 Research Article
Research Article
Disease Characteristics and Neuropsychological Performance in Chronic Epilepsy
Theodora Afrantou1, Eleni Konstantinopoulou1,2*, Roza Lagoudaki1 and Panagiotis Ioannidis1
12nd Department of Neurology, Aristotle University of Thessaloniki, Greece
2Schooll of Psychology, Aristotle University of Thessaloniki, Greece
Eleni Konstantinopoulou, 2nd Department of Neurology, Aristotle University of Thessaloniki, Schooll of Psychology, Aristotle University of Thessaloniki, Greece
Received Date: May 29,2025; Published Date:July 07,2025x
Abstract
Background and Objective: Many factors influence cognitive functioning of epileptic patients, including seizure type, epileptic syndrome, seizure frequency, disease duration and antiepileptic medications. The aim of the present study was to evaluate neuropsychological status in chronic outpatients suffering from epilepsy, to investigate cognitive changes over time and to explore the role of seizure frequency, disease duration and antiepileptic therapy on neuropsychological test performance. Materials and Methods: Forty epileptic patients with various types of epileptic syndromes (20 men and 20 women) and 20 healthy controls matched for age, sex and educational status completed neuropsychological tests estimating memory, attention, processing speed and executive function. Patients and controls were assessed at the beginning of the study. Patient group was reevaluated after twelve months. The serum levels of AEDs were monitored during the 12-month follow-up period and they were in therapeutic levels. Results: Significant differences were observed between the two groups of participants in performance on neuropsychological measures. Duration of epilepsy and seizure frequency were correlated with performance on measures of speed processing and verbal memory, respectively. Polytherapy resulted in lower performance on the immediate condition of the visual memory test. On follow-up examination, patients showed diverse patterns of improvement in neuropsychological performance with respect to seizure presence and antiepileptic therapy. Conclusions: Accounting for factors that may affect cognition such as disease characteristics and antiepileptic therapy, the course of cognitive functioning is an issue of considerable interest in chronic epilepsy.
Keywords:Epilepsy, Neuropsychological Assessment, Seizure Type, Duration, Antiepileptic Treatment
Introduction
Many factors influence cognitive deficits in epileptic patients such as seizure type, seizure syndrome, seizure frequency, disease duration and antiepileptic medications [1-3]. The presence and the location of brain pathology seem to be important factors influencing patient’s cognitive function, although there is no specific neuropsychological profile that characterizes chronic epilepsy [3, 4]. Cognitive impairment varies in severity and type depending on the site of the epileptic discharge and localized discharges seem to have a selective effect [4, 5]. For example, language dysfunction may be observed most frequently in patients with seizures arising from the language dominant hemisphere [3]. Despite the range of epileptic syndromes and the presence of different pathologies, literature concerning neuropsychological function in epilepsy has been focused on cognitive impairments related to temporal lobe epilepsy (TLE) and pathological changes have been associated to the magnitude of cognitive impairment [6, 7].
Furthermore, there is evidence that neuropsychological performance differentiates patients with different types of epilepsy, as for example frontal lobe epilepsy (FLE) and TLE [5, 8-10]. Contradictory findings have also been observed, failing to demonstrate the dissociation between memory and executive test performance when comparing TLE and FLE patients [11]. TLE patients commonly present an ‘‘extratemporal neuropsychological profile”, where cognitive dysfunction extends beyond memory impairment [12-15] and tend to rely on cognitive functions associated to frontal areas during the completion of memory tasks [16]. Additionally, FLE seizure spread is extremely rapid and seizure activity in frontal areas can affect distant temporal brain areas and vice versa [17]. Longitudinal studies offer controversial findings for the course of cognitive impairment in patients with chronic epilepsy. There is evidence indicating progressive cognitive deterioration which impacts a variety of cognitive domains [18-21]. Other researchers observed subtle cognitive disturbance affecting memory and psychomotor speed in a group of newly diagnosed patients, but their neuropsychological test performance remained stable after a five-year interval [22].
