 Research Article
Research Article
Characterization and Processing of U-Bearing Syenitic Rocks from Mbanga Massif, South Region, Cameroon: Insight from Geochemistry, Mineralogy, Radiometry, and H2SO4 Acid Leaching
Raoul Pierre Fodjo1, Nguo Sylvestre Kanouo1*, Francois Bidzang Ndong2 and Emmanuel Archelaus Afanga Basua3
1Department of Mining Engineering and Mineral Processing, National Advanced School of Mines and Petroleum Industries, University of Maroua, Cameroon
2Institut of Geological and Mining Research, Yaounde, Cameroon
3Department of Geology, Faculty of Science, University of Buea, Cameroon
Nguo Sylvestre Kanouo, Department of Mining Engineering and Mineral Processing, National Advanced School of Mines and Petroleum Industries University of Maroua, Cameroon
Received Date: December 01, 2024; Published Date: December 13, 2024
Abstract
U-bearing syenitic rocks cropping at the Mbanga massif in the South Region of Cameroon were geochemically and mineralogially charactarized, before the determination of their radiometric data, and processing of their uranium ores by H2SO4 acid leaching. The syenitic rocks are alkaline syenite, high-K syenite, and alkaline quartz syenite, with the first two rocks being metaluminous and the third, peraluminous. Both rocks show a compositional difference in uranium abundances (133-447 ppm) and those of other elements (exàmples of SiO2: 61.6-66.6 wt.% and Al2O3: 16.9- 17.9 wt.%). The trace and rare earth element’s suites show the predominance of Sr (157.1-999 ppm) and Zr (111-599.7 ppm). The ƩLREE (37.3- 168 ppm), ƩHREE (8.7- 22.2 ppm), ƩLREE/ ƩHREE (1.6-13.8), and Eu/Eu* < 0.4 are variable. Found U- ore minerals (uraninite, uranophane, autunite, coffinite, carnotite, torbernite, and/or coffinite) and other ore minerals (U-rich titanium oxide, zircon, magnetite, and/or ilmenite) are in a gangue made up of chlorite, calcite, quartz, alkaline feldspars, plagioclase, and/or biotite. The uranium contents (< 465 ppm) in the studied U-bearing syenites classify them within low grade ores; suggested to have formed with the aid of circulating hydrothermal fluids source of the precipitated OH, H2O, and OH-H2O bearing uranium ore minerals. The measured raw radiometric values range from 3542-6600 c/s and U3O8, from 0.33-0.59 wt.%. The presence of some minerals in the studied ores and the use of oxidant such as manganese dioxide during the H2SO4 acid leaching, provided elements which positively impact the process by increasing the U extraction with a total recovery of 80-90 %. The maximum leaching temperatures 40-60oC were less, compared to those used to process some other low grade uranium ores.
Keywords: Cameroon; Mbanga massif; syenitic rock; uranium ore; characteristics; H2SO4 acid leaching process
Article Highlights
a. U-bearing syenitic rocks from Mbanga massif were geochemically and mineralogically characterized, and their radiometric features determined before an H2SO3 acid leaching ore processing.
b. They are alkaline syenite, alkaline quartz syenite, and high-K alkaline transitional type syenite dominantly metaluminous suggested to have formed from partial melting of preexisting protoliths.
c. The U-bearing ores are low grade formed with the aid of hydrothermal fluids.
d. Element from the gangue minerals and used oxidant improved the extracting process with a maximum recovery of 80-90 % at 40-60oC leaching temperatures.
Introduction
Uranium is a radioactive-lithophile metal which occurs in many geologic formations; including metamorphic, magmatic, and sedimenatry rocks (e.g., [1-4]). It is relatively high in some plants, water, soils, sediments, bitumens, coals, and fossil woods [5-12]. This element is mined in some sandstones, comglomerates, shales, granites, pegmatites, alaskites, monzonites, limestones, carbonatites, syenites, pelites, soils, sediments, tuffs, metaschists, metacarbonates, and some acid volcanic rocks [4,13-18]. The presence of positive uranium anomalies in rocks, plants, soils, water, coals, and sediments is signified by its radioactivity or high contents; which might save as a guide for uranium deposits exploration [4,6,8,12,19-26]. Many types of uranium deposits are distinguished: quartz-pebble conglomerate deposits, carbonaceous-siliceous-pelite deposits, carbonate type deposits, the vein type deposits, surficial deposits, intrusive deposits, the metasomatic type deposits, the phosphorite and lignite deposits, the hematitic breccia complex deposits, albitite type deposits, sandstone deposits, and unconformity-related deposits [8,13,17,27,28]. In these deposits, uranium is hosted by many ore minerals including: a. Major u-minerals (uraninite, uranite, coffinite, brannerite, davidite, carnotite, pitchblende, and betafite);b. Minor U-minerals (uranotile, chalcolite, clacouranite, gummite, tyuyminite, autunite, uranothorianite, torbernite, uranophane, euxenite, fergusonite, and samarkite, torbernite…), and c. As substitute of other elements in zircon, xenotime, monazite, apatite, and titanite [3,14,27,29-33].
These minerals are either disseminated in their host rocks or carried by specific features such as fractures, veins, lenses, sheetlike or pod-like features, reticules, stockworks, faults, and brecciated- shear zones [4,27,28,30,34-38]. In some sandstone type U-deposits, uranium ore minerals occur as grain coatings and intergranular fillings or as replacements of sand-size and smaller grains [18,38]; as clay aggregates, in volcanic rock fragments, or as replacements of carbonaceous plant debris [39]. The study of U-ore minerals and their hosted structures place a better role in characterizing its deposit. U-ore minerals in some deposits are associated to other mineable minerals such as zircon, thorianite, monazite, xenotime, hematite, magnetite, pyrite, scheelite, pyrrhotite … in a gangue made up of feldspars, chalcedony, plagioclase, sphene, quartz, tourmaline, amphibole…[3,15,27,28,30]. The identification of the above minerals, and their characterization, coupled to other features already presented, are of interests. They play an important role during ore prospecting, grading, mining, and processing, to define a deposit type, and to present its genetic model [8,21,23,40].
U-ore processing techniques include leaching, solid-liquid separation, ion-exchange, and solvent extraction [41-46]. Acidic leaching in particular, is often used during experimental uranium extraction, as it has the advantages of being more effective, requiring lower temperatures and leaching times [45]. The use of sulphuric acid as leaching agent, has shown high leach performance at relatively low cost [45]. It is therefore interesting to use sulphuric acid leaching technique for an experimental extraction of uranium from newly studied U-ore body. In Cameroon, U-ore mineral occurrences are mainly found in Lolodorf (in the south) and Kitongo « Poli » (in the North) [2,13,47,48]. The Kitongo U-ore mineral occurrence has been subjet of many studies (e.g., [2,3,13,48-50]. AIEA [13] proposed that the Kitongo U- ore mineral occcurence is a disseminated type formed as a result of structurally-controlled metasomatic replacement in syn-orogenic granitic plutons of Pan-African age, intruded along a deep-seated fault. The granitic nature of the rock hosting the Kitongo uranium occurrence was confirmed by Kouske et al. [2]. For Kouske et al. [2], the Kitongo U-ore occurrence is an albitite structurally-controlled with metasomatic fingerprintings.
This brecciated-albitite uranium ore occurrence affected by Na-metasomatism (albitization), Ca-metasomatism(calcitization), coffinitization, oxidization, and hematization, encloses uraninite, coffinite, U- Zr-Si, U-Fe-Si, and U-Ti phases in a gangue made up of albite, riebeckite, aegirine, amphibole, monazite, magnetite, calcite, zircon, epidote, apatite and titanite [2]. Saïdou et al. [48,49] carried out natural radioactive measurements in Poli (area of location of the Kitongo uranium ores). Limite published information are available for Lolodorf uranium ore mineral occurrences and other uranium ore mineral occurrences (e.g., those found in d’Awanga and Ngomba, and Mbanga). The first radioactive anomaly found in Lolodorf was evidenced on alkaline syenite during 1979 GMRB (Geologic and Mining Research Board) field survey [47]. Collected and analyzed samples shew the presence of uranothorite, uraninite, pyrite, chalcopyrite, molybdenite, and galena [47]. Survey work carried out by Mega Uranium Ltd (uranium exploration company) gave an estimation of up to 11000 tones of U in the Lolodorf prospect.
