 Research Article
Research Article
Cognitive Trajectories and Psychological Well-Being of Severe SARS-CoV-2 Patients admitted to the Intensive Care Unit: A One-Year Longitudinal Analysis Including Caregiver Perspectives
Luisa Sambati1, Katia Mattarozzi2, Susy Ferrari1,3, Rossella Santoro1, Lucia Cretella1, Tommaso Tonetti2,4, Maria Della Giovampaola2, Martina Bordini2, Paolo Bottausci2, Luciano Romano1, Pietro Cortelli1,3,Luciana Mascia5* and Maria Guarino1*
1UOC Clinica Neurologica Rete Metropolitana (NeuroMet), IRCCS Istituto delle Scienze Neurologiche, Bologna, Italia
2Department of Medical and Surgical Sciences (DIMEC), University of Bologna, Bologna, Italy
3Department of Biomedical and NeuroMotor Sciences (DIBINEM), University of Bologna, Bologna, Italy
4Anesthesiology and General Intensive Care Unit, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna, Italy
5University of Salento, Dipartimento di medicina sperimentale, Lecce Italy
Maria Guarino, UOC Clinica Neurologica Metropolitana (NeuroMet), IRCCS Istituto delle Scienze Neurologiche, Bologna, Italia.
Received Date:April 03,2024; Published Date:April 24, 2024
Abstract
We investigated the cognitive profile and psychological well-being of severe SARS-CoV-2 patients admitted to the Intensive Care Unit (ICU), including caregiver perspectives. We assessed caregivers’ quality of life, psychological distress and satisfaction with the information received by healthcare professionals. Consecutive patients with SARS-CoV-2 infection requiring ICU admission were recruited together with a caregiver. Patients with previous cognitive disorders were excluded. Comprehensive neuropsychological evaluation was conducted at three months (T1) and 12 months (T2) after ICU discharge and both patients and caregivers filled out validated questionnaires.
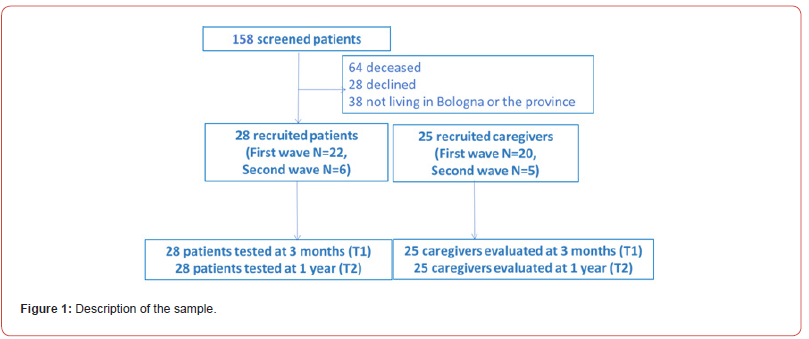
Out of 158 patients admitted to ICU in the study period, 28 met the inclusion criteria. All 28 patients (23 males) completed the assessment at 3 and 12 months. Cognitive performance analysis revealed that 40% had cognitive impairment at T1, decreasing to 33% at T2. Long-term memory was the most impaired domain at T1 and T2. [1) One-way MANOVA showed significant improvement in global cognitive efficacy and phonemic access over time. Lower performances in long-term memory were significantly associated with longer ICU stay, duration of mechanical ventilation, higher Sequential Organ Failure Assessment (SOFA) score and longer treatment with benzodiazepines, opioids, antipsychotic drugs and propofol over time.
Quality of life (QoL) analysis indicated that 71% of patients had a reduced QoL due to physical limitations at T1, which improved 12 months later. Psychological burden-related QoL remained stable. Subjectively perceived QoL worsened over time. Caregiver mental distress was moderate in 3 out of 25 caregivers on SQR-20. Caregivers reported high satisfaction (90%) with communication between caregivers and healthcare staff evaluated by COSM-R.
In conclusion, our study provides valuable insights into the cognitive and psychological well-being of severe COVID-19 patients admitted to the ICU, emphasizing the importance of long-term follow-up. The study sheds light on the potential for cognitive improvement within the initial 12 months post-ICU discharge, suggesting that early and meticulous neuropsychological assessment could guide interventions to enhance prognosis. Further, the role of caregivers as a meticulous investigator of the patient’s disorder emphasizes the need of holistic support.
Introduction
he early stages of the COVID-19 pandemic revealed that nearly 1 in 10 infected individuals required hospitalization and admission to an intensive care unit (ICU) due to acute respiratory distress syndrome (ARDS) necessitating mechanical ventilation [1]. Post- COVID-19 syndrome has since emerged, marked by prevalent and debilitating cognitive impairment and psychological distress [2], especially in ICU patients who experience more severe impairment compared to those not requiring ICU care [3].