Seizure control has a positive effect on cognitive functioning and, moreover, patients who are not well controlled by drug treatment are at risk of cognitive decline [23]. On the other hand, cognitive side effects of antiepileptic drugs (AEDs) are common and may have a negative impact, not only on cognition but also on treatment per se [24-26]. Therefore, the potential contribution of pharmacotherapy to neuropsychological function is an important issue for the clinician to consider. Previous studies highlight the important role of cognitive assessment in patients with chronic epilepsy and suggest that neuropsychological measures offer a practical tool for the evaluation of cognitive decline due to antiepileptic treatment [25, 27, 28]. Since many factors contribute to cognitive difficulties of epileptic patients, in the present study we aimed at exploring the role of disease characteristics in performance on neuropsychological measures. In specific, we investigated associations among seizure frequency, duration of disease, antiepileptic therapy (monotherapy vs polytherapy) and performance on neuropsychological tests assessing memory, attention, psychomotor speed and executive function in a group of patients with chronic epilepsy. Additional aims of the present study were to explore differences between patients suffering from epilepsy and normal individuals and to investigate cognitive changes in neuropsychological functioning over time by reevaluating neuropsychological test performance of participants after a twelve-month interval.
Materials and Methods
Participants
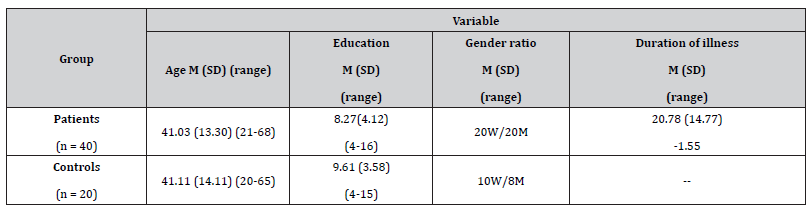
Forty epileptic patients (20 men and 20 women) of mean age 41.03 (SD = 13.30), participated in the present study presenting the following types of epileptic syndromes: (a) Temporal Lobe Epilepsy (TLE, n = 11), (b) Frontal Lobe Epilepsy (FLE, n = 6), (c) Idiopathic Generalized Epilepsy (IGE, n = 5) and (d) Cryptogenic epilepsy (n = 18). The type of epileptic syndrome was determined according to the classification of the International League against Epilepsy (ILAE, 1989). All patients were recruited from the outpatient epilepsy clinic of a university affiliated hospital, located in an urban area in northern Greece. Exclusion criteria were as follows: (1) progressive diseases of the CNS, (2) systemic medical conditions that can affect cognitive functions, (3) history of psychiatric disorder, (4) chronic medication treatment other than AEDs, (5) inability to undergo neuropsychological testing (e.g., due to low educational level).
Considering the characteristics of epilepsy the mean disease duration was 20.78 years (SD = 14.77), with a seizure frequency of, approximately, one seizure per month during the last year (M = 1.13, SD = 4.94). Eighteen patients were seizure free, whereas 22 patients had seizures during the last year. With respect to antiepileptic therapy, 20 patients received monotherapy. The serum levels of AEDs were monitored once during the period of the study in order to verify that the AEDs were in therapeutic levels. During the course of the study the characteristics of epilepsy were stable. In addition, patients did not present prolonged seizures that can lead to status epilepticus responsible for brain damage and severe cognitive deficit [29].
Furthermore, an age-, education- and gender-matched control group of 20 healthy individuals participated in the present study. Healthy participants were recruited from family members of patients and from staff members of the same hospital. Exclusion criteria were neurological disorder, history of psychiatric disorder or other systematic medical condition affecting cognitive functioning. Demographic characteristics of participants are summarized in Table 1.
All participants provided written informed consent for their participation in the study and were treated according to the Declaration of Helsinki ethical guidelines. Neuropsychological tests were administered at baseline and after a twelve-month interval.
Table 1:Descriptive information regarding epilepsy and control groups.
Neuropsychological testing
Neuropsychological assessment of participants took place in a private room within the hospital and lasted approximately 75 minutes. Patients and controls completed commonly used neuropsychological measures of visual and verbal memory, attention, psychomotor speed and executive functions. More specifically, the examination comprised by a Word List Learning Test (WLLT) [30] and a Complex Figure Test (CFT) [30], which measure verbal and visual memory respectively, the Digit Span Test (forward and backward conditions) [30] estimating immediate verbal recall and working memory, the Stroop Color Word Interference Test (SCWT) [31], which is a commonly used for the evaluation of attention, cognitive inhibition and processing speed, the Trail Making Test (TMT) assessing processing speed, attention and executive function [32], as well as a Verbal Fluency task (VF) including semantic and phonemic conditions [33].
