiogenic and anti-angiogenic factors, leading to placental dysfunction with subsequent generalized extension of endothelial injury [24].
Thus, the onset of PRES, even in apparently healthy subjects, might be determined by subacute and transient increases or decreases in blood pressure, due to dysregulation of responsiveness of the vascular bed. The altered vascular permeability is initially reversible, and, once the triggering cause has been removed, the pre-existing physiological condition is restored within a few weeks. Another pathogenetic hypothesis ought to be considered. Gonadal hormones flare up during pregnancy and sharply drop after delivery. Their levels are influenced by maternal age, parity, body mass index (BMI), ethnicity, gender of the foetus, and lifestyle factors. Deviating steroid concentrations during the peripartum may be associated with pathological conditions at brief and long term [25]. Sex steroids are mainly secreted by ovaries, but cerebral production is also described, through de novo synthesis from cholesterol or through resynthesis of local steroid metabolites. In autocrine, paracrine and endocrine ways, they modulate neuronal excitability and brain plasticity. They are involved in brain development and plasticity, influencing cell migration and differentiation, axonal sprouting, synaptogenesis, dendritic branching, and myelination. Their trophic effects emerge early in brain development and keep on acting on adulthood, both in healthy and injured tissues [26]. They stabilize neuronal function, support neuronal viability, prevent neuronal death, through regulation of neuronal gene transcription, action on GABA-A receptors, inhibition of glutamate-mediated toxicity, reduction of NF-kappa-B activation, expression of inducible nitric oxide synthase and production of inflammatory mediators, promoting anti-oxidant activity via preventing lipid peroxidation and scavenging free radicals. Oestrogen increases cerebral blood flow and angiogenesis, through release of endothelium- derived relaxing factor, antagonism of endothelin-related vasoconstriction, hyperpolarization of vascular smooth muscle, calcium antagonist effect. Moreover, progesterone modulates the expression of aquaporin 4 channels, reducing brain oedema, and activates the expression of brain neurotrophic factor [27]. During pregnancy hormonal levels have a role in inducing and maintaining tolerance to paternal alloantigens to prevent rejection of the foetus. A shift towards Th1 dominance, and a fall in Th2 and Treg cells, followed by altered cytokine pattern in the first weeks following delivery, have been reported. Indeed, all these changes may result in the worsening of Th1 and Th17-type autoimmune diseases after delivery. In the postpartum period remarkable decrease of Leukaemia Inhibitory Factor Receptor (LIF-R), Latency-Associated Peptide Transforming Growth Factor beta-1 (LAP TGF-beta-1), C-C motif Chemokine 28 (CCL28), Oncostatin M (OSM) and Fibroblast Growth Factor 21 (FGF21) are detected, together with decrease of Interleukin (IL) 6 and IL-10, while Tumor Necrosis Factor ligand superfamily member 11 (TRANCE), Tumor Necrosis Factor ligand superfamily member 12 (TWEAK), and C-C motif Chemokine/Eotaxin (CCL11) increase [28]. Therefore, all the described cascade of events, hormonal changes included, may contribute to PRES development. Their entity and persistence are crucial in determining its extension and severity. The prevalence and incidence of PRES may be underestimated, considering that this potentially pathological milieu may develop after delivery.
MRI of the brain is the gold standard diagnostic tool. It allows early detection of diffuse vasogenic oedema of the white matter, its posterior site, in the parieto-occipital regions, extent of damage, differential diagnosis with other pathological conditions. The peculiar lesions of PRES are symmetrical, hypointense in T1-weighted sequences, hyperintense in T2-weighted and T2 FLAIR sequences, isointense or mildly hyperintense in DWI. They have a watershed pattern [29]. Apparent diffusion coefficient (ADC) maps may show normal or increased diffusion in the case of vasogenic edema (signal hyperintensity), restricted in the case of cytotoxic edema (signal hypointensity). Following gadolinium administration, linear or perimetral enhancement (gyrus-like) was observed in 20% of patients. PRES is considered mild when cortical and subcortical white matter oedema is present, without mass effect, herniations, hemorrhages, minimal involvement of another region (cerebellum, brainstem, basal nuclei). Moderate PRES is defined by the presence of confluent oedema extending from the cortex to the deep white matter without extension to the periventricular regions or mild involvement of two of the other regions indicated above (cerebellum, brainstem and basal nuclei). A mild mass effect may be present, without herniations or midline shifts, hemorrhages. Severe PRES is characterized by confluent oedema extending from the cortex to the ventricles, midline shift or herniation due to oedema or hemorrhage, involvement of three other regions (cerebellum, brainstem, and basal ganglia) [30]. As mentioned above, DWI sequences and ADC maps are useful for distinguishing vasogenic edema from cytotoxic edema, typical of hypoperfusion in cases of cerebral infarction or other conditions, such as inflammatory, demyelinating and space-occupying lesions. However, small areas of restriction of diffusion and large areas of vasogenic oedema are found in 15-33% of PRES patients [31,32]. These, hyperintense in DWI, hypointense in ADC maps, indicate cytotoxic edema and are predictive of incomplete recovery and poor prognosis [33]. The study of intracranial vessels with MRI with angio sequences, CT angiography and transcranial Doppler ultrasound are indicated for the differential diagnosis with reversible cerebral vasoconstriction syndrome, which also appears with intense headache in the postpartum period [34]. Cerebral hemorrhages are found in 10–30% of cases.