Reports of persistent cognitive impairment following ICU discharge in COVID-19 patients have been described [4,5,6] but the variability in cognitive assessment tools, ranging from surveys to telephone assessments, challenges the determination of the true prevalence of cognitive impairment in this population.
The profile of post-COVID-19 cognitive impairment, often assessed through cognitive screening tests (e.g., the MoCA or Mini– Mental State Examination), administered either in person [6,7,8,9] or by telephone [10], limits the reliability of the data. Comprehensive neuropsychological assessment, although carried out in a few studies, suggests the involvement of processing speed and longterm memory [4], potentially compromising executive functions.
Psychological distress is a further concern, affecting over 50% of the patients with critical SARS-CoV-2 infection, manifesting as anxiety, depression, trauma and stress-related disorders [4,11]. Caregivers facing isolation and inability to communicate with the patients also experience heightened psychological distress [12].
Given the extensive impact of SARS-CoV-2 infection on various mental dimensions, including both cognitive and emotional domains, there is an urgent need for a comprehensive longitudinal investigation, encompassing cognitive assessment, quality of life evaluation, and an exploration of psychological well-being among patients who have undergone critical SARS-CoV-2. The pivotal role of the caregivers, an examination of their quality of life and psychological well-being, in parallel to their interactions with ICU staff, stand as a crucial facet to be further explored. Hence, the primary objective of this study is to analyse the prevalence and nature of cognitive deficits, as well as the trajectories of quality of life and psychological well-being in a prospective, consecutive cohort of COVID-19 patients who received ICU care and mechanical ventilation due to ARDS.
To achieve these objectives, this study proposes an in-depth evaluation employing a comprehensive assessment of patients’ cognition, encompassing patients and caregivers’ psychological well-being and healthcare interactions, thus contributing to a more comprehensive understanding of the pandemic’s consequences. This assessment was conducted at two distinct intervals, 3 and 12 months post ICU discharge, highlighting the potential persistence of cognitive impairments and exploring the impact of time on recovery trajectories. Furthermore, this study aims to identify the clinical and biometric factors associated with cognitive impairments, thereby contributing to the understanding of the mechanisms underlying such deficits.
Methods
During the first and second wave of SARS-CoV-2 infection (i.e., from March 1 to April 30, 2020, and from November 1 to 30, 2020) all consecutive patients aged 18 years or older, with SARS-CoV-2 infection confirmed by PCR on oro-nasopharyngeal swab, requiring ICU admission and mechanical intubation for acute respiratory distress syndrome (ARDS) [13], and discharged, were enrolled together with a caregiver from Sant’Orsola-Malpighi University Hospital of Bologna, Northern Italy. As caregivers we considered family members or close relatives who provide care voluntarily to patients at home. Exclusion criteria were: previous neurological and psychiatric diseases, presence of cognitive impairment or dementia, substance or alcohol abuse, assessed by in-depth caregiver interview and clinical records evaluation. Patients not living in Bologna or the province were also excluded because they could not undergo on-site clinical and neuropsychological evaluations as pandemic travel restrictions were current at that time. Patients were recruited on ICU admission (T0), when the following clinical and biometric data were collected: age, sex, PaO2/FiO2 ratio (P/F ratio), C-reactive protein (CRP), procalcitonin test (PCT), interleukin- 6 (IL-6), d-dimer, Sequential Organ Failure Assessment (SOFA) score, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, Richmond Agitation-Sedation Scale (RASS) score, sedative drugs used and days of treatment, days of mechanical ventilation and length of ICU stay. Worst P/F, worst SOFA score, worst APACHE II score, were also collected.
Cognitive function, quality of life and psychological well-being assessment
At three months (T1) and 12 months (T2) following ICU discharge, patients underwent a rigorous and standardized neuropsychological evaluation, along with self-reported questionnaires pertaining to quality of life (QoL) and psychological well-being. The neuropsychological battery, which encompassed a range of cognitive domains, was administered by two trained neuropsychologists (S.F., R.S.) in sessions lasting approximately 60 to 90 minutes each.
Likewise, caregivers completed validated questionnaires that gauged various dimensions of psychological well-being at the corresponding time point. At T1, an additional aspect of the caregiver experience, namely the degree of satisfaction with their interaction with ICU staff, was also assessed. For the purpose of classifying cognitive impairment, well-established criteria outlined in the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) for minor and major neurocognitive disorders were employed [14].