Statistical analysis
Kolmogorov-Smirnov test was used to test quantitative variables for normality of their distribution. Group differences in baseline performance were tested using analyses of variance (ANOVA). Regression analyses were used to estimate the role of disease characteristics (duration and seizure frequency) in baseline neuropsychological performance. Independent samples t-test was conducted in order to explore the role of antiepileptic therapy in neuropsychological test performance at baseline. Finally, paired samples t-test was used to explore differences in performance on tests scores between baseline and follow-up examinations. All p values were 2-sided and were considered significant when < 0.05. Statistical analysis was performed using the SPSS software.
Results
Neuropsychological performance of epileptic patients and controls
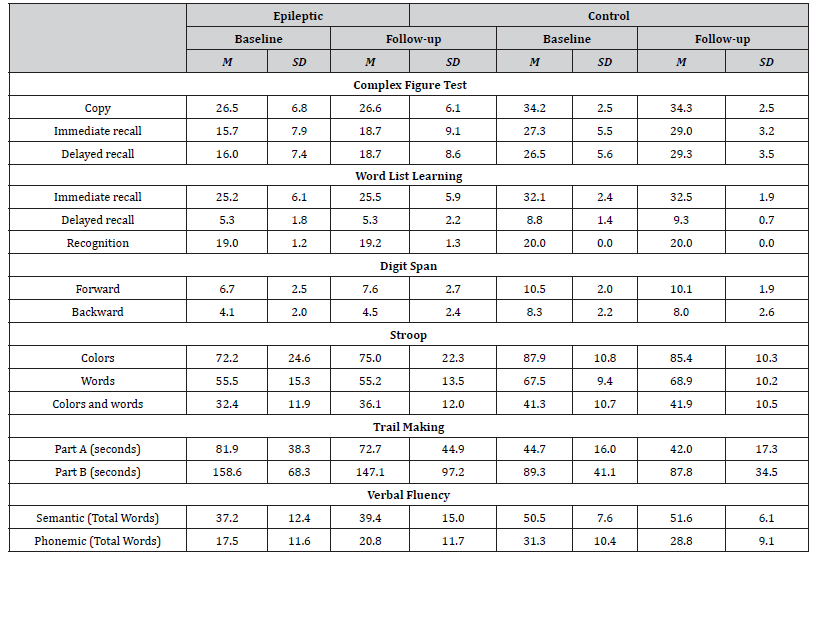
Healthy individuals scored higher than patients on all neuropsychological measures administered (Table 2). More specifically, significant differences emerged in favor of the control group on the copy trial [F(1, 58) = 25.87, p = .000, η2 = .328], on the immediate recall [F(1, 58) = 21.58, p = .000, η2 = .319] and the delayed recall [F(1, 58) = 25.67, p = .000, η2 = .358] trials of the CFT, as well as on the immediate recall [F(1, 58) = 22.33, p = .000, η2 = .296], delayed recall [F(1, 58) = 44.70, p = .000, η2 = .457] and recognition trials of the WLLT [F(1, 58) = 10.66, p = .002, η2 = .167]. In addition, participants of the control group scored significantly higher on the forward [F(1, 58) = 35.50, p = .000, η2 = .401] and backward [F(1, 58) = 52.83, p = .000, η2 = .499] conditions of the Digit Span Test and needed less time to completed the part A [F(1, 58) = 13.37, p = .001, η2 = .201] and the part B [F(1, 58) = 14,04, p = .000, η2 = .226] of the Trail Making Test than patients. Moreover, participants of the control group outperformed patients on the word [F (1, 58) = 10.49, p = .002, η2 = .171], colour [F (1, 58) = 12,43, p=.001, η2=.190] and colour-word [F (1, 58) = 7.63, p = .008, η2 = .128] conditions of the SCWT. Finally, healthy participants produced more words than patients on semantic [F (1, 58) = 17.74, p = .000, η2 = .251] and phonemic conditions of the Verbal Fluency Test [F (1, 58) = 15.43, p = .000, η2 = .225].
Duration of disease, seizure frequency and anti-epileptic therapy
Duration of epilepsy predicted baseline performance on neuropsychological measures estimating speed processing of visual stimuli and more specifically, on part A of the TMT [b = .455, t(38) = 2.98 p = .005] and on color condition of the SCWT [b = -.372, t(38) = -2.34, p = .025]. Seizure frequency (number of seizures per month), significantly predicted patients’ performance on the delayed recall trial of the WLLT [b = -.340, t (38) = -2.14, p = .040]. With respect to anti-epileptic therapy, patients on polytherapy scored significantly lower (M = 12.28, SD=7.76) on immediate recall trial of CFT than those on monotherapy (M=19.071, SD = 9.37) [t (38) = 2.171, p = .039].
