 Research Article
Research Article
Pyrometallurgy And Electrometallurgy of Rare Earths – Part B: Cell Design, Construction, Rate Processes, And Unit Operation
Muhammad Musaddique Ali Rafique1,2,3*
1Massachusetts Institute of Technology, Cambridge, MA 02139, USA
2National Aeronautics and Space Administration, 39B, Merritt Island, Florida, USA
3Eastern Engineering Solutions Inc. Cambridge, MA 02139, USA
Muhammad Musaddique Ali Rafique, National Aeronautics and Space Administration, 39B, Merritt Island, Florida, USA.
Received Date: February 02, 2023; Published Date: February 20, 2023
Abstract
Production of rare earths by metallothermic reduction and its variants is presented in an earlier study and its analysis is performed indicating major type and sequence of reactions. Diagrams required and procedures to establish them are also described. Their role and importance are also described. A comparison is also made to identify parameters of importance. This study is continuation of a previous study in which cell design to extract metals is described. Parameters to design and construct cell (metallothermic and electrothermic) are determined and used. Rate processes (heat transfer, mass transfer, charge transfer and simultaneous processes) occurring inside a cell, its operation for a specific reaction and sequence for a specific metal (Nd) are described. Outline of calculations outlining cell efficiency, output and yield are enumerated for individual (Nd, Ce, Dy) metal. Optimization and method to achieve is described. Procedures to control and production is also described. Here preliminary graphs are presented.
Keywords: Rate processes; Heat transfer; Mass transfer; Efficiency; Yield
Introduction
Essentially, pyrometallurgical processes involves design of furnace for melting, holding, and refining of metal oxides and compounds while electrowinning and electrorefining (after pyrometallurgy) involves design and control of electrochemical cell (room or high temperature) operated by the fundamental principles of current density, its control, distribution, and electromotive force (EMF). Its detail may be found in specialized texts [1-7] and literature [8-11]. This study is continuation of previous study of author [12] on the subject describing extraction and refining of rare earths from their ores by metallothermic reduction. In this area of emphasis is cell design and rate processes (heat, mass, and activity coefficient determination) which lead towards economic production of these metals from their source. Various graphs and carts are presented lying emphasis on the efficient extraction and refining of rare earths. Nd-Fe foundry alloy is taken as an example to describe construction and operation of cell. Unit operation including piping and instrumentation diagrams (P&IDs) is described to explain different (Elution and electrowinning) circuits. Lastly, a future outlook is presented to calculate individual efficiency and throughput.
Fundamentals
Cell design of rare earth electrowinning process depends on type of metal won (Nd [8], Sm, W, Th, U, Ce, Sc), its quantity required, production rate, type of electrolyte, concentration of metal in electrolyte and flow rate of electrolyte.
Cell design
Cell design primarily consists of outlining parameters of its construction based on its holding capacity. It may be carried out in various ways such as production rate, throughput, efficiency, yield but design based on holding capacity constitutes the simplest. It is presented here.
Consider a typical cell of 5 tons / day. For this, considering rectangular dimensions (as they ease out operation and maximizes yield).
Rate processes
Research progress in development and processing of rare earths may be described following [13,14]. The density of a typical metal as a function of concentration and temperature is presented by the following equation (Figure 1).