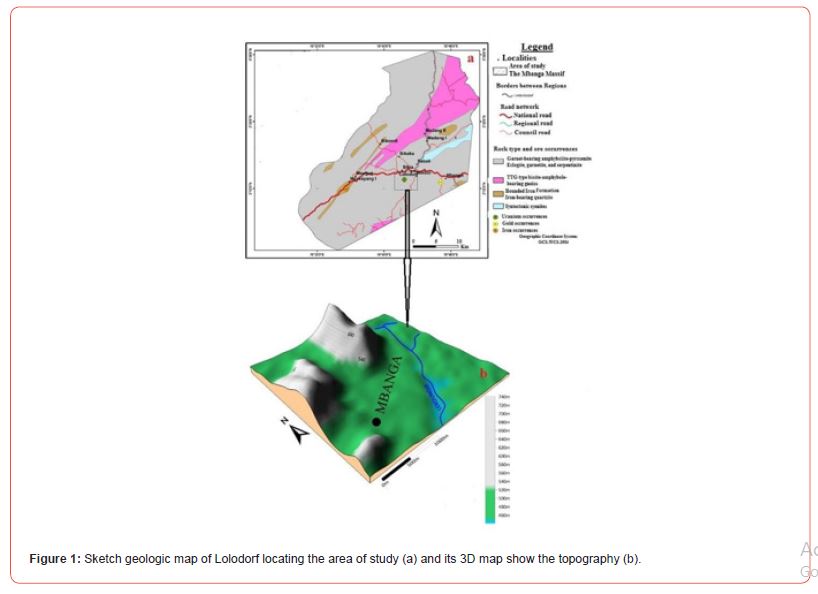
Natural radioactivity measurements in uranium and thorium bearing zones in Lolodorph, recorded significant 226Ra, 232Th, and 40K radiations [24,49]. The Mbanga Massif found at about 43 Km SE to the Lolodorf uranium ore occurrences has not yet been studied in detail. Information characterizing the U-bearing ores and their host rocks are lacking. The U-bearing ores has not yet been processed. In this paper, we present geochemical, and mineralogical data characterizing targeted U-bearing syentic rocks cropping in the Mbanga massif. Radiometric analytical data are coupled the other obtained results (this study) to evidence the presence of uranium ore minerals and evaluate the potential. The identified and sampled ores are processed by H2SO4 acid leaching to extract uranium.
Geological Settings
The Mbanga Massif (Figure 1) found at about 43 km SE of Lolodorf in the South Region of Cameroon, is part of the Congo Craton (Ntem Complex), precisely the Nyong Unit (a mega lithostructurale unit composed of rocks of the Craton and those the PanAfrican Mobile Belt [51]). This large NNE-SSW trending band of dominantly migmatized gneisses bordering the NW corner of the Congo Craton [51], encloses rocks aged Archean to Paleoproterozoic [52-55]. They are migmatitic orthogniesses (TTGs), meta-gabbros, amphibolites, metagranodiorite, pyroxenites, metadolerites; garnetites, eclogites, felsic gneisses of volcanic to volcano- sedimentary origin, quarzites, charnockites, nepheline syenites, alkaline meta-syenites, and bounded iron formation (BIF) (e.g., [55-72]). Migmatites, chanockites, and meta-volcano- sedimentary rocks are Archean in age [52,53]. Rocks in Lolodorf comprise gneisses, clinopyroxene-bearing metasyenites, pyroxenites, and TTG [55,59] largely covered by very thick to thick ferralitic, hydromorphic and less-developped soils, and alluviums [73]. Meta- mafic rocks in Lolodorf (melanocratic, fine to coarse-grained, and localy foliated) form dykes, sills, and lenses in charnockitic gneisses [59,73]. These rocks are composed of garnet, clinopyroxene, amphibole, quartz, plagioclase, apatite, ilmenite, and rutile [74]. Amphibolites and metadolerite outcroping in Ako’Ozam-Njabilobe have the following composition [69]: a. Amphibolites are composed of amphibole, garnet, plagioclase, iron oxides, quartz, and/or biotite; and b. Metadolerites are dominantly composed of plagioclase, pyroxene, and amphibole, with few magnetite, hematite, quartz, biotite, and microcline.
Garnet amphibolite cropping Eséka is mainly made up of amphibole, garnet, plagioclase, biotite, and pyroxene with few titanite, quartz, epidote, and opaque minerals [72]. Amphibolites from Akom II is mainly and respectively composed of green hornblende, plagioclase, garnet, biotite, and quartz in association with very few proportions of zircon, titanite, apatite, and opaques minerals [71]. Metagranodiorite found in the Bongue area is composed of K-feldspar, quartz, biotite, plagioclase, and opaque minerals [68]. Ngovayang quartzites (banded ferruginous quartzites, massive ferruginous quartzites, and leucocratic quartzites) have the following composition [63] banded ferruginous quartzites is composed of quartz, magnetite, hematite, martitite, and ilmentite; massive ferruginous quartzites mainly occurring as fractured filling materials are composed of quartz, hematite, and magnetite; leucocratic quartzites always in association with the two names quartzites, lack iron oxides and other coloring minerals. Magnetite gneiss found in the Nyong Unit is weakly to moderately foliated, coarse-grained, and dominantly composed of quartz and magnetite in association with amphibole, plagioclase, clinopyroxene within a biotite- feldspar- quartz rich groundmass with few sulphide disseminations [67]. Rocks found in Edéa part of the Nyong Unit are composed of:
a. Garnet-rich micaschist (biotite, muscovite, garnet porphyroblasts, quartz, plagioclase, with monazite and oxides forming the accessory minerals);
b. Pyroxene-rich gneiss (quartz, plagioclase, orthopyroxene, hornblende, biotite, and garnet with oxides as accessory minerals);
c. Garnet-rich charnokitic gneiss (quartz, plagioclase, orthopyroxene, biotite, garnet porphyroblasts surrounded by quartz-plagioclase- biotite-rich rim; monazite and oxides are accessoiry minerals);
d. Garnet and amphibole-rich gneiss (hornblende, quartz, plagioclase, k-feldspar, orthopyroxene, garnet, and biotite with zircon and apatite being the accessoiry minerals);
e. Pyrigarnite (quartz, plagioclase, k- feldspar, orthopyroxene with rim of quartz and plagioclase, hornblende, garnet porphyroblasts with rims of quartz+k-feldspars, plagioclase+pyroxene, and plagioclase+quartz; apatite, rutile and oxides are the accessory minerals);
f. Garnet-rich amphibolite (amphibole, pyroxene, quartz, garnet, plagioclase; apatite, zircon and oxides form the accessory suite [64].
Syenitic bodies of peralkaline to alkaline nature found within rocks of the Nyong Unit include: Pan-African aged nepheline syenites at the Rocher du Loup and Mont des Elephants [57,75] and the Early Proterozoic clinopyroxene syenites in the Doum-Lolodorf area [57,59]. The syenites cut across the greenstone belts and the members of TTGs suite [56]. The Eburnean (2.05: [53]) metmorphism affected both the oldest rocks (e.g., TTGs: 2.9-2.8 Ga) of the Nyong Unit and Early Proterozoic (2.3 Ga: [76]) syenitic intrusions [59]. The Pan-African age nepheline syenites are a band of three small plutons found along the Atlantic coast; these rocks are composed of nepheline, microcline-perthite, and plagioclase, as the main minerals suite [75]. The clinopyroxene syenites form numerous intrusions that form a NE–SW oriented elongated composite intrusive body covering nearly 70km [57]. They are layered melanocratic to leucocratic with diverse composition; with the melanocratic syenites predominantly composed of diopside-hedenbergite series and perthitic alkali feldspar, minor amounts of amphibole, biotite, and sodic plagioclase [59]. The accessory minerals suite includes titanite, apatite, ilmenite, and magnetite; whereas, secondary phase minerals are chlorite, biotite, actinolite, albite, calcite and magnetite [59]. The leucocratic clinopyroxene syenites are composed of perthitic alkali feldspar and lesser amounts of sodic plagioclase with titanite, apatite, ilmenite and magnetite forming the accessory minerals suite [59]. Late metamorphic overprinted secondary minerals such as garnet and biotite, and low-grade alteration secondary minerals (chlorite, sericite, calcite, actinolite and leucoxene) are found [59].