These are of different magnitudes, from microbleeds in susceptibility- weighted sequences (SWI), to minute focal hemorrhages (<5 mm), subarachnoid hemorrhages at the level of the cerebral sulcus, focal hematomas of variable size [31,35]. By SWI images, they may be detected in 64% of PRES cases [36].
Arterial Spin Labeling MRI showed hyperperfusion in the majority of PRES patients. However, conflicting results are reported on perfusion images. Considering time of imaging, hyperperfusion is detected in acute phase, hypoperfusion in subacute phase [37]. The evolution of PRES is usually benign and is closely linked to the timeliness of diagnosis and therapy. However, residual vascular parenchymal brain damage may be observed [31].
There are no specific indications regarding treatment. Certainly, the elimination of any triggering factors and the normalization of blood pressure values are essential to avoid ischemic and/or haemorrhagic complications in the brain. Membrane stabilizing drugs and anti-oedema drugs can also help, if necessary, to promote the resolution of cerebral oedema. However, steroids may even contribute to worsening of clinical conditions [38]. A complete regression of clinical manifestations has been described in 35 to 100% of cases. In the case of neurological complications, the regression rate is lower (49-75%), over a period ranging from 5-7 days to 17 months. Predictive factors of malignant PRES are related to Glasgow Coma Scale < 8, clinical worsening despite treatment of intracranial pressure, radiological severity [39]. Recurrences were described in about 2-4% of the cases, even in 8% of the cases [40-42]. The prognosis is generally favorable, with rapid recovery in most cases (75-90%). The mortality rate is 6-36% and is mainly related to cerebral hemorrhage, acute hydrocephalus, marked cerebral edema [7,35,39,41,43]. The risk of epilepsy and stroke is higher in patients with PRES positive history case [44,45].
Conclusions
PRES is a recently described, little-known and often undiagnosed syndrome in gynecology and obstetrics services. Although almost always related to eclampsia, pre-eclampsia and HELLP syndrome, it has also been described in women who have recently given birth with normal blood pressure values and without other risk factors. The case of PRES come to our observation involved a woman who had recently given birth in good health. Medical history was negative regarding the presence of risk factors for PRES. The available clinical and laboratory data ruled out a condition of eclampsia, even in its atypical forms, as well as the presence of other morbid conditions. It is hypothesized that a condition of altered vascular bed responsiveness with temporary and subacute arterial pressure changes, due to autonomic dysregulation, hormonal imbalance, triggered the onset of PRES. Lastly, we do not exclude that constitutional meiopragic status, together with increased venous stasis, because of excessive body weight gain, may have further contributed to PRES pathogenesis, accounting for the peculiar localization in posterior and watershed areas. The initial symptom was headache, initially interpreted as a complication of spinal anesthesia. Its persistence and the subsequent appearance of seizures led to the hypothesis of a different genesis. Close monitoring of symptoms coordinated management involving a multidisciplinary team and targeted diagnostic investigations (MRI) allowed a precise diagnosis and an adequate therapy with a favorable prognosis for the patient. Further studies are needed to deep the knowledge on PRES. The incidence may be underestimated. On a predisposing asset, abrupt modifications of vascular tone and hormonal levels may trigger clinical manifestations and account for the radiological findings. Health education of healthcare professionals is pivotal for early recognition of clinical features and prompt treatments to reduce maternal morbidity and mortality and neurological sequelae in the short and long term.
Acknowledgements
Consent was obtained from the patient and submission was approved by ethics committee.
Disclosure
The authors report no conflicts of interest in this work.
References
- Hinchey J, Chaves C, Appignani B, J Breen, L Pao, et al. (1996) A reversible posterior leukoencephalopathy syndrome. New Engl J Med 334(8): 494–500.