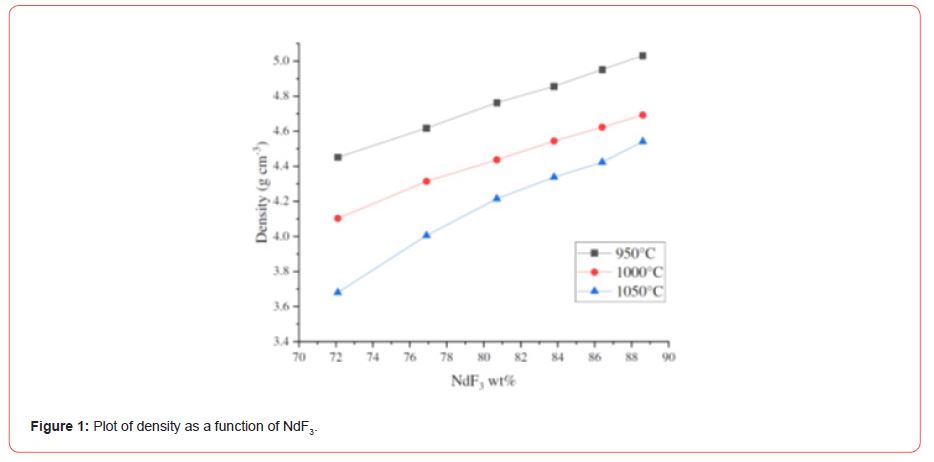
𝜌 = −6.53212 × 10−4𝑐2 − 0.06987𝑐 + 1.31265 × 10−5𝑇2 − 0.05297𝑇 + 1.69004 × 10−4𝑐𝑇+ 43.14013
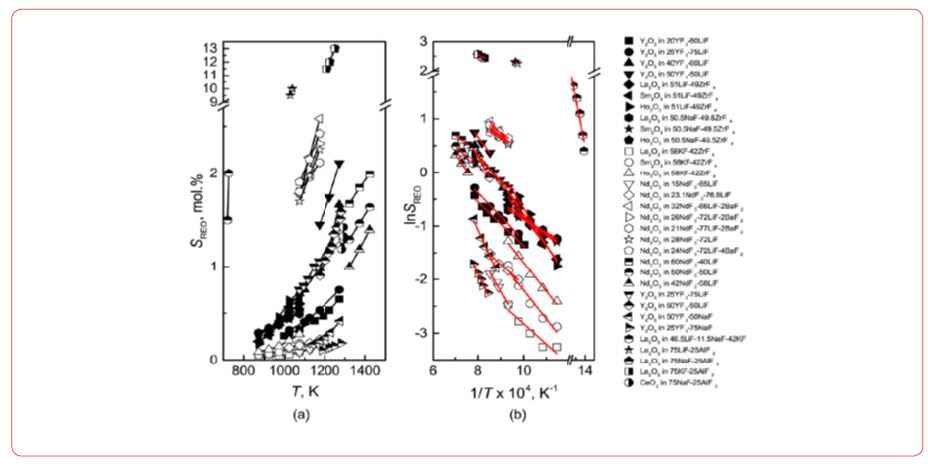
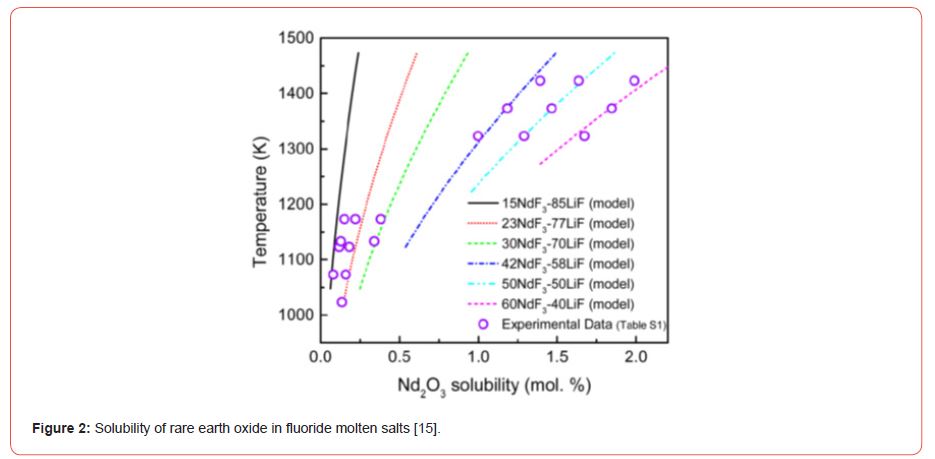
Solubility and dissolution of rare earth oxide in the fluoride melt [15]
Rare earths in their oxide forms may exhibit solubility and may be dissolved in their oxide and fluoride electrolyte melts (Figure 2).
Nd2O3 solubility in fluoride melts with different NdF3-LiF compositions and at different temperatures [15].