QoL assessment was carried out through a multifaceted approach. This included the Short Form Health Survey (SF-12) [15] and the 5-level EQ-5D version (EQ-5D-5L) EuroQol Group in 2009. Additionally, a patient-centered perspective was incorporated using the Schedule for the Evaluation of Individual Quality of Life (SEIQoL) [16].
Emotional assessment was evaluated through the Hospital Anxiety and Depression Scale (HADS) [17], a widely adopted in strument particularly relevant for critically ill patients, as evidenced by its applicability in similar contexts [18,19]. Caregivers were engaged through the completion of the SQR-20 Self-Reporting Questionnaire (SQR-20)20 for the evaluation of physical and psycho-emotional symptoms. Furthermore, an adapted version of a questionnaire originally designed to assess satisfaction with received information and healthcare provider relationship, i.e. the COSM-R (Communication medical doctor – patients – Revised (COSM-R) [21,22], was employed to gauge caregivers’ perspective on these aspects.
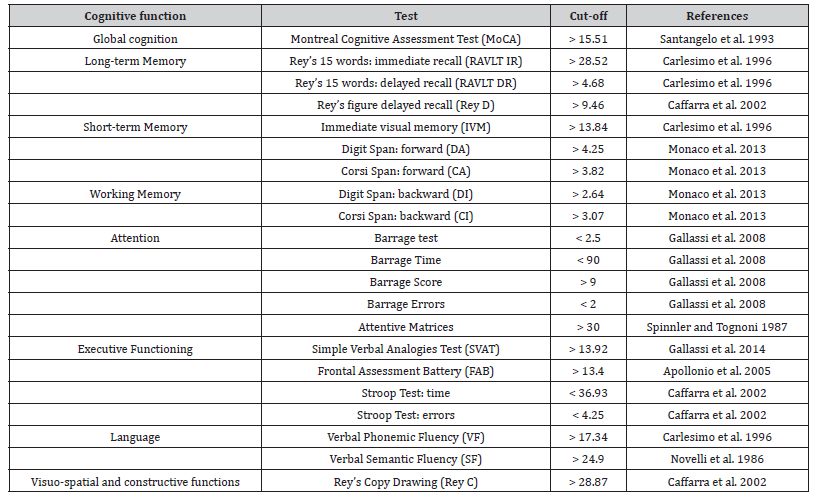
Table 1 outlines the data collection timeline and the questionnaires for both patients and caregivers. Additionally, Table 2 details the specific components of the neuropsychological evaluation of patients.
Table 1:Protocol of the study.
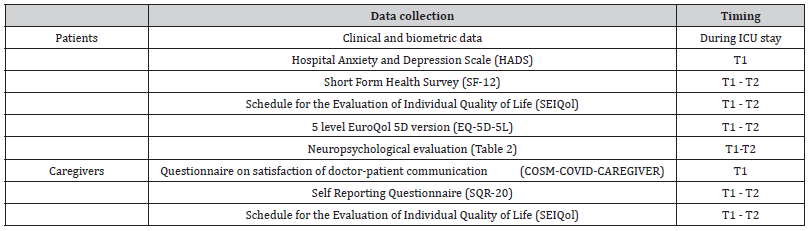
Table 2:Neuropsychological evaluation.
The current study was approved by the Ethics Committee of the Local Health Authority of Bologna, Italy (reference number 533/2020/Oss/AOUBo) and performed in accordance with the principles of good clinical practice. Participation of each patient was contingent upon obtaining written informed consent.
Statistical analysis
Initial data exploration encompassed descriptive statistics to derive concise numerical summaries of neuropsychological tests, questionnaires, and clinical data. Pearson correlations were conducted to discern potential associations between continuous variables. In order to elucidate temporal variations, separate multivariate analysis of variance (MANOVA) tests, with time as a within-subject factor (T1 vs. T2), were executed to assess cognitive and affective scores. Qualitative data stemming from open-ended responses to SEIQoL were subjected to qualitative analysis.
Results
Out of 158 patients admitted to our ICU during the study period, 28 met the inclusion criteria. All 28 patients (23 males) were included and completed the clinical assessment both at 3 and 12 months. The sample recruitment and procedure overview and demographic variables are reported in Fig.1 and Table 2a, respectively.
