 Research Article
Research Article
Investigation of The Frequency and Risk Factors of Hyponatremia in Patients Receiving Levetiracetam Treatment
Faruk Incecik* and Ozlem M Herguner
Divisions of Neurology, Department of Pediatrics Çukurova University, Adana, Turkey
Faruk Incecik, Divisions of Neurology, Department of Pediatrics Çukurova University, Adana, Toros mah. 78186 sok., Yeşilpark Evleri, Kat: 7, no: 13, Adana, Turkey.
Received Date: July 04, 2022; Published Date: July 22, 2022
Background: Levetiracetam (LEV) is a widely used antiepileptic drug (AED) in the treatment of various type of seizures. The side effects of
LEV are somnolence, asthenia, dizziness, mood changes, kidney dysfunction, rhabdomyolysis, minor infections, and thrombocytopenia. Recently,
hyponatremia due to LEV therapy has been reported.
The Aim: The goal of the study was to evaluate frequency and risk factors associated with hyponatremia in LEV administration.
Method: One hundred and fiftytwo children were enrolled. The risk factors were analysed.
Results: Among the 152 patients, 79 (52.0%) were boys and 73 (48.0%) were girls, and the mean age was 90.37 ± 46.43 months (24-188
months). Of the 152 patients, 64 (42.1%) were treated monotherapy, and 88 (57.9%) polytherapy. We detected hyponatremia in one patient (0.7%).
The serum sodium level was 130 mmol/l. The patient was asymptomatic, and LEV dose was 25 mg/kg. To investigate the etiology of hyponatremia
in the patient, syndrome of inappropriate antidiuretic hormone secretion tests were performed and found normal. There were no significant
differences in the hyponatremia between the patients treated with LEV.
Conclusion: We detected that LEV may cause hyponatremia. When treating patients with LEV, clinicians should closely monitor serum sodium
level. To the best of our knowledge, this is the first study of hyponatremia associated with LEV therapy in children.
Keywords:Levetiracetam; Hyponatremia; Children
Abbreviations:AEDs: Antiepileptic Drugs; LEV: Levetiracetam; GABA: Gammaaminobutyric Acid; ILAE: International League Against Epilepsy; CT: Cerebral computed tomography; SIADH: Antidiuretic Hormone Secretion
Background
Epilepsy is one of the common chronic illnesses of childhood. Antiepileptic drugs (AEDs) are used for its long-term treatment. This therapy may be associated with several side effects. Levetiracetam (LEV) is a new broad spectrum antiepileptic agent with favourable efficacy and low profile of toxicity in epilepsy treatment. LEV does not bind to plasma proteins, and is eliminated by the kidneys [1]. It has been argued that LEV can act on the N-type Ca2 channel and can reverse the gammaaminobutyric acid (GABA) and glycine-gated currents [2].
Side effects include somnolence, asthenia, dizziness, mood changes, minor infections, skin rashes, thrombocytopenia, interstitial nephritis and rhabdomyolysis [1-5]. Recently hyponatremia has been recognized to be one of the adverse effects of LEV [6-10]. It is known that hyponatremia is caused by the use of AEDs such as carbamazepine and oxcarbazepine [11]. To our knowledge, there are a few case reports of LEV associated hyponatremia in the literature [6,7,9,10]. However, there are no studies of LEV associated hyponatremia. Therefore, we planned a study designed to identify the frequency and risk factors associated with hyponatremia during LEV therapy in children with epilepsy.
Methods
This retrospective study was performed in the pediatric neurology department of our hospital between January 2016 and January 2018. Patients with symptoms and signs of illnesses other than epilepsy (eg, kidney, liver, gastrointestinal, endocrine, and metabolic). In addition, patients who received carbamazepine, oxcarbamazepine and other medication (such as diuretics), which can cause hyponatremia, were not included in the study. The patients were divided into two groups according to their therapy. Group 1 consisted of 64 patients who treated with only LEV, group 2 consisted of 88 patients treated with add on LEV. Group 1 has a history of new-onset epilepsy and treated monotherapy with LEV. Group 2 was treated add-on LEV included patients with more treatment refractory epilepsy, who were taking 1-3 AEDs, and had been diagnosed with intractable epilepsy. The institutional ethics committee approved the study protocol. Possible risk factors that may have a role in the hyponatremia prognosis such as age, sex, number of the antiepileptic drugs (mono or polytherapy), dosage of LEV, intellectual disability, neurological abnormality, neuroimaging findings, and etiology were investigated. We classified seizures according to the International League Against Epilepsy (ILAE) criteria. According to the guidelines of ILAE, seizures were divided partial, generalized, and mixed seizures. Aetiologies of the epilepsies were divided into 3 groups as symptomatic, idiopathic, and cryptogenic. Cerebral computed tomography (CT) and/or magnetic resonance imaging (MRI) studies were performed in all patients. Considering the related neuroradiologic studies, the patients were divided into 2 groups: normal and abnormal. The tests used to determine the degree of mental retardation of the patients were the Wechsler Intelligence Scale for Children (standardized for Turkish children) in patients aged 6 to 16 years and the Stanford Binet (American format) for patients aged 2 to 6 years. Levetiracetam was initially administered in a twice daily administration with a starting dose of 10 mg/kg per day. Further doses were titrated until patients were seizure-free. Patients who were started at the beginning of the study and followed for at least 12 months were included in the study. Serum concentration of LEV could not do in our hospital. Retrospectively, patients whose serum sodium levels were examined before the start of treatment and 3 and 12 months after treatment were recorded. A sodium level lower than and equal to134 mEq/L was defined as hyponatremia.