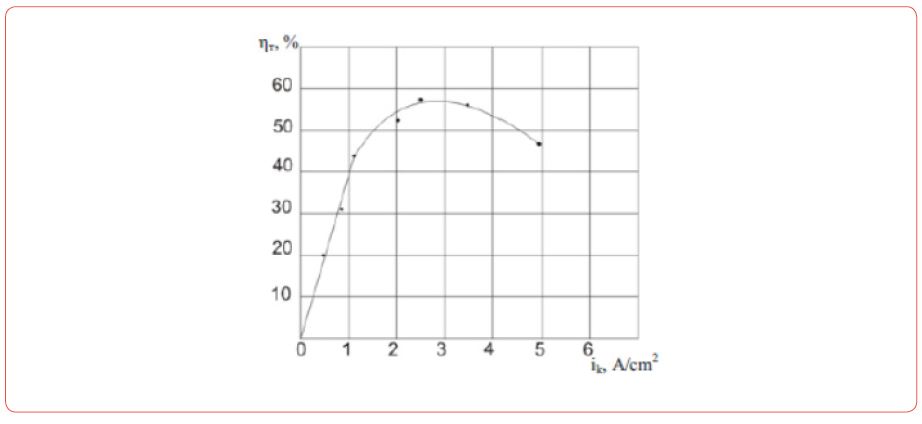
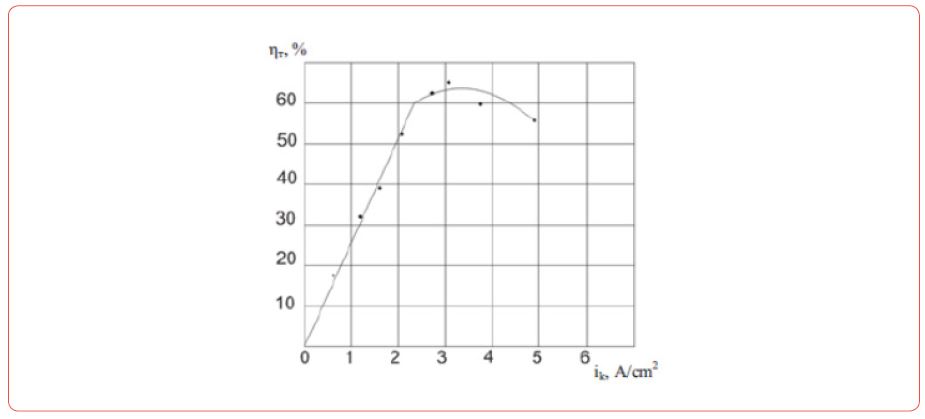
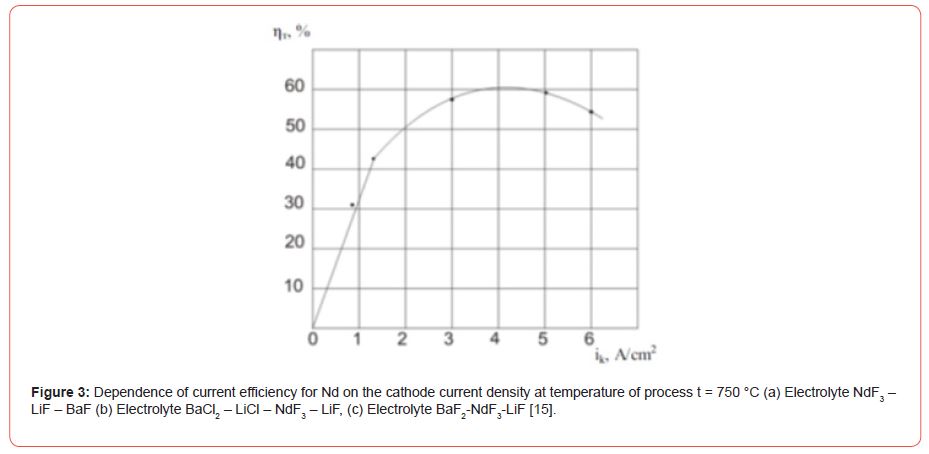
Production of Nd-Fe foundry alloy by the electrolysis of neodymium fluoride
(Figure 3) During the electrolysis of these melts at the electrodes the following reactions are taking place: at the graphite anode [16].