Neuropsychological performance over time
Overall, patients’ test scores were significantly higher in followup, as compared to baseline neuropsychological examination (Table 2). However, this increment in performance during the follow-up assessment was significant on immediate [t(39) = -3.89, p=.001] and delayed recall [t(39) = -3.43, p=.002] trials of CFT, on forward condition of digit span test[t(39) =-3.72, p=.001], on color-word condition of the SCWT [t(39) =-3.31, p=.003] and on phonemic verbal fluency [t(39) =-2.55, p=.016]. With respect to healthy participants, significant differences between baseline and follow-up test performance were observed only on the immediate [t (19) =-3.61, p=.002] and delayed [t (19) =-3.20, p=.005] recall trials of CFT.
Table 2:Mean performance of patients and healthy participants at baseline and follow-up neuropsychological testing.
Discussion
Neuropsychological deficits in patients with chronic epilepsy depend on many factors and their magnitude vary among patients [1, 26, 34], whereas no specific neuropsychological profile has been identified. Moreover, in chronic epilepsy cognitive impairment is observed and attributed to widespread network changes [35-37], but remains unclear whether these changes are a result of epilepsy per se or due to antiepileptic therapy. Furthermore, it is essential to evaluate and record the emergence and course of cognitive deficits, in order to propose interventions and evaluate overall treatment.
In the present study, normal individuals outperformed epileptic patients on all neuropsychological measures administered. Moreover, disease duration was correlated with performance on measures of processing speed. This finding agrees with previous studies suggesting that increased disease duration affects cognition negatively [35-38]. In addition, literature supports the presence of a relationship between seizures and cognitive deficits [39]. In our study, seizure frequency (number of seizures per month) was negatively correlated with patients’ performance on the delayed recall trial of the WLLT. This finding indicates a possible negative seizure effect on patients’ ability to recall previously learned verbal information and/or their ability for strategy implementation [40].
Previous studies demonstrate the impact of antiepileptic drugs on cognition suggesting that excessive reduction of neuronal excitability may result in side effects such as psychomotor slowness, poor attention and memory, which are common side effects of sodium channel blockage and increasing GABAergic inhibitory activity [41]. Moreover, the risk of cognitive side effects rises with polytherapy and increased AED dosages/blood levels, which is sometimes necessary to control epilepsy [2]. Furthermore, the reduction of number of drugs or switches from polytherapy to monotherapy may lead to behavioral and cognitive improvement [41, 42, 43]. It is also suggested that cognitive side effects may serve as important components in overall treatment evaluation [44]. Even in the presence of relatively mild cognitive side effects, many everyday situations and aspects of daily activity can be negatively affected [27], such as for example in learning and school environments, during driving or operating machinery. In the present study though, significant differences were found in favor of monotherapy group in performance on one test index only, namely the immediate recall trial of the visual memory test.
Our final goal was to examine cognitive functions after one year without any change on the characteristics of epilepsy. During followup examination, significant improvements in patients’ performance were observed on tests assessing visual memory, short-term verbal memory and executive functions. After the twelve-month interval higher performance on the visual memory test was also observed in the control group. Improvements in performance on neuropsychological measures that were observed in epilepsy, as well as in control groups, could be attributed to learning effects and to increased familiarity with assessment procedures at followup examination. However, it is important to mention that in the present study retesting took place once, a year post baseline, and alternative test versions were administered to participants in order to minimize learning effect. Moreover, the fact that disease characteristics, such as seizure frequency and antiepileptic treatment, were stable throughout the study could account, at least in part, for improvements observed in neuropsychological test performance of participants with epilepsy.
The small sample size suggests an important limitation of the present study. Moreover, the role of seizure frequency and antiepileptic therapy in neuropsychological test performance alterations over time was not explored. Future longitudinal studies are needed in order to clarify the impact of disease characteristics and antiepileptic therapy on the course of cognitive functioning in epilepsy. Furthermore, the administration of alternative versions of neuropsychological measures after the twelve-month interval does not eliminate completely practice effects suggesting another limitation of the present study.
Conclusion
Cognitive functioning in patients with chronic epilepsy warrants careful consideration. The clinician should take into account possible changes in neuropsychological status over time. In patients with stable disease course and unchangeable disease characteristics improved and/or stable cognitive functioning can be observed after a twelve-month interval and further cognitive decline seems to be improbable. Moreover, the present findings suggest that seizure presence and antiepileptic therapy are key factors which should be taken into consideration for the evaluation of cognitive function in chronic epilepsy. Since cognitive dysfunction in epileptic patients can be attributed to multiple interconnected factors including antiepileptic medications, the primary aim of any pharmaceutical intervention is to achieve the best seizure control with minimal side effects.