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) version 21.0 (IBM SPSS Corp.; Armonk, NY, USA). Descriptive statistical methods (mean, median) as well as qualitative data were compared using the chi-square test. P < .05 was considered as a significance level.
Result
One hundred fiftytwo children, mean age 90.37 ± 46.43 months (24-188 months), were included in this study. Among the 152 patients, 79 (52.0%) were boys and 73 (48.0%) were girls. Of the 152 patients, 64 (42.1%) were treated monotherapy, and 88 (57.9%) polytherapy. Hyponatremia was detected only in 1 (0.7%) patient. The patient was a 5-year-old girl and she had mild intellectual disability. She was only receiving LEV therapy. The serum sodium level measured at 3 months of treatment was 130 mmol/l (normal range; 135-145 mmol/l) and. The patient was asymptomatic, and LEV dose was 25 mg/kg. To investigate the etiology of hyponatremia in the patient, syndrome of inappropriate antidiuretic hormone secretion (SIADH) tests were performed and found normal. We thought that hyponatremia may be due to LEV therapy. LEV treatment was discontinued, and the serum sodium level normalized after 3 days.
Table 1:Summary of demographics data and risk factors for hyponatremia in study.
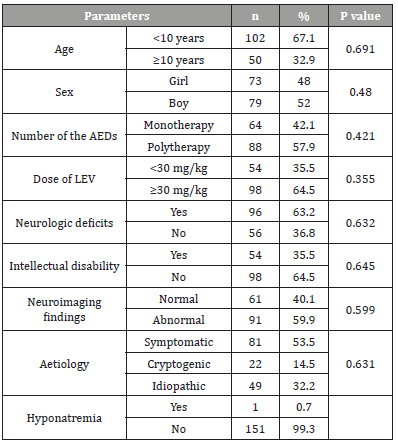
AEDs: Antiepileptic drugs; LEV: Levetiracetam
There was no correlation between hyponatremia and age, sex, number of the AEDs, LEV dose, intellectual disability, neurological abnormality, abnormal cerebral magnetic resonance findings, and aetiology. Table 1 shows demographics data and the associations between hyponatremia and risk factors (Table 1).
Discussion
Levetiracetam is an AED, used for the treatment of epilepsy, either monotherapy or add-on therapy. LEV has a favorable pharmacological profile, with almost complete absorption after oral administration, linear pharmacokinetics, and a low extent of metabolism. Levetiracetam is unlikely to interact with other AEDs, because it has a low protein binding [1,2]. Comparison with the other AEDs, LEV use is gradually increasing due to its favourable pharmacokinetics and safety.
The most frequently observed adverse effects were somnolence and behavioural. However, LEV induced thrombocytopenia, interstitial nephritis, rhabdomyolysis, fulminant liver failure, and rise of gama glutamyl transferase have been reported in patients in literature [1,2,5]. Recently, a few cases have been reported hyponatremia due to LEV treatment [6,8,10]. In previously reports, hyponatremia was reported to occur with the use of antiepileptic drugs (AEDs) such as carbamazepine, oxcarbazepine, valproate, lamotrigine, and lacosamide [11-14]. In the recent a report, Rosca et al. [10] reported a 73-year-old man with epilepsy in whom hyponatremia was induced by only LEV. Nasrallah et al. [6] described hyponatremia due to LEV therapy in a 65-year patient. The patient was receiving LEV and pantoprazole. Belcastro et al. [8] observed a patient developing hyponatremia with LEV monotherapy. The other case was described by Cordoba Lopez et al. [7]. Arı et al. [9] reported a 74-year-old man with hyponatremia induced only LEV therapy. In the literature, to our knowledge, this is the first report of hyponatremia due to LEV therapy in the children.
Since epilepsy is a common disease requiring long-term treatment with AEDs, adverse effects such as hyponatremia can be a major problem [11,12,14]. Especially carbamazepine and oxcarbazepine were associated with a vastly increased risk [11,15]. Both carbamazepine and oxcarbazepine are known to cause hyponatremia with an occurrence of 4.8-31.3% and 0.14- 73.3%, respectively [11,16]. Although not fully elucidated, SIADH is believed to be the major contributor of AEDs-induced hyponatremia [16,17]. However, some studies have demonstrated that AEDsinduced hyponatremia can occur without ADH levels being affected [18,19], Some antiepileptic drugs may have a direct effect on renal tubules and/or increase the tubular response to ADH [16]. Of the previously published cases with LEV-induced hyponatremia, four had a predisposition to SIADH [6-10]. Similar to the case of Belcastro et al. [8], we could not detect SIADH as the cause of hyponatremia. Hyponatremia due to lev therapy develops rarely and generally progresses asymptomatically. Similar to the cases reported in the literature [6,8,10] in our case it was asymptomatic.