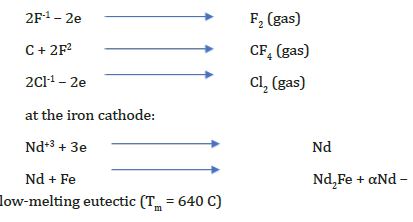
Production of Nd-Fe foundry alloy by the electrolysis of neodymium oxide
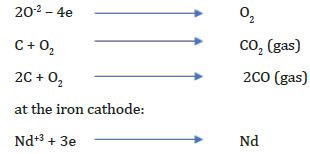
During the electrolysis of neodymium oxide in fluoride melts at the electrodes the following reactions are taking place at the graphite anode [16]

Coefficients
Following coefficients are important, must be considered and calculated for effective electrometallurgical operations. Activity coefficients, diffusion coefficients, equilibrium separation coefficients, effective separation coefficients.
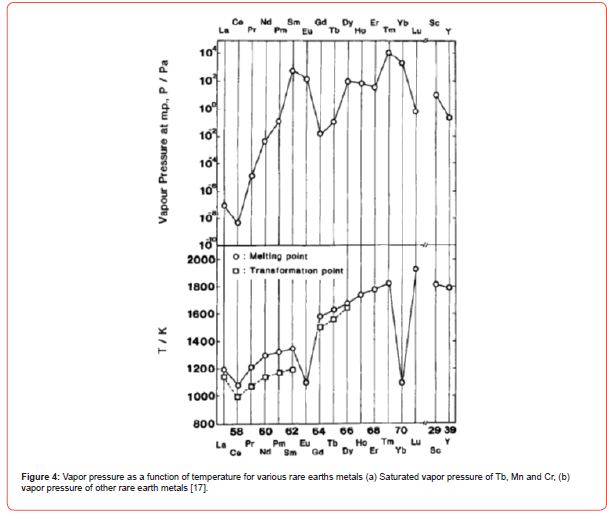
Vapor pressure
(Figure 4) Vapor pressure as a function of temperature for various rare earths metals
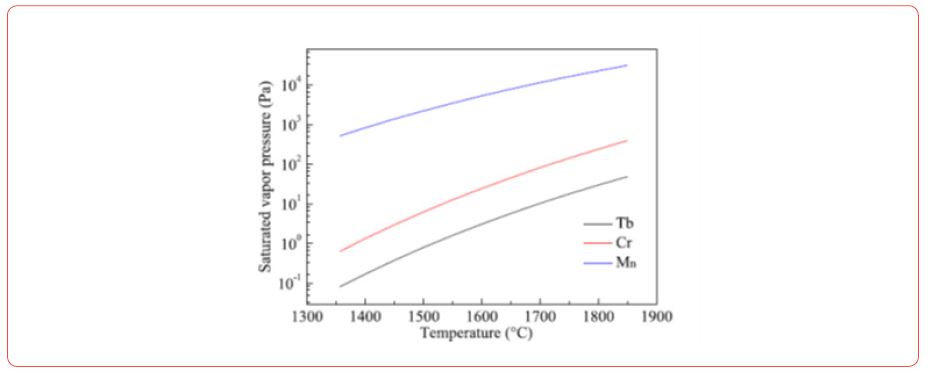
• Saturated vapor pressure of Tb, Mn and Cr,
• vapor pressure of other rare earth metals [17]
Cell efficiency
Cell efficiency may be calculated by measuring the concentration of reactant and product species in electrolyte. An excellent study documents these in Cerium [18]. Interested reader is referred to it for further details.