Acknowledgement
None.
Conflict of Interest
None.
References
- Novak A, Vizjak K, Rakusa M (2022) Cognitive Impairment in People with Epilepsy. J Clin Med 11(1): 267.
- Wang L, Chen S, Liu C, Lin W, Huang H (2020) Factors for cognitive impairment in adult epileptic patients. Brain Behav 10(1): e01475.
- Holmes GL, Lenck Santini PP (2006) Role of interictal epileptiform abnormalities in cognitive impairment. Epilepsy Behav 8(3): 504-515.
- Morrisson SE., Bakhtin L (2007) Neuropsychological features of lesion-related Epilepsy in Adults: An Overview. Neuropsychol Rev 17: 385-403.
- Mc Donald CR, Delis DC, Norman MA, Wetter SR, Tecoma ES, et al. (2005) Response inhibition and set shifting in patients with frontal lobe epilepsy or temporal lobe epilepsy. Epilepsy Behav 7(3): 438-446.
- Hermann B, Seidenberg M, Lee EJ, Chan F, Rutecki P (2007) Cognitive phenotypes in temporal lobe epilepsy. J Int Neuropsychol Soc 13: 12-20.
- Dabbs K, Jones J, Seidenberg M, Hermann B (2009) Neuroanatomical correlates of cognitive phenotypes in temporal lobe epilepsy. Epilepsy Behav 15(4): 445-51.
- Upton D, Thompson PJ (1996) General neuropsychological characteristics of frontal lobe epilepsy. Epilepsy Res 23(2): 169-177.
- Ziaei M, Arnold C, Thompson K, Reutens DC (2023) Social Cognition in Temporal and Frontal Lobe Epilepsy: Systematic Review, Meta-analysis, and Clinical Recommendations. J Int Neuropsychol Soc 29(2): 205-229.
- Exner C, Boucsein K, Lange C, Winter H, Weniger G, et al. (2002) Neuropsychological performance in frontal lobe epilepsy. Seizure 11: 20-32.
- Cahn Weiner D, Wittenberg D, McDonald C (2009) Everyday cognition in temporal lobe and frontal lobe epilepsy. Epileps Disord. 11(3): 222-227.
- Martin RC, Sawrie SM, Gilliam FG, Palmer CA, Faught E, et al. (2000) Wisconsin Card Sorting performance in patients with temporal lobe epilepsy: clinical and neuroanatomical correlates. Epilepsia 41(12): 1626-1632.
- Keller SS, Baker G, Downes JJ, Roberts N (2009) Quantitative MRI of the prefrontal cortex and executive function in patients with temporal lobe epilepsy. Epilepsy & Behavior 15: 186-195.
- Bell B, Lin JJ, Seidenberg M, Hermann B (2011) The neurobiology of cognitive disorders in temporal lobe epilepsy. Nature Rev Neurol 7: 154-164.
- Giovagnoli A (2001) Relation of sorting impairment to hippocampal damage in temporal lobe epilepsy. Neuropsychologia 39: 140-50.
- Centeno M, Vollmar M, O'Muircheartaigh J, Stretton J, Bonelli S.B, et al (2012) Memory in frontal lobe epilepsy: An fMRI study. Epilepsia 53(10): 1756-64.
- Exner C, Boucsein K, Lange C, Winter H, Weniger G, et al. (2002) Neuropsychological performance in frontal lobe epilepsy. Seizure 11: 20-32.
- Seidenberg M, Pulsipherm DT, Hermann B (2007) Cognitive progression in epilepsy. Neuropsychol Rev 17: 445-454.
- Helmstaedter C, Elger CE (2009) Chronic temporal lobe epilepsy: a neurodevelopmental or progressively dementing disease? Brain 132: 2822-2830.
- Kanner AM, Helmstaedter C, Sadat Hossieny Z, Meador K (2020) Cognitive disorders in epilepsy I: Clinical experience, real-world evidence and recommendations. Seizure 83: 216-222.
- Helmstaedter C, Sadat-Hossieny Z, Kanner A.M, Meador K.J (2020) Cognitive disorders in epilepsy II: Clinical targets, indications and selection of test instruments. Seizure. 83: 223-231.
- Taylor J, Baker G.A (2010) Newly diagnosed epilepsy: Cognitive outcome at 5 years. Epilepsy Behav. 18(4): 397-403.