Materials and Methods
This part presents materials and methods used to obtained sample’s analytical and ore processing results. Rock petrography, hold rock geochemistry, XRD and SEM mineralogy, and U radiometry were used for sample analysis. The H2SO4 acid leaching was used to process the uranium ores.
Samples collection and preparation
Thirty fresh rocks were sampled with the aid of a geologic hammer and a 50kg mallet. They were sampled on located and described outcrops. The geographic coordinates of each outcrop and sampling point were recorded with the aid of a GPS. The collected samples were labeled and carried to the laboratory for preparation and analyses. The sample preparation took place at the Institute of Geologic and Mining Research (IGMR) in Yaoundé, Cameroon. This sample preparation includes: fresh rock thin sectioning for petrographic (three for each rock type) and SEM (scaning electromicroprobe) mineralogical analysis (one for each rock type), and a powdering of part of the fresh rocks for geochemical and XRD mineralogical, and U-rich ore bodies for radiometric analyses, and processing by leaching. Prepared thin sections at the IGMR were characterized in this research and sent to Fance for SEM analysis. Sample preparation for geochemical and XRD mineralogical analyse was as followed: fresh rock samples were sun dried before being crushed and milled. Each sample was broken with the aid of a mallet to obtain a much smaller size fragment. These fragments were introduced in a FRITSCH type jaw crusher to crush at a grain size of 5mm. Before and after any crushing the crusher was cleaned with a pulverizer and ethyl alcohol to avoid any contamination. The obtained fragments were milled to a grain size of less than 1μm. The milling machine was cleaned before introducing a new sample.
Petrography
Sampled fresh rock fragments were macroscopically described, based on the following parameters: color, texture, structure, mineralogical composition, the presence or absence of U- ore minerals, and the relationship between these minerals and other minerals. Prepared thin sections were observed under a polarizing microscope. The parameters used to characterize each thin section include: texture, structure, mineral color, shape, pleochroism, relief, extinction angle, proportion, polarizing ton, relation between the minerals, and composition.
Geochemical analysis
Five prepared rock powders (one for each rock type) were sent for major, minor, trace and rare earth elements geochemical analysis in Ireland. This analysis was carried out at the OMAC geochemical laboratory of the ALS Geochemistry group in Ireland. The major and minor element oxides composition was determined with the aid of an energy Phillips PW 1840 brand device X-ray fluorescence spectrometer (XRF). The trace and rare earth element composition was determined with a VG- Plasma Quad STE ICP-MS (Inductively Coupled Plasma Mass Spectrometry). Analytical uncertainties are 1% for major elements, 5 and 10 % for trace elements, 5% for REE > 10 ppm and 10% for REE <10 ppm. The XRF analysis was follows: 1 g of the rock powder and 8 g of lithium tetra borate were weighed, introduced into a platinum/gold alloy called a crucible, and mixed. The mixture was homogenized, and 1 ml of lithium bromide was added with the aid of a micropipette.
The obtained solution was put in an electric laboratory furnace at 1150°C for 20min. The heated solution was removed, and poured into an appropriate crucible to obtain a molten pearl, which was allowed to cool for few minutes in ambient air. The analysis was launched following the instructions of the used X-ray florescence spectrometer and after having entered the value of the loss on ignition, followed by those of other quantified chemical elements in the analysis program. The results appear 5min afterwards on the connected computer monitor. The ICP-MS analysis was as follow: 10 g of each rock powder was collected and oven dried at 100°C. A pellet was prepared with the dried sample before being dissolved in a high-pressure Teflon bomb using a 1/1 mixture of HF and HClO4 at 180°C. The dissolved pellet was brought to a solution of HNO3 under standard internal conditions; and pured into a mixture made up of HNO3, 6NHCl and HF. The obtained solution was diluted. This solution was quantified 24 hours after dilution in order to prevent absorption of High field strength elements (HFSE) onto the sample vial. Geochemistry softwares suchas (Minpet, Geoxdraw, Ersoy 2010, XLSTAT and Excel were used to treat the obtained results.
Mineralogical analyses
XRD mineralogy
The mineralogical analyses carried out on samples from the Mbanga U-bearing syenitic rocks, include the X-ray diffractometry (XRD) and scanning electronic microscopic analysis (SEM). These analyses were carried out at the Interdisciplinary Laboratory of Continental Environments in Lorraine (France, Western Europe). The XRD mineralogical analysis was carried out on rock powders to determine the different minerals and their proportion. It was carried out at the Interdisciplinary Laboratory of Continental Environments found at the University of Lorraine, Lorraine in the NE of France (West of Europe). The analysis was done with the aid of two powder diffractometers: a. Brand Bruker, operating with a copper tube (λ=1.54Å) and b. Operating with a cobalt tube (λ=1.79Å).
The analysis was as follows: the X- ray beams produced by accelerating the electron into a copper target and directed toward the sample, where the atoms deflect them. Angles of constructive interference diffracted X-ray into the detector which transduced the X-ray intensity and plotted in form of curves (diffractogram). Diffractogram presented peaks at very specific diffraction angles. The position of these peaks is a true signature of the arrangement of atoms inside a crystal (distance between atoms, and between intracrystalline planes). The empirical relation that connects the angles at which the peaks were observed and the distances between atomic planes is the Bragg’s law. X-ray diffraction makes it possible to distinguish products having the same chemical composition but different atomic arrangements. On the other hand, phases of different chemical nature but of same atoms arrangement present great similarities, for instance, diffraction peaks located at the same angular positions. The identification procedure was done in two stages: the first stage (search) consists of comparing the peaks obtained with those present in the database that includes several hundreds of thousand records. This step was followed by a validation step (match) with respect to the chemical composition. The user must have a good knowledge of the samples and database, in order to remove ambiguities and confusion when carrying out validation.
SME mineralogy
Thin sections characterized under the polarizing microscope in IGMR (Institute of Geologic and Mining Research) in Yaoundé (Cameroon), were sent France for SEM mineralogical analysis. This analysis was carried out at the Interdisciplinary Laboratory of Continental Environments found at the University of Lorraine, Lorraine in the NE of France (West of Europe). This was done with the aid of an FEI Quanta 200 FEG brand environmental scanning electron microscope and Hitashi S-2600N conventional scanning electron microscope, coupled with an energy dispersive spectrometer (EDS). The analytical procedure was a follows: The thin sections were covered with carbon to make their surface conductive to electrons. The thin sections were then imaged and mapped with a Hitashi S-2600N electron microscope. This phase was coupled with an energy dispersive spectrometry (EDS) analysis carried out with a working voltage ranging from 10 to 20 kV. The scanning electron microscope used a very fine electron’s beam emitted which scanned point by point, the surface of the thin section. Electromagnetic lenses focused the electron beam on the thin section. The interaction between electrons and sample caused the formation of lower energy secondary electrons; which were amplified, detected, and converted into an electrical signal. This process was carried out at each point of the sample by scanning the microscope. This lead to a complete imaging of the sample by reconstructing the topography and providing information on the morphology of the surface.