Mass transfer
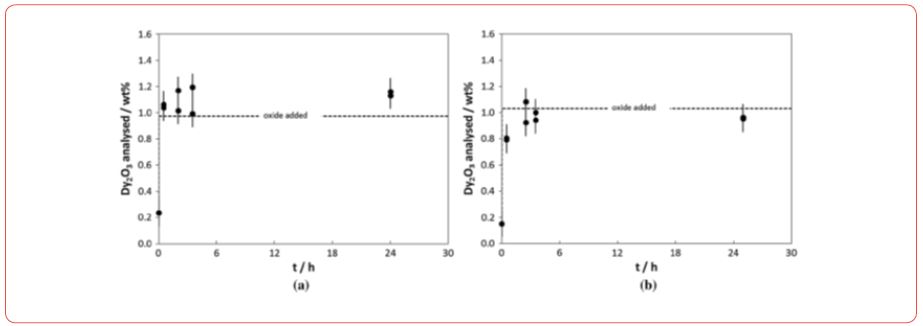
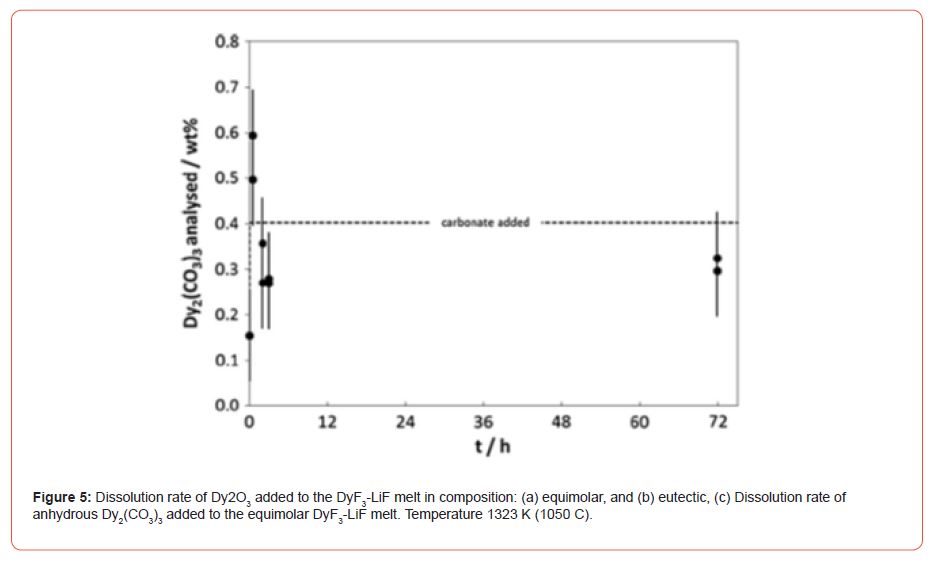
Mass transfer coefficients, solubility of rare earth oxides in molten fluorides, oxide solubility determinations, oxide solubility diagrams. Prior to the proper oxide solubility determinations and diagram development, the equilibration time for oxide source dissolution, e.g., Dy2O3 and Dy2(CO3)3, was determined. It was determined by adding a certain amount of the oxide source (below solubility limit) and sample analysis thereafter at certain intervals. The time of equilibrium dissolution is measured by noting the time at which the analyzed oxide content becomes constant. Below figures (Figure 5) show the results obtained when Dy2O3 was added to the DyF3-LiF melts at different compositions, i.e., DyF3 (50 mol pct)-LiF (50 mol pct) and DyF3 (20 mol pct)-LiF (80 mol pct), at a working temperature of 1323 K (1050 C). The results showed that in the case of the eutectic composition longer dissolution times than in the equimolar composition are needed, in which case, all Dy2O3 added is dissolved well within 30 minutes.