Cognitive Performance
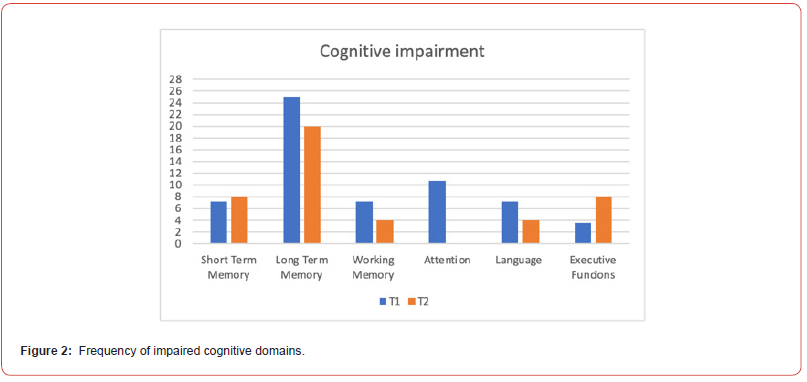
Of the 28 patients, 39% (11/28) had a neurocognitive disorder at T1, overall minor except one who had a major neurocognitive impairment. At T2, 33% (9/28) presented with a neurocognitive disorder, overall mild. The only patient with T1 major neurocognitive disorder remained stable at 12 months. Long-term memory was the most impaired domain (25% of patients), followed by attention (11%), working memory (7%), language (7%) and executive functions (4%) at T1. At T2, long-term memory was still the most frequent deficit (20 %), followed by executive functions (8%), working memory (4%) and language (4%). Attention was never impaired at T2. Praxis was never impaired either at T1 or T2 (Figure 2).
A one-way MANOVA, with time as a within-subject factor (T1 vs. T2), showed a significant difference on cognitive performance, F(6,18) = 2.98, p = 0.03, ηp2 = 0.49. Specifically, global cognitive efficiency, i.e. MoCa score, F(1,23) = 6.77, p = 0.02, ηp2 = 0.23, and phonemic access, i.e. phonemic fluency score, F(1,23) = 9.93, p = 0.004, ηp2 = 0.30, improved over time (25.17 ±2.53 vs. 26.40 ±2.51; 35.41 ± 9.80 vs. 41.25 ± 15.23 respectively) (Table 3).
Table 2a:Clinical characteristics of the cohort of the patients.
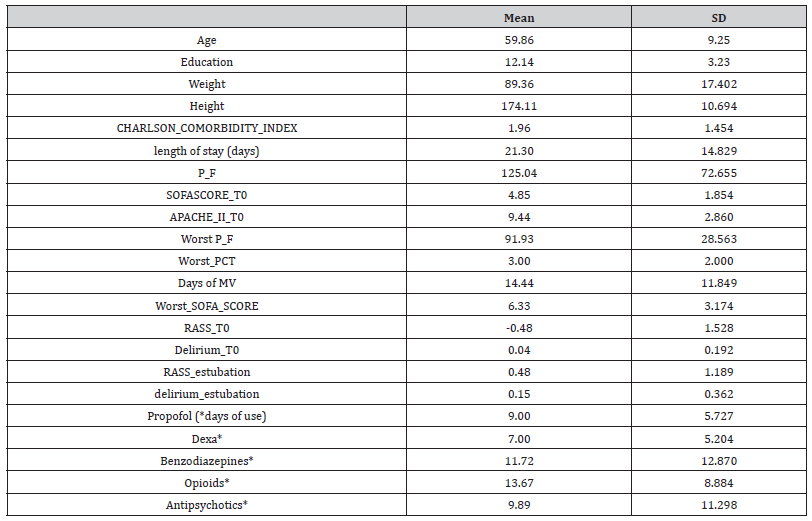
Table 3:Neuropsychological evaluation at T1 and T2
Lower performances in long-term memory were significantly associated with longer ICU stay, (RAVLT Delayed recall T1, r: -.491 p=.009 – RAVLT Immediate recall T2, r: -.529 p=.009), duration of mechanical ventilation (RAVLT Immediate recall T1 r=-.464, p=.015 – RAVLT Immediate recall T2 =-.550, p=.007, RAVLT Delayed recall T1 r: -.529, p=.005), higher Sequential Organ Failure Assessment (SOFA) score (RAVLT Immediate recall T2 r=-.450, p=.031, RAVLT Delayed recall T1 r=-.503, p=.007 – T2 =-.490, p=.018) and longer treatment with benzodiazepines (RAVLT Immediate recall T1 r=- .477, p=.045 – T2 =-.543, p=.036, RAVLT Delayed recall T1 r=-.504, p=.033) , opioids (RAVLT Delayed recall T1 r=-.490, p=.009 – RAVLT Immediate recall T2 =-.564, p=.005) antipsychotic drugs (RAVLT Delayed recall T1 r=-.593 p=.007) and propofol (RAVLT Delayed recall T1 r=-.518, p=.007 – RAVLT Immediate recall T2 =-.539, p=.008).