Radiometric test
Five 5 samples from U-ore bodies identified by Nu Energie uranium exploration company in 2007 were collected and a radiometric test was performed on them. The radiometric test was carried out at the Orano calibration laboratory in Bessines (Gartempe, France, Western Europe). This was done with the aid of a tank containing the sample to be tested, a source that emits gamma radiation, and gamma radiation detector (radiometric probe). Radiations produced by radioactive element (e.g., U) since decay were measured with radiometric probe. During this measuring phase, the used device recorded each spectrum in the form of counts/s (c/s) (unit of radioactive emissions). Indeed, if no emission is found in the area surveyed by the beam rays, the intensity of the radiation will remain stronger, but with the presence of emissions in the beam, the intensity of the radiation will be attenuated. The irradiation detected by the detector can then make it possible to calculate the value of the parameter sought, i.e. the conversion of the c/s into equivalent concentrations of uranium, which is therefore considered to be a reasonable representation of the content of uranium in situ. Thus, the eU3O8 equivalent uranium analyzes (“e” for equivalent) determined are expressed in parts per million (ppm) or percentage by weight (wt.%).
Ore processing by acid leaching
The method used to process the samples collected from U-ore bodies is H2SO4 acid leaching. The samples underwent the following operations: crushing, milling, leaching, solid/liquid separation and washing as described in Guettaf et al. [43]. The collected samples were milled to a grain size of close to 1 mm. 20 g of this powder was mixed with 60 ml of sulfuric acid in a beaker. The sulfuric acid attacked and dissolved the ore to liberate U. To significantly reduce the leaching time, compounds such as manganese oxide was added into the solution, and heated (at 40-60oC) to extract the uranium in a reduced form, poorly soluble in acid solutions. After leaching, the solids and liquids were separated, and the solids were washed to allow recovery of the adhering leach solution. Solvent extraction process were used to separate uranium from the leaching solutions. The used active agent during solvent extraction is an organic amine salt diluted in kerosene, which can selectively extract uranium ions by forming an organic complex, insoluble in water. The formed organic complex was separated from the aqueous phase by a continuous settling technique in a funnel after shaking the mixture to extract the uranium. The organic complex with its low density staid in suspension in the aqueous phase. Two beakers were used to recover the two phases so as to separate through a funnel tap.
Results
This part presents, major, trace, and rare earth elements results for the identified syenitic rocks, after their nomenclature and petrography. XRD and SEM mineralogical results are also presented. In addition to the geochemical and mineralogical results, are those of radiometric tests, and of uranium separation from the ores by H2SO4 acid leaching.
Nomenclature and petrography of metasyenitic rocks cropping at the Mbanga massif
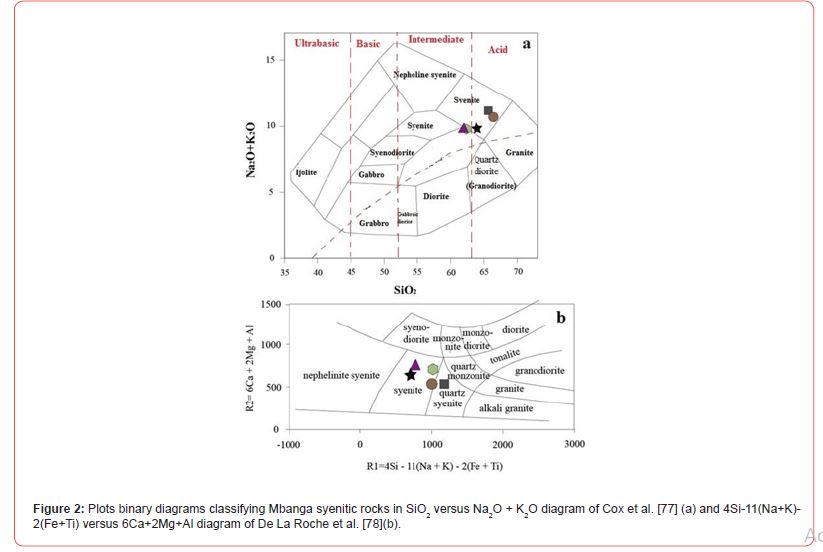
The major element oxide results for five rock samples plotted in Cox et al. [77] SiO2 versus Na2O+K2O binary diagram fall all in alkaline field (Figure 2a). Three plots fall in alkaline syenite field and two, on the dividing line. In 4Si-11(Na+K)-2(Fe+Ti) versus 6Ca+2Mg+Al diagram of De La Roche et al. [78] (Figure 2b), one sample is plotted in quartz syenite field; whereas, four samples were mainly plotted in syenite field. The studied samples can be named alkaline syenite, alkaline quartz syenite, alkaline transitional type syenite (the one plotted on the dividing line between syenite and quartz syenite). The macrophotographs and microphotographs of samples from the named rocks are presented in Figures 3 & 4, respectively. The alkaline syenite (Figure 3a) is whitish in color, weakly structure, coarse-grained, and composed of alkali feldspar, plagioclase, with very few amphibole, and biotite (Figure 4a-b). The texture is grano-lepido-pneumatoblastic to heterogranular. Felspar form subhedral laths. This mineral with plagioclase laths host dark spots which could be burning due to U radioactivity.
These burning form halos in some amphibole crystals. The alkaline quartz syenite (Figure 3b) is also whitish in color but, host much spots of ferromagnesian mineral than alkaline syenite. It is also weakly structure but, much structured than the alkaline syenite. This rock like alkaline syenite is coarse-grained, and granoblastic to heterogranular. It is composed of alkali feldspar, plagioclase, with few quartz and clinopyroxene (Figure 4b-c). The alkaline transitional syenite (Figure 3c) is much structured than the two previously described syenites. This rock is granoblastic to heterogranular and composed feldspar-plagioclase rich band alternating with large feldspar-plagioclase-clinopyroxene bands (Figure 4d-e). Feldspar and plagioclase host dark spots-burning.

Geochemistr
The major, minor, trace, and rare earth element composition of each syenitic rocks (named alkaline syenite, alkaline quartz syenite, alkaline transitional type syenite) are presented separately.
Major, minor element oxide and CIPW mineralogical composition
The major, minor element and CIPW mineralogical composition in Table 1, show some variations. The oxide composition in the three groups of alkaline syentic rocks show the predominance of SiO2 which is 66.55 wt.% in quartz syenite, 65.85 wt.% in transitional type syenite, but, ranges from 61.29 to 63.98 wt.% in syenite (Table 1). This value is followed by that of Al2O3 (16.98-17.95 wt.%), Na2O (4.74-9.69 wt.%), and K2O (0.13-6.34 wt.%), respectively. The Na2O and K2O contents are 7.32 wt.% and 3.29 wt.% in alkaline quartz syenite, 4.74 wt.% and 6.34 wt.% in alkaline transitional type syenite, and range, from 6.37 to 9.69 wt.% and 0.13 to 3.32 wt.% in alkaline syenite. The Na2O+ K2O is 10.61 wt.% in alkaline quartz syenite, 11.08 wt.% in alkaline transitional type syenite, ranges from 9.67 to 9.85 wt.% in alkaline syenite whose Na2O+ K2O/Al2O3 ratios ranging from 0.55 to 0.57, is less than that of alkaline quartz syenite (0.59), and alkaline transitional type syenite (0.62). The K2O/Na2O ratio is 0.45 in alkaline quartz syenite, 1.34 in alkaline transitional type syenite, and ranges from 0.013 to 0.45 in alkaline syenite. The other quantified oxides include Fe2O3, MnO, MgO, CaO, TiO2, and P2O5 are dominantly below 1 wt.%, excepting the values of Fe2O3 and CaO which are greater than 1.5 wt.% with CaO above 2.8 wt.% in MGA03 and MGA04. These two samples also enclose the highest LOI content. The calculated Al2O3/TiO2 ratio is 138.1 in alkaline quartz syenite whose CaO+ Na2O+ K2O (11.98 wt.%), (Na2O+ K2O)-(0.37* SiO2-14.43) (0.42 wt.%), and Mg# (41.73) are different those of alkaline transitional type syenite, and alkaline syenite. The Mg# (29.65), CaO+ Na2O+ K2O (12.61 wt.%), and (Na2O+ K2O)- (0.37* SiO2-14.43) (1.2 wt.%) for alkaline transitional type syenite are within the range 28.29-38.42, 12.39-13.09 wt.%, and 0.58-1.27 wt.%, respectively for alkaline syenite. The CIPW normative mineralogical results (Table 2) are variable with the predominance of albite (40-82 wt.%), and the presence of quartz (4.4-10.41 wt.%) in all the syenitic rocks. The lowest albite value is found in in alkaline transitional type syenite which also encloses the highest orthoclase and quartz. Other remarkable features are the extreme variation orthoclase contents (0.77-37.47 wt.%), and the different in proportion between alkaline feldspars (orthoclase+albite: 73.4-82.8 wt.%) over anorthite (3.9-8.0 wt.%).