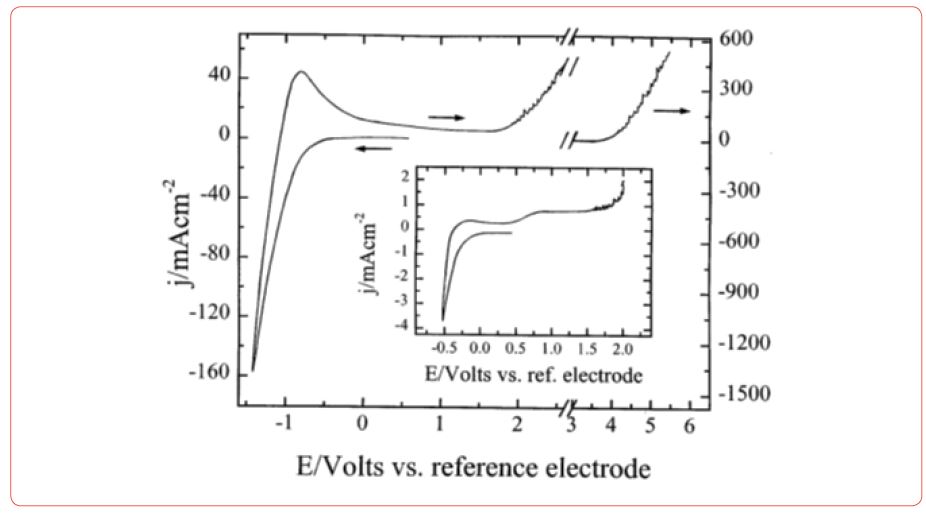
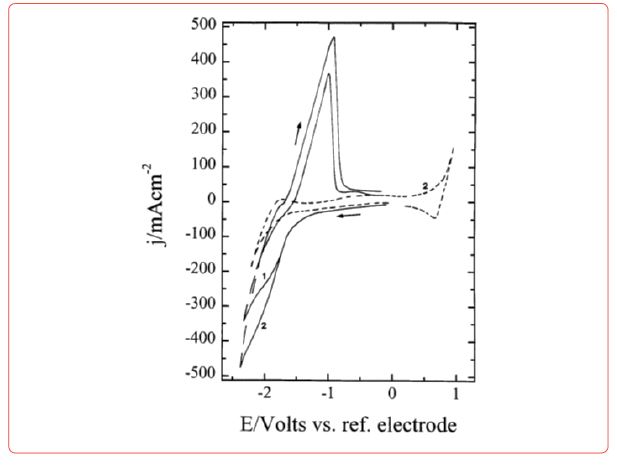
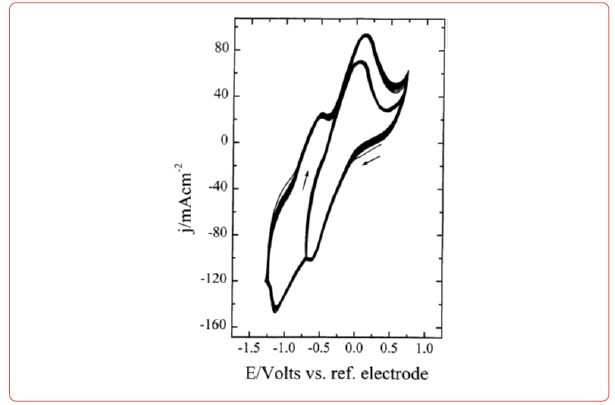
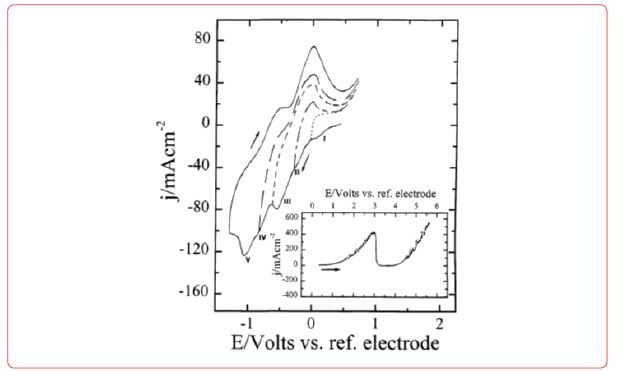
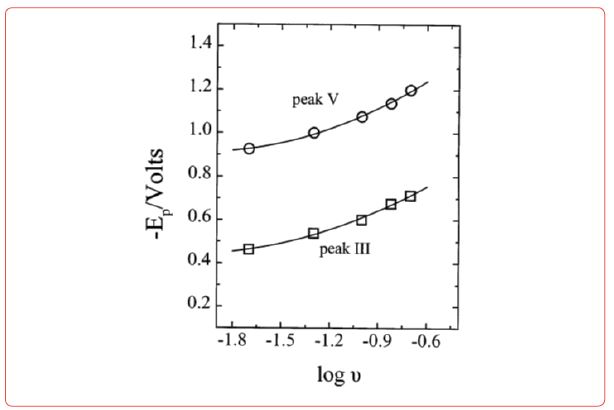
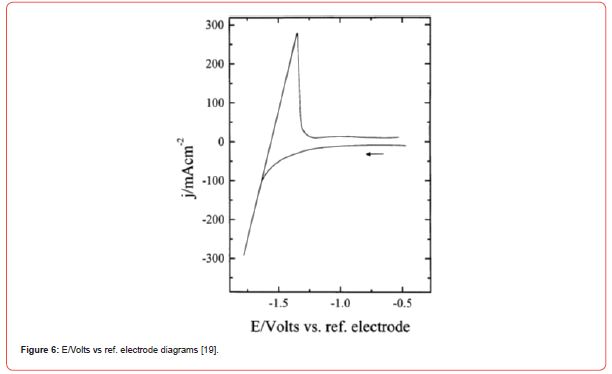
Diagrams for electrodeposition of Nd from its salts are described below [19] (Figure 6).