No clinical studies of hyponatremia have been reported with its use in the literature. Therefore, we conducted a study designed to identify the frequency and risk factors associated with hyponatremia during LEV therapy in children with epilepsy. We detected, one of 152 patients (0.7%) had hyponatremia during LEV therapy. So far, to our knowledge, this is the first study of hyponatremia secondary to LEV treatment in the literature. We detected no significant difference between the risk factors and hyponatremia during LEV therapy.
Conclusion
In conclusion, we found a case with hyponatremia due to LEV treatment, and the patient was asymptomatic. Thus, patients taking LEV and presenting these risk factors should be particularly monitored for laboratory evidence of hyponatremia. Future studies are needed to determine the frequency and risk factors of this side effect, as well as to determine whether hyponatremia is due to SIADH or an alternative mechanism.
Ethics Approval and Consent to Participate
An ethical approval was granted by the Institutional Ethics Committee of Cukurova University.
Conflict of Interest
No conflict of interest noted by the authors
Funding
The authors have not received any kind of financial support in the conduct or publication of this research.
Author’s Contribution
Both the authors both authors contributed at every stage of the article.
References
- Grosso S, Cordelli DM, Franzoni E, Coppola G, Capovilla G, et al. (2007) Efficacy and safety of levetiracetam in infants and young children with refractory epilepsy. Seizure 16: 345-350.
- Patsalos P (2000) Pharmacokinetic profile of levetiracetam: toward ideal characteristics. Pharmacol Ther 85: 77-85.
- Kimland E, Höjeberg B, von Euler M (2004) Levetiracetam induced thrombocytopenia. Epilepsia 45: 877-878.
- Tan TC, de Boer BW, Mitchell A, Delriviere L, Adams LA, et al. (2008) Levetiracetam as a possible cause of fulminant liver failure. Neurology 71(9): 685-686.
- Hurwitz KA, Ingulli EG, Krous HF (2009) Levetiracetam induced interstitial nephritis and renal failure. Pediatr Neurol 41: 57-58.
- Nasrallah K, Silver B (2005) Hyponatremia associated with repeated use of levetiracetam. Epilepsia 46: 972-973.
- Cordoba Lopez A, Granado Martinez D, Perez Frutos MD, Jimeno Torres B (2010) Levetiracetam-associated hyponatremia. Med Clin (Barc) 135: 429-430.
- Belcastro V, Costa C, Striano P (2008) Levetiracetam-associated hyponatremia. Seizure 17: 389-390.
- Arı H, Kahraman F, Acaban MB (2015) The first case of levetiracetam-induced and tolvaptan-resistant hyponatremia. Turk Kardiyol Dern Ars 43: 284-287.
- Rosca EC, Simu M (2018) Levetiracetam-induced hyponatremia. Acta Neurol Belg 118: 123-124.
- Berghuis B, van der Palen J, de Haan GJ, Lindhout D, Koeleman BPC, et al. (2017) Carbamazepine and oxcarbazepine-induced hyponatremia in people with epilepsy. Epilepsia 58: 1227-1233.
- Branten AJ, Wetzels JF, Weber AM, Koene RA (1998) Hyponatremia due to sodium valproate. Ann Neurol 43: 265-267.
- Gandhi S, McArthur E, Mamdani MM, Hackam DG, McLachlan RS, et al. (2016) Antiepileptic drugs and hyponatremia in older adults: two population based cohort studies. Epilepsia 57: 2067-2079.
- Gupta SS, Patti R, Lindsay D, Raheja H, Kupfer Y (2018) Lacosamide: Associated Hyponatremia. Am J Ther 25: e729-e730.
- Dong X, Leppik IE, White J, Rarick J (2005) Hyponatremia from oxcarbazepine and carbamazepine. Neurology 65: 1976-1978.
- Lu X, Wang X (2017) Hyponatremia induced by antiepileptic drugs in patients with epilepsy. Expert Opin Drug Saf 16: 77-87.
- Liamis G, Milionis H, Elisaf M (2008) A review of drug-induced hyponatremia. Am J Kidney Dis 52: 144-153.
- Wales JK (1975) Treatment of diabetes insipidus with carbamazepine. Lancet 2: 948-951.
- Sachdeo RC, Wasserstein A, Mesenbrink PJ, D'Souza J (2002) Effects of oxcarbazepine on sodium concentration and water handling. Ann Neurol 51: 613-620.
-
Faruk Incecik* and Ozlem M Herguner. Investigation of The Frequency and Risk Factors of Hyponatremia in Patients Receiving Levetiracetam Treatment. Arch Neurol & Neurosci. 12(5): 2022. ANN.MS.ID.000796.
-
Hyponatremia, Levetiracetam Treatment, Pediatrics, Neurology, Antiepileptic Drug, Somnolence, Asthenia, Dizziness, Mood Changes, Kidney Dysfunction, Rhabdomyolysis, Minor Infections, Thrombocytopenia, Children, Epilepsy.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