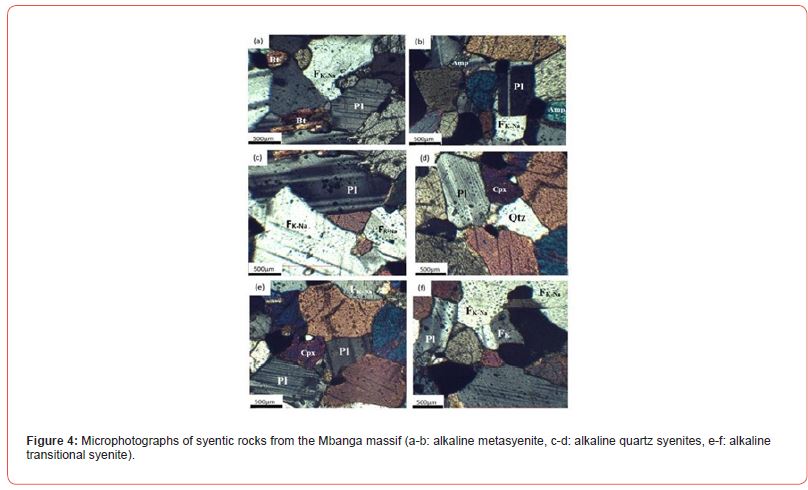
Table 1: Oxides composition and CIPW norms for the syenitic rocks cropping at the Mbanga massif.
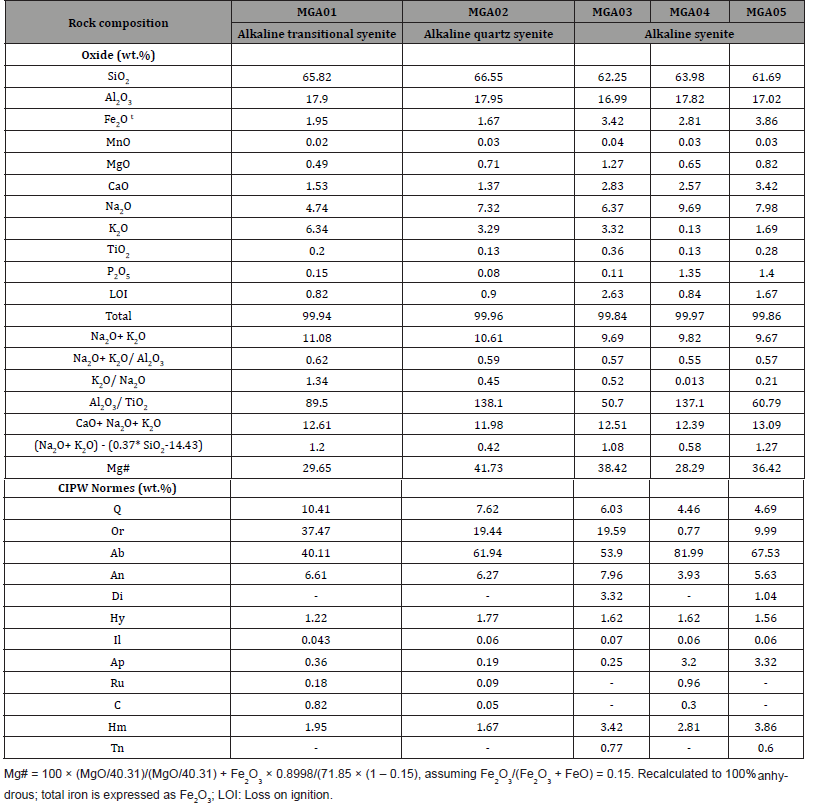
Table 2: Trace and rare earth element contents in the alkaline syenitic rocks cropping at the Mbanga massif, calculated ratios and anormalies.
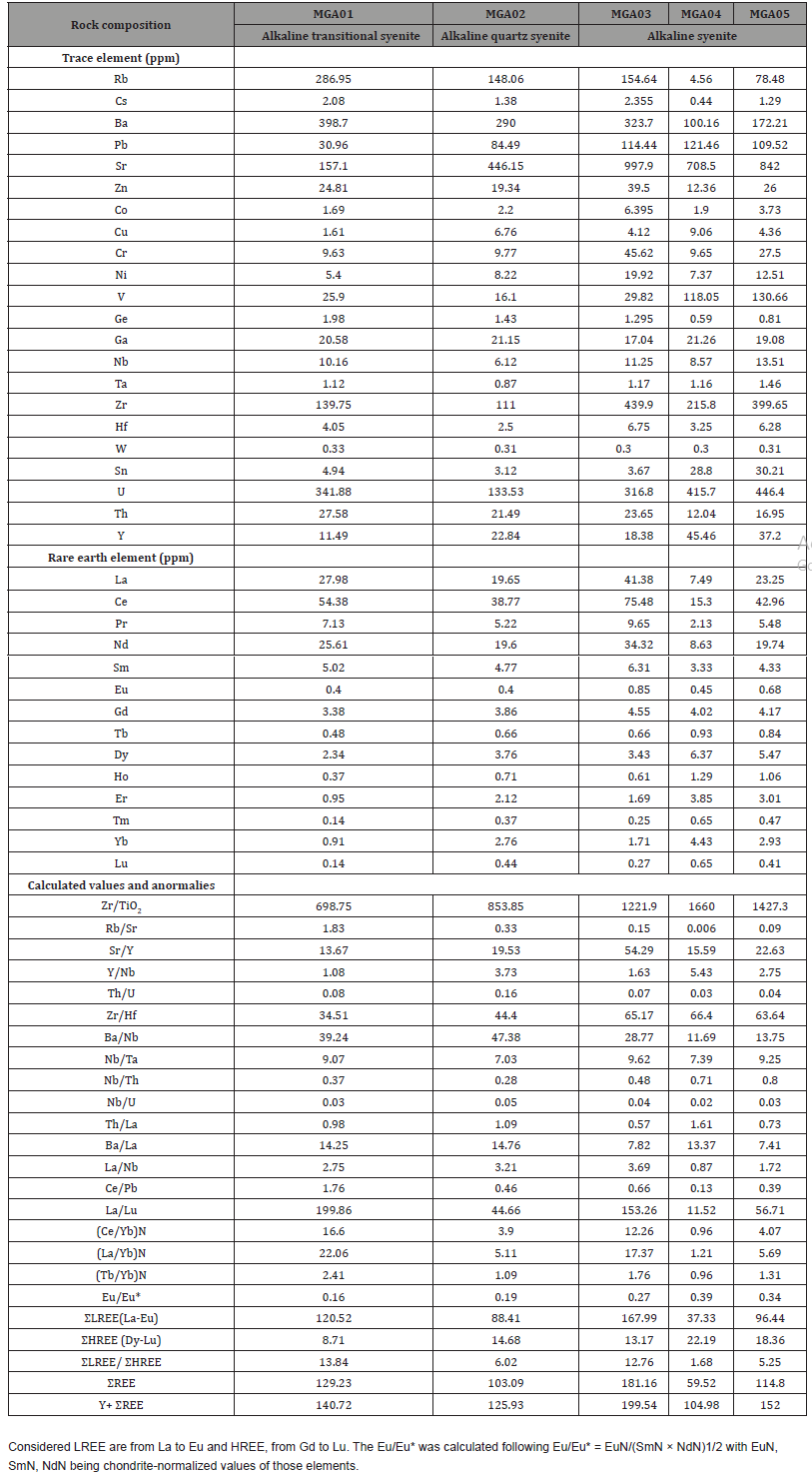
Trace elements
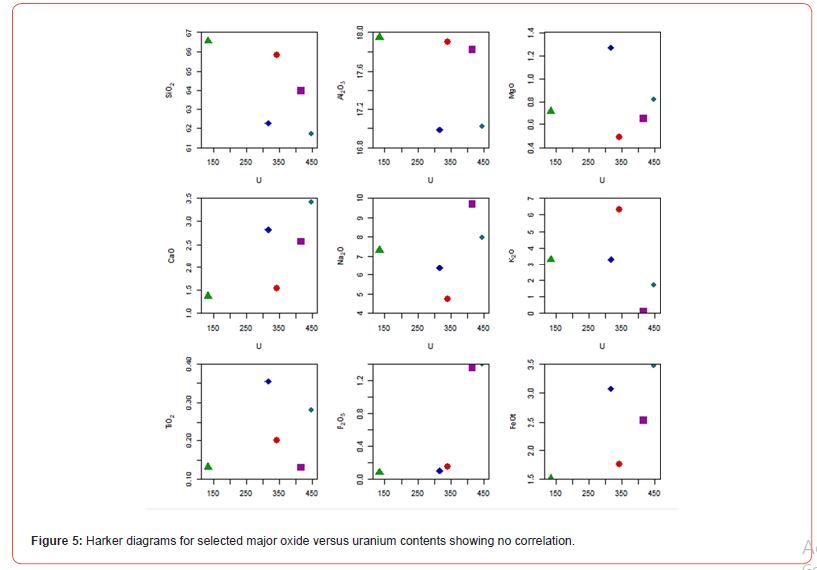
The trace composition in the studied U-bearing alkaline syenitic rocks (Table 2) are variable with the predominance of Sr whose content is 446.15 ppm in alkaline quartz syenite, 842.0 ppm in alkaline transitional type syenite, and ranges from 708.5 to 997.9 ppm in alkaline syenite. It is follow by that of U, Ba, Zr and Rb whose contents are 133.5 ppm, 290.0 ppm, 111.0 ppm, and 148.1 ppm in alkaline quartz syenite, 341.9 ppm, 398.7 ppm, 139.8 ppm, and 287.0 ppm in alkaline transitional type syenite, and range from 316.8 to 446.4 ppm, 100.1 to 323.7 ppm, 215.8 to 439.9 ppm, and 4.56 to 154.48 ppm in alkaline syenite. The highest U content was quantified in MGA05. U versus oxide plot diagrams (Figure 5), generally show a scattered behavior. Elements such as Pb, V, Zn, Ga, Th, Y, Cr, and Nb whose contents range from 6.1 to 130.7 ppm are significantly variable from one syenitic rock to another. The highest contents within these elements suites, are those of Pb and V whose concentration is 84.49 ppm, and 16.10 ppm in alkaline quartz syenite, 30.96 ppm, and 25.90 ppm in alkaline transitional type syenite, and range from 109.5 to 121.5 ppm, and 29.8 to 130.7 ppm in alkaline syenite. The last elemental suite is composed of Sn, Ni, Hf, Co, Cu, Ge, Ta, and Cs. These elements contents generally range from 0.3 to 30.21 ppm with the highest value being that of Sn and Ni found in part of the alkaline syenite. The calculated trace element ratios (Ba/ Nb: 11.6-47.4; Nb/U: 0.02-0.05; Zr/Hf: 34.5 -65.2; Sr/Y: 13.6 – 54.3; Nb/Ta: 7.0-9.6; Th/U: 0.03- 0.16; Rb/Sr: 0.006-1.83; Y/Nb: 1.0-5.5) show an extreme variation.
Rare earth elements
The rare earth composition in the studied U-bearing alkaline syenitic rocks (Table 2) shows the predominance of light rare earth elements (La-Eu) over heavy rare earth elements (Gd-Lu). Within the light rare earth elements (LREE) suite, La, Ce, and Nd are the highest quantified elements. The La, Ce, and Nd is 19.65 ppm, 38.77 ppm, and 19.60 in alkaline quartz syenite, 27.98 ppm, 54.38 ppm, and 