A significant correlation between lower performances in phonemic fluency and longer duration of mechanical ventilation (T1 r=-.493, p=.009), longer treatment with benzodiazepines (T1 r=- .543, p=.020), antipsychotic drugs (T1 r=-.629 p=.004) and opioids (T1 r=-.528, p=.005) was observed at T1. At T1 longer term treatment with antipsychotic drugs was related with lower scores on MoCa (r=-.662 p=.002) and digit span backward (r=-.640, p=.003).
Quality of life and psychological distress in patients
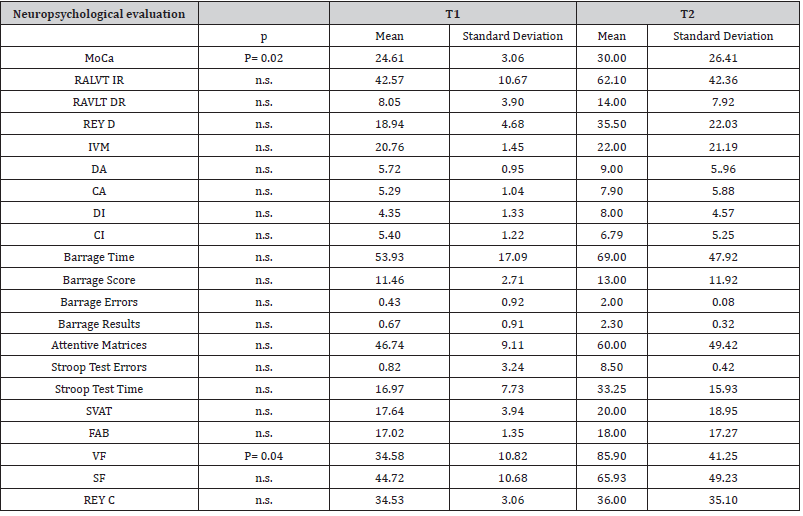
A total of 71 % of patients (24 out of 28 patients) had a reduced QoL due to physical limitations at T1 (SF-12 PCS score < 54.1, 42.42 ± 9.17), which tended to improve 12 months year later, F(1,23) = 3.59, p = 0.07, ηp2 = 0.14, (45.97 ± 8.36). A proportion of 57.14% of patients (16 out of 28) had a reduced QoL due to psychological burden at T1 (SF-12 MCS score < 52.9, 50.77 ± 8.78), which remained stable 12 months later, F(1,23) = 0.05, p>.05, ηp2 = 0.02, (51.22 ± 9.56) [Table 4a].
Table 4a:Quality of life (QoL) and psychological distress in patients and caregivers
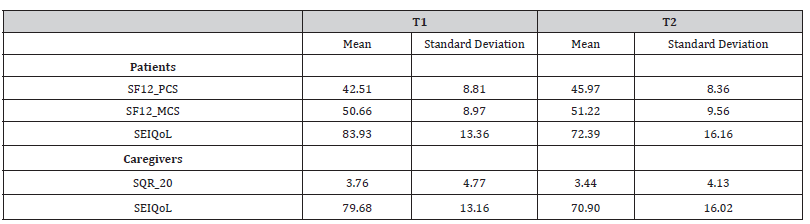
SF-12 PCS scores at T2 were significantly related to higher SOFA scores (r=.643, p=.001) and to lower digit span backward scores (r=.651, p=.001). SEIQoL showed a worsening of subjectively perceived quality of life between T1 and T2, F(1,23) = 17.91, p < 0.001, ηp2 = 0.44, (83.07 ± 13.52 vs. 72.39 ± 16.16). Concerning SEIQoL categories, at T1 patients reported hobbies as the most important category to consider in the quality of their life. At T2, besides hobbies, the most important categories were also personal realization and feeding. No significant relations were detected between SF-12 MCS, SEIQoL, QoL and psychological wellbeing and patients’ cognitive scores. No significant differences were found over time on EQ-5D score [Table 4b].
Table 4b:Health-related quality of life questionnaire in patients.
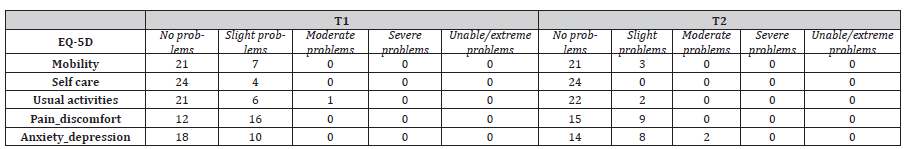
EQ-5D score at T1 was related to longer duration of treatment with benzodiazepines (T1 r=.505, p=.033) and with antipsychotics (T1 r=.507, p=.027).
Quality of life and affective outcomes in caregivers
Moderate mental distress was detected in 3 out of 25 caregivers on SQR-20 (SQR-20 >8). No significant differences were found over time on SQR-20 score and SEIQoL [Table 4].