- Meador KJ (2002) Cognitive outcomes and predictive factors in epilepsy. Neurology 58(8): 21-6.
- Meador KJ (2008) Cognitive Effects of Levetiracetam versus Topiramate. Epilepsy Cur. 8(3): 64-5.
- Witt JA, Elger CE, Helmstaedter C (2013) Which drug-induced side effects would be tolerated in the prospect of seizure control? Epilepsy Behav 29(1): 141-3.
- Loring DW, Marino S, Meador KJ (2007) Neuropsychological and behavioral effects of antiepileptic drugs. Neuropsychol Rev 17: 413-425.
- Helmstaedter C (2013) The impact of epilepsy in cognitive function. J Neurol Neurosurg Psychiatry 84:
- Barr W (2007) Epilepsy and Neuropsychology: Past, Present, and Future. Neuropsychol Rev 17: 381-383.
- Vingerhoets G (2006) Cognitive effects of seizures. Seizure 15: 221- 6.
- Kosmidis MH (2010) Battery of Neuropsychological Tests. Thessaloniki, Greece: Aristotle University of Thessaloniki (Unpublished tests).
- Zafiri M, Kosmidis MH (2008) Effects of demographic factors on the “Stroop conflict”. Psychology: Journal of the Hellenic Psychological Society, Special Issue: Psychometric Assessment: Applications to Neuropsychology and Education (Guest ed. Panagiotis Simos). 15: 319-341.
- Vlachou CH, Kosmidis ΜΕ (2002) The Trail Making Task in the Greek population: Preliminary normative data. Psychology. 9(3): 336-352.
- Kosmidis MH, Vlachou CH, Panagiotaki P (2004) The verbal fluency task in the Greek population: Normative data, and clustering and switching strategies. J Int Neuropsychol Soc 10: 164-172.
- Elger C.E, Helmstaedter C, Kurthen M (2004) Chronic epilepsy and cognition. Lancet Neurol 3: 663-672.
- Holmes GL (2015) Cognitive impairment in epilepsy: the role of network abnormalities. Epileptic Disord. 17(2): 101-116.
- Vlooswijk MCG, Vaessen MJ, Jansen JFA, de Krom MCFTM, Majoie HJM, et al. (2011) Loss of network efficiency associated with cognitive decline in chronic epilepsy. Neurology 77(10): 938-944.
- Vaessen MJ, Jansen JFA, Vlooswijk MCG, Hofman PAM, Marian Majoie HJM, et al. (2012) White Matter Network Abnormalities Are Associated with Cognitive Decline in Chronic Epilepsy. Cereb. Cortex 22 (9): 2139-2147.
- Chapman Black L, Schefft BK, Howe SR, Szaflarski JP, Privitera HYMD (2010) The effect of seizures on working memory and executive functioning performance. Epilepsy Behav 17: 412-9.
- Dodrill C (2004) Neuropsychological effects of seizures. Epilepsy Behav 5(1): 21-24.
- Mitrushina M, Boone KB, Razani J, D’Elia LF (2008) Normative Data for Neuropsychological Assessment, (2nd ) Oxford University Press: New York. Pp: 357-372.
- Shehata GA, Bateh AEM, Hamed SA, Rageh TA, Elsorogy YB (2009) Neuropsychological effects of antiepileptic drugs (carbamazepine versus valproate) in adults males with epilepsy. Neuropsych Dis Treat 5: 527-533.
- Cavanna AE, Ali F, Rickards HE, MeCorry D (2010) Behavioral and cognitive effects of antiepileptic drugs. Discov Med 9(45): 138-144.
- Witt JA, Helmstaedter C (2017) How can we overcome neuropsychological adverse effects of antiepileptic drugs? Expert Opin Pharmacother 18(6): 551-554.
- Eddy CM, Rickards HE, Cavanna AE (2011) The cognitive impact of antiepileptic drugs. Ther Adv Neurol Disord 4(6): 385–407.
-
Theodora Afrantou, Eleni Konstantinopoulou*, Roza Lagoudaki and Panagiotis Ioannidis. Disease Characteristics and Neuropsychological Performance in Chronic Epilepsy. Arch Neurol & Neurosci. 17(5): 2025. ANN.MS.ID.000925.
-
Epileptic patients, Antiepileptic medications, Neuropsychological function, Pathological changes, Extratemporal neuropsychological profile, Antiepileptic drugs, History of psychiatric disorder, Demographic characteristics, Helsinki ethical, Verbal memory
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