25.61 ppm in alkaline transitional syenite, range from 7.49 to 41.38ppm, 15.30 to 75.48 ppm, and 8.63 to 34.32 ppm in alkaline syenite. The highest La, Ce, and Nd contents are quantified in part of the sampled alkaline syenite (MGA03). The lowest light rare earth element contents are those of Pr, Sm, and Eu whose values range from 2.13 to 9.65ppm, 3.33 to 6.31 ppm, and 0.4 to 0.85 ppm, respectively. Within the heavy rare earth elements (HREE) suite, the highest quantified contents are those of Gd and Dy. The Gd and Dy contents are 3.86 ppm and 3.76 ppm in alkaline quartz syenite, 3.38ppm and 2.34ppm in alkaline transitional type syenite, range from 4.02 to 4.55ppm, and 3.43 to 6.37ppm in the alkaline syenite. The lowest contents are those of Yb (0.91-4.43 ppm), Er (0.95- 3.85 ppm), Ho (0.37-1.29 ppm), Tb (0.48-093 ppm), Tm (0.14-0.65 ppm), and Lu (0.14-0.65 ppm), respectively. The calculated values for Eu/Eu* is less than 1 in all the studied syenitic rocks; which shows a negative Eu anomaly. The (La/Yb)N, (Ce/Yb)N, and (Tb/ Yb)N ratios range from 1.2 to 22.1, 0.96 to 16.60 and 0.96 to 2.41, respectively with the highest values found in MGA01(alkaline transitional type syenite) and the lowest in MGA04 (alkaline syenite). The total LREE (ƩLREE: 37.3-168.0 ppm) and HREE (ƩHREE: 8.7- 22.2 ppm) with the highest and lowest values found in alkaline syenite. The calculated La/Lu, ƩREE, Y+ ƩREE, and ƩLREE/ ƩHREE range from11.5 to 199.7, 59.5 to 181.2 ppm, 104.9 to 199.6 wt.%, 1.6 to 13.9, respectively.
Spider diagram and REE patterns
The primitive mantle-normalized multi-element patterns (N-MORB), normalization values after Sun and McDonough [79] spider diagram (Figure 6a) of Mbanga alkaline syenitic rocks are characterized by variable behavior of incompatible elements (LILE: K, Rb, Sr, and Ba; and HFSE: Hf, Zr, Ti, Nb, REE Th, U and Ta). Within the large-ion lithophile elements (LILE) suites, Sr and Rb show a weak positive anomaly, whereas, Ba anomaly is weekly negative, and that of K, strong negative. Within the high-field-strength-element (HFSE) suite, U shows a strong positive anomaly, whereas, Zr shows a weak postive or no anomaly. The normalized REE patterns (Figure 6b) dominantly show a decrease from La to Sm, although plot for MGA04 is different, with its positive Sm anomaly. A negative Eu anomaly is noted before a slow decrease from Gd to Er for most of the spider diagrams. Almost flattening feature is noted above Er.