At T1 SQR-20 score was related to T1 patients’ SF12-MCS score (r=-.515, p=.008) and T2 SEIQoL total score (r=-.637, p=.001), while at T2 SQR-20 score was related to T2 patients’ SF12-MCS score (r=-.576, p=.020).
At T2 the caregiver SEIQoL total score was related to patients’ SEIQoL total score (r=.540, p=.031).
Concerning SEIQoL, at T1 caregivers reported mental health and autonomy as the most important category in the quality of their life, conversely at T2 the most important categories were hobbies and autonomy. Moreover, lower caregiver QoL (T2 SEIQoL) was related to higher patients’ SOFA score (r=-.611; p=.012) and to LTM impairment (RAVLT Delayed T1 r=.463; p=.071, RAVLT Delayed T2 r=.459; p=.074). Concerning the communication between caregivers and healthcare staff evaluated by COSM-R, 90% of caregivers reported high satisfaction (mean score 40.36 ± SD 8.18).
Discussion
In this study, we reported a comprehensive analysis of neuropsychological performances, quality of life, and psychological well-being in severe COVID-19 patients admitted to the ICU and requiring mechanical ventilation, examined at 3 and 12 months post-discharge. Additionally, we explored the relationship between patients’ conditions and the quality of life and psychological well-being of caregivers.
Several studies have focused on cognitive functions after COVID-19 infection, but the large use of cognitive screening tests as well as the frequent use of telephone assessments limit the reliability of the incidence rate of cognitive impairment. Furthermore, few studies tested long-term cognitive performances with complete neuropsychological assessment in ICU COVID-19 survivors19. Our emphasis on face-to-face comprehensive neuropsychological assessments sets this study apart. Our findings documented that 40% of patients were cognitively impaired at 3 months decreasing to 33 % at 12 months. Our data confirm that extensive neuropsychological evaluation is necessary to detect the most consistent cognitive impairment frequency19 (up to 43%) compared to screening tests [23] (18%), both at baseline and at 12 months after ICU discharge [24]. Furthermore, beyond screening tests, also the administration of questionnaires for subjective cognitive symptoms is not recommended to define specific alterations in cognitive domains. In fact, about one-third of COVID-19 patients, regardless of the acute disease severity, reported high levels of subjective cognitive dysfunction which was not associated with results from objective cognitive evaluation but with psychological and demographic factors [25]. Thus, to define specific alterations in cognitive domains, the use of complete neuropsychological batteries is crucial to characterize the cognitive impairment to provide the best intervention in ICU discharged patients.
In our study, long-term memory was the most impaired domain both at 3 and 12 months, followed by attention, working memory, language and executive functions, instead praxis was never impaired, suggesting a predominant frontal-temporal subcortical cognitive dysfunction. This pattern is consistent with memory impairment detected in ARDS patients with or without COVID infection [26]. Long-term cognitive impairments in non-COVID ICU survivors were strictly related to hypoxemia, cardinal feature of ARDS of pulmonary etiology [27], suggesting that ARDS-mediated neurological damage could involve cytokine storm following lung injury or sepsis [28]. Mechanical ventilation was also considered an independent factor related to persistent cognitive impairment, diminished quality of life, and depression [29].
In our sample all patients had acute ARDS and all were mechanically ventilated, thus the predominant memory deficit we found could be explained by the peculiar sensitivity of medial temporal lobes to hypoxic injury.
We also observed a slight reduction in cognitive impairment frequency over time, similarly to patients with milder COVID as described by Ferrucci et al. 4 as well as in non-COVID ICU survivors [30]. Long-term memory impairment was significantly associated with higher SOFA score, longer mechanical ventilation, stay in ICU and psychotropic drugs use at 3 months. The association between long-term memory impairment, higher SOFA score and longer stays in ICU persisted at 12 months of follow-up, emphasizing the lasting impact of these clinical factors on cognitive outcomes. Our study confirmed that at 3 months post-ICU discharge patients reported a reduced QoL, primarily due to physical limitations, which tended to improve 12 months later [31-32]. However, health-related quality of life due to psychological burden remained stable 12 months later. Subjectively perceived quality of life worsened over time. Self-as sessed health-related quality of life was associated with duration of treatment with benzodiazepines and with antipsychotic drugs, but not cognitive scores. In our study, the majority of caregivers did not report symptoms related to mental distress either at baseline or at 1 year of follow-up. However, consistent with previous reports [33], physical and psycho-emotional symptoms reported by caregivers were related to patients’ lower QoL. Moreover, lower caregiver QoL was related to higher patients’ memory impairment. Generally, caregivers reported a good degree of satisfaction concerning the communication with healthcare staff.