Unit operations
Unit operations consist of drawing procedures to operate plant, optimize them, making P&IDs, setting them, calculating reactions and their sequences, and calculating efficiencies and throughput.
Piping and Instrumentation Diagrams (P&IDs)
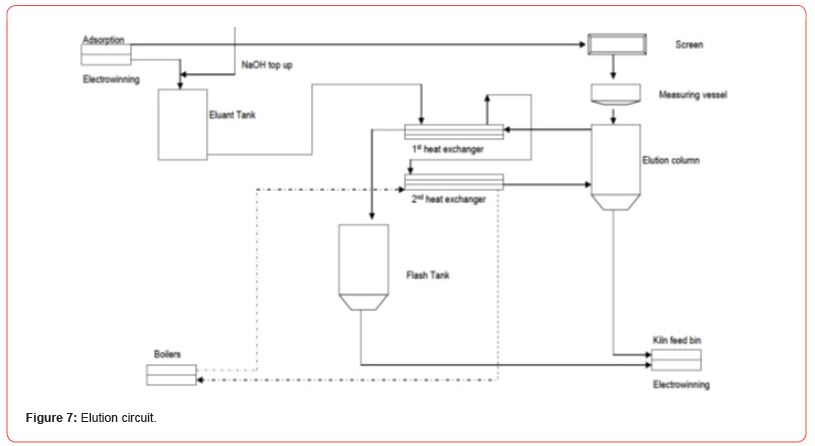
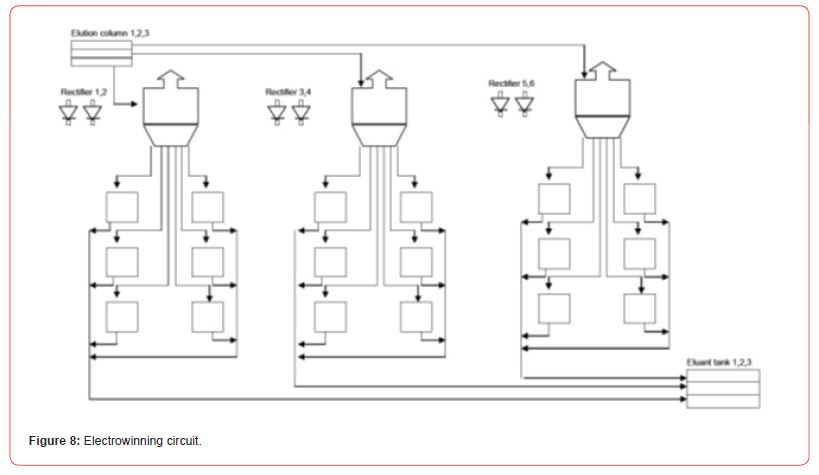
Piping and Instrumentation Diagrams (P&IDs) for rare earths. For a typical 5 ton / day plant, for example optimized P&IDs are developed. Example P&IDs for Au processing plants are shown here [20] (Figures 7-9).