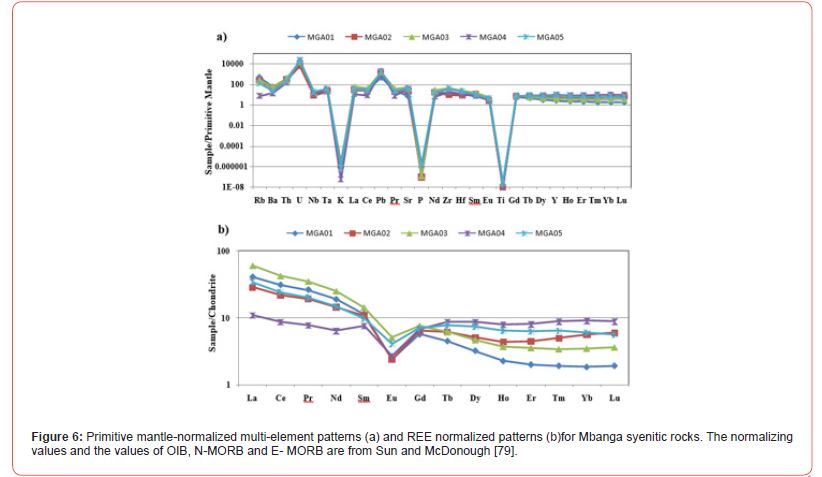
Mineralogy
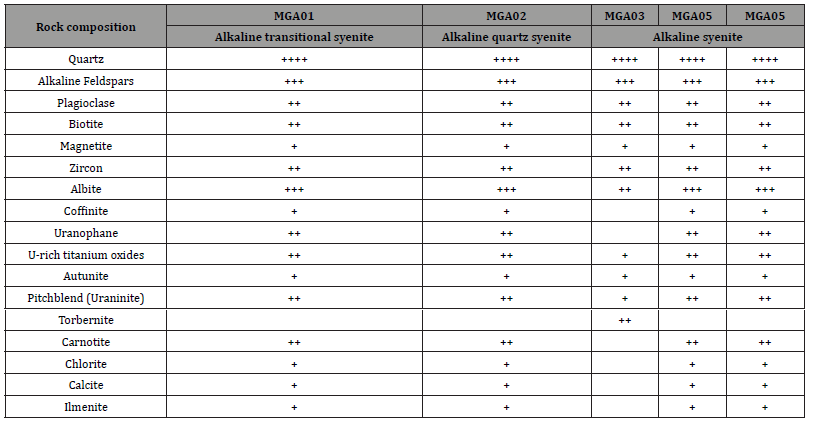
Table 3 XRD mineralogical data for U-ore in the alkaline syenitic rocks cropping at the Mbanga massif (++++: very abundant, +++: abundant, ++: less abundant, and +: trace).
XRD mineralogy
XRD mineralogical results for the studied syenitic rocks show three groups of minerals (Table 3): a. The gangue minerals, composed of quartz, alkaline feldspar, albite, plagioclase, chlorite, calcite and biotite; b. The u-ore minerals made up of uraninite, uranophane, autunite, carnotite, and torbernite with coffinite in trace or absent; and c. Other ore minerals including, u-rich titanium oxides, zircon, magnetite, and ilmenite.
Excepting some of the gangue minerals (quartz, alkaline feldspar, albite, plagioclase, and biotite), the proportion of U-ore minerals and other ore minerals varies from one sample to another. Within the U-ore minerals suite, torbernite was exclusively found in MGA03 associated with uraninite, autunite, and U-rich titanium oxides but no coffinite, carnotite and uranophane. This sample encloses zircon, and magnetite; but lack chlorite, calcite, and ilmenite. The other samples MGA01, MGA02, MGA04, and MGA05 enclose the same U-ore minerals (uraninite, U-rich titanium oxides, uranophane, carnotite, coffinite, and autunite) with the predominance of the first four minerals over the two last. Ore minerals (zircon, magnetite, and ilmenite) and gangue minerals (chlorite and calcite) are dominantly in trace.
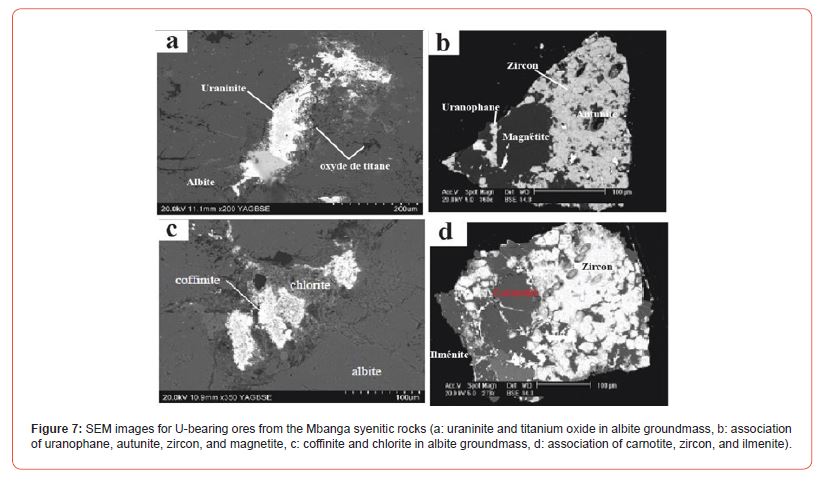
SEM mineralogy of the U ores
The SEM mineralogical results in Figure 6 shows that the uranium ore minerals are uraninite, uranophane, autunite, coffinite, and carnotite. These minerals occur in association with chlorite, zircon, ilmenite, titanium oxide, and/or magnetite mainly found in albite groundmass. Individually, uraninite is elongated, zoned (with a brighter core surrounded by less bright rim), and associated with titanium oxide (Figure 7a). Uranophane and autunite forming anhedral to sub-rounded crystals or disseminated, are associated with magnetite and zircon (Figure 7b). Autunite shows a clear zoning (with a dark core surrounded by two different rims) probably due to different chemical composition. Coffinite, sub-hedral in shape and much brighter, is associated to chlorite (Figure 7c). Carnotite anhedral, less bright, and partly hosting brigther micro-veinlets and spots, is associated with zircon and ilmenite.
Radiometric features
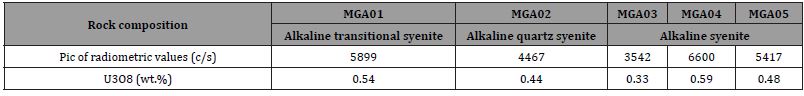
The radiometric results (Table 4, Figure 8) representing the raw measured values, shows that the highest recorded radiometric values 6600 c/s and 5899 c/s were found on MGA04 (from alkaline syenites) and MGA01 (from alkaline transitional type syenite), respectively. The lowest values 3452 c/s and 4467 c/s were found on MGA03 (from alkaline syenite) and MGA02 (from alkaline quartz syenite). The raw measured radiometric values transformed to uranium oxide (U3O8) concentration in each syentic rocks show variation. The highest U3O8 concentrations U3O8 (0.59 wt.%) and U3O8 (0.54 wt.%) were found in MGA04 and MGA01; and the lowest, 0.33 wt.% in MGA03. Intermediate values 0.44 wt.% and 0.48 wt.% were found in MGA02 and MGA05, respectively.
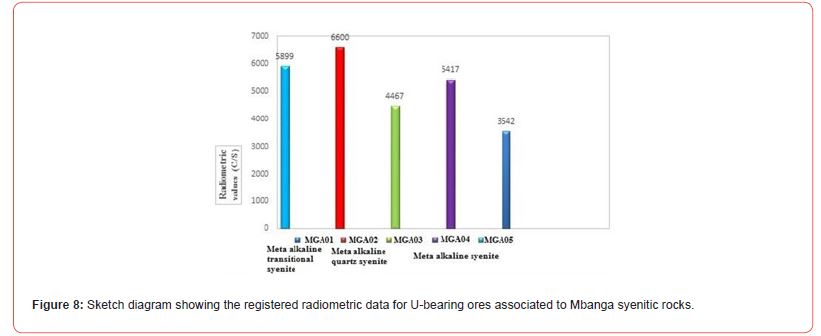
Table 4 Pic of radiometric values (c/s) and that of triuranium octaoxide (wt.%) for U-bearing ores found in alkaline syenitic rocks cropping at the Mbanga massif.
Acid Leaching of U ores
The acid leaching of the Mbanga syenitic U-ore bodies was facilitated by the high proportion of uranium-ore minerals (mainly the primary uranium oxides) and the presence of iron ore mineral such as magnetite and ilmenite in all the processed samples. Fe increases the U extraction by lowering the acid concentration in the dissolving solution. Most of the primary uranium ore minerals found in the processed samples were in their reduced form which therefore lead to their fast dissolution. Oxidants such as manganese dioxide used during the leaching process facilitate the transformation U4+ found in the U ore minerals to U6+. The presence of gangue minerals such as quartz, feldspars, amphibole, chlorite, biotite, calcite do not negatively impact the leaching process.
Discussion
Geochemical, mineralogical, radiometric, and leaching results are used to characterize each of the U-bearing syenitic rock. Part of these results is used for petrogenetic evaluation. The other part is used to determine the ores grade and their economic importance.