Our study presents valuable insights into the cognitive and psychological well-being landscape of COVID-19 survivors who underwent ICU care. Despite its contributions, several key limitations warrant consideration. Notably, our sample size was modest and the absence of baseline cognitive function data limited our ability to discern pre-existing cognitive deficits. Nonetheless, we undertook meticulous measures, including comprehensive anamnesis and clinical chart review, to minimize this limitation and ensure a robust evaluation of cognitive status.
The absence of a control group is a shared limitation observed in numerous studies within the same domain. The COVID-19 pandemic has hindered the recruitment of control participants for in-person assessments due to prevailing safety concerns. This limitation highlights the challenging research landscape during the pandemic and must be taken into account when interpreting our findings.
Turning to our strengths, our study stands out for including an extensive neuropsychological battery and patient-centered perspective measures to comprehensively gauge health-related quality of life and psychological well-being. In summary, our findings reveal a significant prevalence of cognitive impairment among COVID-19 ICU survivors, as assessed through an in-depth face-toface neuropsychological evaluation, with particular involvement of long-term memory deficits, exerting a discernible influence on quality of life as confirmed by caregiver assessment. Our data demonstrated cognitive improvement within the initial 12 months post-ICU discharge. Interestingly, the neuropsychological profile and the associated factors align with those observed in non-COVID ICU survivors, implicating the broader framework of post-intensive care syndrome rather than a distinct role for COVID-19 infection [27,28].
Furthermore, our study underscores the interconnectedness between patient well-being and caregiver experiences. We observe that caregivers’ psychological well-being is strongly linked to patient quality of life and memory performance. In conclusion, our study improves the understanding of cognitive and psychological outcomes in COVID-19 ICU survivors. This insight bears significance in guiding clinical practices, suggesting that early and meticulous neuropsychological assessment could facilitate the identification of patients who might benefit from cognitive therapies, ultimately enhancing the prognosis of this debilitating complication.
Acknowledgement
None.
Conflicts of Interest
There is no conflict of interest.
References
- CDC COVID-19 Response Team (2020) Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep 69(12): 343-346.
- Ceban F, Ling S, Lui LMW, et al. (2022) Fatigue and cognitive impairment in post-COVID-19 Syndrome: A systematic review and meta-analysis. Brain Behav Immun 101: 93-135.
- Ollila H, Pihlaja R, Koskinen S, et al. (2022) Long-term cognitive functioning is impaired in ICU-treated COVID-19 patients: a comprehensive controlled neuropsychological study. Crit Care Lond Engl 26(1):223.
- Ferrucci R, Dini M, Rosci C, et al. (2022) One-year cognitive follow-up of COVID-19 hospitalized patients. Eur J Neurol 29(7):2006-2014.
- García-Sánchez C, Calabria M, Grunden N, et al. (2022) Neuropsychological deficits in patients with cognitive complaints after COVID-19. Brain Behav 12(3): e2508.
- Raman B, Cassar MP, Tunnicliffe EM, et al. (2021) Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine 31:100683.
- Pistarini C, Fiabane E, Houdayer E, Vassallo C, Manera MR, et al. (2021) Cognitive and Emotional Disturbances Due to COVID-19: An Exploratory Study in the Rehabilitation Setting. Front Neurol 12:643646.
- De Lorenzo R, Conte C, Lanzani C, et al. (2020) Residual clinical damage after COVID-19: A retrospective and prospective observational cohort study. PloS One 15(10): e0239570.
- Rass V, Beer R, Schiefecker AJ, et al. (2021) Neurological outcome and quality of life 3 months after COVID-19: A prospective observational cohort study. Eur J Neurol 28(10): 3348-3359.
- Baig AM. (2020) Neurological manifestations in COVID-19 caused by SARS-CoV-2. CNS Neurosci Ther 26(5):499-501.
- Mattioli F, Stampatori C, Righetti F, Sala E, Tomasi C, et al. (2021) Neurological and cognitive sequelae of Covid-19: a four month follow-up. J Neurol 268(12): 4422-4428.
- Jafari-Oori M, Ebadi A, Moradian ST, Jafari M, Dehi M, et al. (2022) Psychiatric distress in family caregivers of patients with COVID-19. Arch Psychiatr Nurs 37:69-75.
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. (2012) acute respiratory distress syndrome: the Berlin Definition. JAMA 307(23):2526-2533.
- DSM-5. Manuale diagnostico e statistico dei disturbi mentali - Biondi M. (cur.). Accessed September 20, 2023.
- Ware J, Kosinski M, Keller SD (1996) A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 34(3): 220-233.