- Narbone MC, Musolino R, Granata F, Mazzù I, Abbate M, et al. (2006) PRES: Posterior or potentially reversible encephalopathysyndrome? Neurological Sciences 27:187–189.
- Fugate JE, Claassen DO, Cloft HJ, Kallmes DF, Kozak OS, Rabinstein AA (2010) Posterior reversible encephalopathy syndrome: associated clinical and radiologic findings. Mayo Clin Proc 85:427–432. doi:10.4065/mcp.2009.0590.
- Yamamoto H, Natsume J, Kidokoro H, Ishihara N, Suzuki Met al. (2015) Clinical and neuroimaging findings in children with posterior reversible encephalopathy syndrome. Eur J Paediatr Neurol EJPN Off J Eur Paediatr Neurol Soc 19:672–678.
- Fugate JE, Rabinstein AA (2015) Posterior reversibile encephalopathy syndrome: clinical and radiological manifestations, pathophysiology and outstanding questions. Lancet Neurol 14:914-925.
- Fischer M, Schmutzhard E (2017) Posterior reversible encephalopathy syndrome. J Neurol 264:1608–1616.
- Legriel S, Schraub O, Azoulay E, Hantson P, Magalhaes E, et al. (2012) Determinants of recovery from severe posterior reversible encephalopathy syndrome. PLoS One 7: e44534.
- Kozak OS, Wijdicks EF, Manno EM, Miley JT, Rabinstein AA (2007) Status epilepticus as initial manifestation of posterior reversible encephalopathy syndrome. Neurology 69: 894– 897.
- Lee VH, Wijdicks EF, Manno EM, Rabinstein AA (2008) Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol 65:205–210.
- Hinduja A (2020) Posterior reversible encephalopathy syndrome: clinical features and outcome. Front Neurol 11:71.
- Zhou LP, Liu LY, Li H, Yang-Yang Wang, Ying Liu, et al. (2019) Establishment and utility assessment of posterior reversible encephalopathy syndrome early warning scoring (PEWS) scale establishment and utility assessment of PEWS scale. BMC Neurol 19: 30.
- Frick D, Huecker M, Shoff H (2017) Posterior reversible encephalopathy syndrome presenting as stroke mimic. Clinical practice and cases in emergency medicine 1:171–174.
- Ismail FS, van de Nes J and Kleffner I (2021) A broad spectrum of posterior reversible encephalopathy syndrome ‑ a case series with clinical and paraclinical characterisation, and histopathological findings. BMC Neurol 21:386.
- Hinduja A, Habetz K, Raina S, Ramakrishnaiah R, Fitzgerald RT (2016) Predictors of poor outcome in patients with posterior reversible encephalopathy syndrome. Int J Neurosci 1–10.
- Liman TG, Bohner G, Endres M, Siebert E (2014) Discharge status and inhospital mortality in posterior reversible encephalopathy syndrome. Acta Neurol Scand 130: 34–9. 368
- Rabinstein AA, Mandrekar J, Merrell R, Kozak OS, Durosaro O, (2012) Blood pressure fluctuations in posterior reversible encephalopathy syndrome. J Stroke Cerebrovasc Dis 21: 254–258.
- Archana H (2020) Posterior Reversible Encephalopathy Syndrome: Clinical Features and Outcome. Frontiers in Neurology 11:71.
- Chen SP, Fuh JL, Wang SJ (2011) Reversible cerebral vasoconstriction syndrome: current and future perspectives. Expert Rev Neurother 11: 1265-1276.
- Koyama Y (2013) Endothelin systems in the brain: involvement in pathophysiological responses of damaged nerve tissues. BiolMol Concepts 4: 335-347.
- Feske SK (2011) Posterior reversible encephalopathy syndrome: a review. Semin Neurol 31:202–215.
- Bartynski WS (2008) Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol 29:1043-1049.
- Nelke C, Schulte-Mecklenbeck A, Pawlitzki M, Rolfes L, Räuber S, et al. (2021) The Innate Immune Response Characterizes Posterior Reversible Encephalopathy Syndrome. Journal of Clinical Immunology 41:1229–1240.
- Bartynski WS, Boardman JF (2008) Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol 29: 447–455.
- Umapathy A, Chamley LW, James JL (2020) Reconciling the distinct roles of angiogenic/antiangiogenic factors in the placenta and maternal circulationof normal and pathological pregnancies. Angiogenesis 23: 105-117.
- Dukic J, Ehlert U (2023) Longitudinal Course of Sex Steroids From Pregnancy to Postpartum Endocrinology 164: 1–15.
- Rehbeina E, Hornunga J, Sundström Poromaa I, Derntla B (2021) Shaping of the Female Human Brain by Sex Hormones: A Review. Neuroendocrinology 111:183–206.