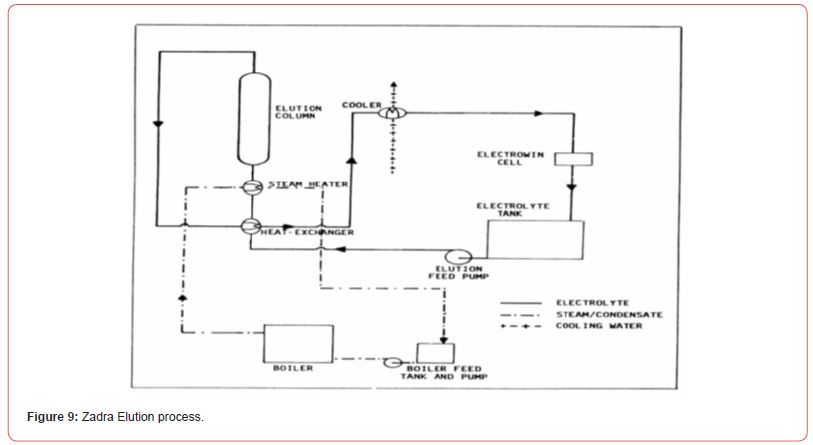
Efficiency and throughput
Efficiency and throughput of individual cells comprising of circuits and P&IDs constitutes an important part of the process and an important rare determining step. These must be carefully calculated and will be presented in subsequent studies with details about each and every individual step.
Conclusion
Various relations may be used to drive and arrive at optimum relations for efficient electrometallurgical operation of cell for efficient extraction and refining of rare earth metals from their salts. These in turn may depend on various intrinsic factors such as activity, heat, and mass transfer coefficients.
Acknowledgement
None.
Conflicts of Interest
The authors do not declare any conflicts of interest.
References
- Lucas J (2014) Rare Earths: Science, Technology, Production and Use.
- Habashi F (2013) Extractive metallurgy of rare earths. Canadian Metallurgical Quarterly 52(3): 224-233.
- Krishnamurthy N, CK Gupta (2015) Extractive Metallurgy of Rare Earths, Second Edition.
- Paunovic M, M Schlesinger (2006) Fundamentals of Electrochemical Deposition.
- Popov K, B Grgur, SS Djokić (2007) Fundamental Aspects of Electrometallurgy. Springer US.
- Zelikman AN, OE Kreĭn, GV Samsonov (2018) Metallurgy of Rare Metals. Nova Science Publishers, Incorporated.
- Qi D (2018) Hydrometallurgy of Rare Earths: Extraction and Separation.
- Yang Y (2020) Recovery of rare-earth element from rare-earth permanent magnet waste by electro-refining in molten fluorides. Separation and Purification Technology 233: 116030.
- Gupta CK, N Krishnamurthy (1992) Extractive metallurgy of rare earths. International Materials Reviews 37(1): 197-248.
- Balaram V (2019) Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geoscience Frontiers 10(4): 1285-1303.
- Xie FA (2014) Critical review on solvent extraction of rare earths from aqueous solutions. Minerals Engineering 56: 10-28.
- Rafique M (2021) Pyrometallurgy and Electrometallurgy of Rare Earths – Part A: Analysis of Metallothermic Reduction and its Variants.
- Liu H (2020) Research Progress in Preparation and Purification of Rare Earth Metals. Metals 10(10): 1376.
- Samuel Senanu, Arne Petter Ratvik, Henrik Gudbrandsen, Ana Maria Martinez, Anne Støre, et al. (2021) Dissolution and Online Monitoring of Nd and Pr Oxides in NdF3–PrF3–LiF Electrolytes. Metals 11(2): 326.
- Guo X, J Sietsma, Y Yang (2014) Solubility of rare earth oxides in molten fluorides. ERES, pp. 149-155.
- Makaseev Y (2017) Production of Nd-Fe Foundry Alloy by Electrolysis in Molten Salts. MATEC Web of Conferences 96: 00009.
- Isshiki M (1996) Purification of rare earth metals. Vacuum 47(6): 885-887.
- Thomas N B (1986) An investigation of some aspects of cerium electrowinning.
- Stefanidaki E, C Hasiotis, C Kontoyannis (2001) Electrodeposition of neodymium from LiF–NdF3–Nd2O3 Electrochimica Acta 46(17): 2665-2670.
- Lunga AL (2008) Optimizing the operating conditions of gold elution and electrowinning for Tau Lekoa stream at Kopanang gold plant.
-
Muhammad Musaddique Ali Rafique*. Pyrometallurgy And Electrometallurgy of Rare Earths – Part B: Cell Design, Construction, Rate Processes, And Unit Operation. Adv in Mining & Mineral Eng. 1(1): 2023. AMME.MS.ID.000502.
-
Thermal effects, Shale’s permeability, Temperature, Sandstone, Teflon, permeability curves, Mineralogical properties
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