- Hickey AM, Bury G, O’Boyle CA, Bradley F, O’Kelly FD, et al (1996) A new short form individual quality of life measure (SEIQoL-DW): application in a cohort of individuals with HIV/AIDS. BMJ 313(7048): 29-33.
- Zigmond AS, Snaith RP (1983) The hospital anxiety and depression scale. Acta Psychiatr Scand 67(6): 361-370.
- Bjelland S, Jones K (2022) A Systematic Review on Improving the Family Experience After Consent for Deceased Organ Donation. Prog Transplant Aliso Viejo Calif 32(2): 152-166.
- Godoy-González M, Navarra-Ventura G, Gomà G, et al (2023) Objective and subjective cognition in survivors of COVID-19 one year after ICU discharge: the role of demographic, clinical, and emotional factors. Crit Care Lond Engl 27(1): 188.
- Harding TW, de Arango MV, Baltazar J, et al (1980) Mental disorders in primary health care: a study of their frequency and diagnosis in four developing countries. Psychol Med 10(2): 231-241.
- Solari A, Mattarozzi K, Vignatelli L, et al (2010) Development and validation of a patient self-assessed questionnaire on satisfaction with communication of the multiple sclerosis diagnosis. Mult Scler Houndmills Basingstoke Engl 16(10): 1237-1247.
- Solari A, Grzeda M, Giordano A, et al (2014) Use of Rasch analysis to refine a patient-reported questionnaire on satisfaction with communication of the multiple sclerosis diagnosis. Mult Scler Houndmills Basingstoke Engl 20(9): 1224-1233.
- Latronico N, Peli E, Calza S, et al (2022) Physical, cognitive and mental health outcomes in 1-year survivors of COVID-19-associated ARDS. Thorax 77(3): 300-303.
- Honarmand K, Lalli RS, Priestap F, et al (2020) Natural History of Cognitive Impairment in Critical Illness Survivors. A Systematic Review. Am J Respir Crit Care Med 202(2): 193-201.
- Pihlaja RE, Kauhanen LLS, Ollila HS, et al (2023) Associations of subjective and objective cognitive functioning after COVID-19: A six-month follow-up of ICU, ward, and home-isolated patients. Brain Behav Immun – Health 27:100587.
- Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, et al. (1999) Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med 160(1): 50-56.
- Mikkelsen ME, Christie JD, Lanken PN, et al (2012) The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med 185(12): 1307-1315.
- Winklewski PJ, Radkowski M, Demkow U (2014) Cross-talk between the inflammatory response, sympathetic activation and pulmonary infection in the ischemic stroke. J Neuroinflammation 11: 213.
- Jackson JC, Hart RP, Gordon SM, et al (2003) Six-month neuropsychological outcome of medical intensive care unit patients. Crit Care Med 31(4): 1226-1234.
- Hopkins RO, Weaver LK, Collingridge D, Parkinson RB, Chan KJ, ed al (2005) Two-year cognitive, emotional, and quality-of-life outcomes in acute respiratory distress syndrome. Am J Respir Crit Care Med 171(4): 340-347.
- Deana C, Vetrugno L, Cortegiani A, et al (2023) Quality of Life in COVID-Related ARDS Patients One Year after Intensive Care Discharge (Odissea Study): A Multicenter Observational Study. J Clin Med 12(3): 1058.
- Gil D, Tiscar C, Gómez M, et al (2022) Health-related quality of life and stress-related disorders in COVID-19 ICU survivors: Are they worse than with other causes of ARDS? J Intensive Med 2(2): 103-109.
- 33 Schittek GA, Bornemann-Cimenti H, Sandner-Kiesling A. Current Insights in Intensive & Critical Care Nursing Wellbeing of ICU patients with COVID-19 Intensive & Critical Care Nursing 65 (2021) 103050 doi.org/10.1016/j.iccn.2021.103050
-
Luisa Sambati1, Katia Mattarozzi, Susy Ferrari, Rossella Santoro, Lucia Cretella, Tommaso Tonetti, Maria Della Giovampaola, Martina Bordini, Paolo Bottausci, Luciano Romano, Pietro Cortelli, Luciana Mascia* and Maria Guarino*. Cognitive Trajectories and Psychological Well- Being of Severe SARS-CoV-2 Patients admitted to the Intensive Care Unit: A One-Year Longitudinal Analysis Including Caregiver Perspectives. Arch Neurol & Neurosci. 16(4): 2024. ANN.MS.ID.000895.
-
Acute Polyradiculoneuritis, Neurology, Guillain-barre; Fann teaching hospital, neuropathy, cranial nerves, immunotherapy, Neuroscience, neurogenic syndrome, epidemiological, Dysphonia.
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