- Brotfain E, Gruenbaum SE, Boyko M, Kutz R, Zlotnik A and Klein M (2016) Neuroprotection by Estrogen and Progesterone in Traumatic Brain Injury and Spinal Cord Injury. Current Neuropharmacology 14: 641-653.
- Bränn E, Edvinsson A, Rostedt Punga A, Inger Sundström-Poromaa I, Skalkidou A (2019) Inflammatory and anti-inflammatory markers in plasma: from late pregnancy to early postpartum. Scientific Reports 9:1863.
- Bartynski WS, Boardman JF (2007) Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. Am J Neuroradiol 28: 1320–1327.
- McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS (2007) Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol 189: 904-912.
- Liman TG, Bohner G, Heuschmann PU, Endres M, Siebert E (2012) The clinical and radiological spectrum of posterior reversible encephalopathy syndrome: the retrospective Berlin PRES study. J Neurol 259: 155-164.
- Zhang N, Yang L, Han A, Wang Y, Zhao G, Wang Y and Chen T (2023) Advances in imaging findings of preeclampsia-related reversible posterior leukoencephalopathy syndrome. Front. Neurosci., Brain Imaging Methods 17.
- Moon SN, Jeon SJ, Choi SS, Song CJ, Chung GH, Yu IK, et al (2013) Can clinical and MRI findings predict the prognosis of variant and classical type of posterior reversible encephalopathy syndrome (PRES)? Acta Radiol 54:1182-1190.
- Ducros A (2012) Reversible cerebral vasoconstriction syndrome. Lancet Neurol 11:906-917.
- McKinney AM, Sarikaya B, Gustafson C, Truwit CL (2012) Detection of microhemorrhage in posterior reversible encephalopathy syndrome using susceptibility-weighted imaging. Am J Neuroradiol 33: 896–903.
- Fazeli S, Noorbakhsh A, Imbesi SG, Bolar DS (2022) Cerebral perfusion in posterior reversible encephalopathy syndrome measured with arterial spin labeling MRI. NeuroImage: Clinical 35:103017.
- Farooq S, Testai FD (2019) Neurologic complications of sickle cell disease. Current Neurology and Neuroscience Reports 19(4):17.
- Akins PT, Axelrod Y, Silverthorn JW, Guppy K, Banerjee A, Hawk MW (2014) Management and outcomes of malignant posterior reversible encephalopathy syndrome. Clin Neurol Neurosurg 125:52-57.
- Garg A, Elmashala A, Roeder H (2022) Early readmissions after hospitalization for posterior reversible encephalopathy syndrome. Neurology 99:18.
- Servillo G, Striano P, Striano S, Tortora F, Boccella P, De Robertis E, Rossano F, Briganti F & Tufano R (2003) Posterior reversible encephalopathy syndrome (PRES) in critically obstetric patient. Intensive Care Med 29:2323-232.
- Sweany JM, Bartynski WS, Boardman JF (2007) “Recurrent” posterior reversible encephalopathy syndrome: report of 3 cases--PRES can strike twice! J Comput Assist Tomogr 31:148-156.
- Alhilali LM, Reynolds AR, Fakhran S (2014) A multi-disciplinary model of risk factors for fatal outcome in posterior reversible encephalopathy syndrome. J Neurol Sci. 347: 59-65.
- Hefzy HM, Bartynski WS, Boardman JF, Lacomis D (2009) Hemorrhage in posterior reversible encephalopathy syndrome: imaging and clinical features. AJNR Am J Neuroradiol 30:1371–1379.
- Seitz A, Parauda SC, Omran SS, Schweitzer AD, Liberman AL, et al. (2023) Long-term risk of seizure after posterior reversible encephalopathy syndrome. Annals of Clinical and Translational Neurology 10: 610–618.
- Parauda SC, Zhang C, Omran SS, Schweitzer AD, Murthy SB, et al. (2022) Risk of Stroke after Posterior Reversible Encephalopathy Syndrome. Stroke 53: 3313–3319.
-
Fiori Patrizia, Miele Antonella, Capaldo Guglielmo and Del Medico Francesco etc all... Posterior Reversible Encephalopathy Syndrome: Clinical Case and Literature Review. Arch Neurol & Neurosci. 16(5): 2024. ANN.MS.ID.000898.
-
Acute Polyradiculoneuritis, Neurology, Guillain-barre; Fann teaching hospital, neuropathy, cranial nerves, immunotherapy, Neuroscience, neurogenic syndrome, epidemiological, Dysphonia.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