Characteristics and petrogenesis the U-bearing syenitic rocks
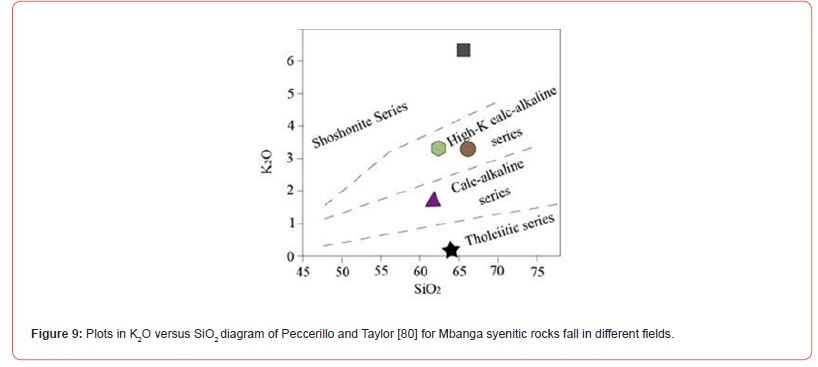
The geochemical composition in Mbanga syenitic rocks varies from one sample to another; which might be due to the availability of these elements in those rocks formation environments; with part of this environment being much enriched in some elements that others. The difference is also visible from the CIPW norms with a proportional difference within the CIPW minerals (the presence of minerals like apatite, diopside, and rutile in some samples, and their absence in others). They were all plotted in alkaline field in Cox et al. [78] TAS diagram (Figure 2a), but, fell in separate fields in De La Roche et al. [79] (Figure 2b), Peccerillo and Taylor [80] (Figure 9), and Shand [81] (Figure 10) discriminating diagrams. They were classified as A-type syenites due to relative high Na2O+K2O (9.6-11.08 wt.%) slightly less than values (≥11.08 wt.%) in Mindif A-type syenites found at about 25 km SE of Maroua [82], but in some case, within the range 7.2-12.1 wt.% in Doum- Lolodorf clinopyroxene alkaline (high-K) syenites found in the Nyong Unit in the South Region of Cameroun [59]. The difference between the studied syenitic rocks and both Mindif A-type syenites and Doum- Lolodorf clinopyroxene syenties is also geochemical and consequently the CPIW norms; which therefore might testifies a different in formation history. The alkaline transitional syenite is high-K type as its following features (K2O=6.34 wt.% > 4%, Na2O+K2O= 11.08 wt.%, and K2O/Na2O=1.34) are similar to those of high-K clinopyroxene syenites presented in [59]. This rock and one alkaline syenite (NGA03) are plotted in high-K field in Peccerillo and Taylor [80] SiO2 versus K2O diagram (Figure 9), which therefore support its high-K nature. There are many geochemical differences between the named high-K syenites (this study) and those presented in Tchameni et al. [59]; notably, the difference in SiO2, Fe2O3, Al2O3, MgO, CaO, V, U, REE, Rb, Ba, Cu, Y… This therefore suggests a difference in nature and composition of the melts source of the studied high-K syenites and those of syenites published in Tchameni et al. [59]; and makes correlative interpretations difficult.
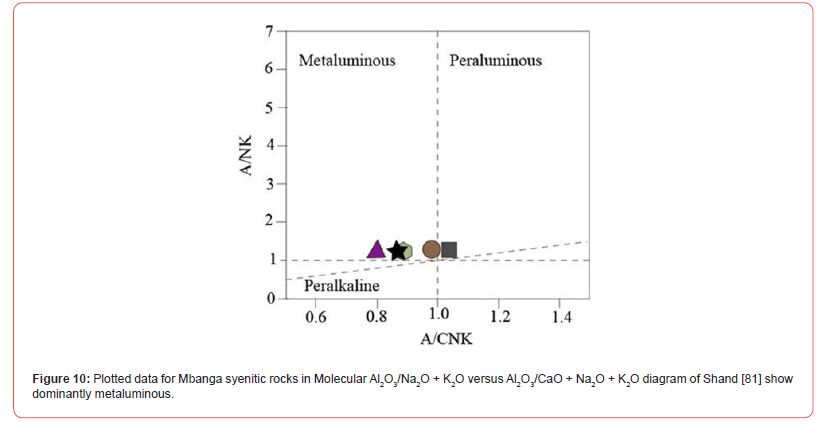
The studied syenites dominantly fell in metaluminous field in Shand [81] diagram (Figure 10), excepting the alkaline quartz syenite whose plot fell in peraluminous field. They are plotted all I-Stype, in Figure 11, which might show a cooling from partial fusion of sedimentary or igneous protoliths. Part of these rocks notably the high-K calc-alkaline syenite could be originated from mixing of basaltic with felsic magmas derived from metasediments, if base on Tchameni et al. [59] studies. The presence of normative quartz in all the syenites suggests that they were crystallized from silica- oversaturated melts. The geochemical features of syenitic rocks have been used to determine the condition under which these rocks were formed; notably the nature of their cooling melt, fingerprinted events, and tectonic settings [59,82,84-86]. The proportion of LILE and HFSE in syenites for example has been used to elucidate the evolutary history of those rocks cooling magmas [85,86]. For Heilimo et al. [85], the negative Eu anomaly presented by a syenitic rock suggests that this rock was formed through feldspar fractionation process during evolution of a basaltic magma. Feldspar fractionation is probably the process which governed the formation Mbanga alkaline syenites, as the Eu anomalies are all negative.
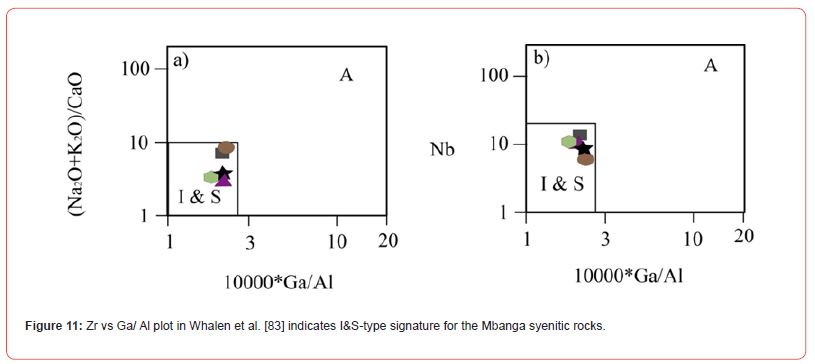
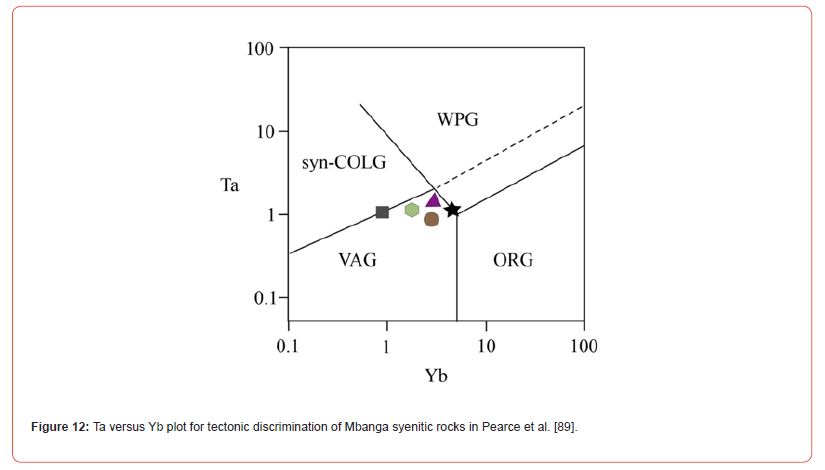
Syenites formed from mantle source magmas exhibit relatively high MgO (5.69 wt.%), Cr (210 ppm), and Ni (92 ppm) abundances (e.g., Saima Alkaline syenitic Complex in NE China: [88]), whereas, those elements values are relatively low in syenites cooled from continental source magmas (e.g., Mindif alkaline syenites, Far North Cameroon: [82]; and Doum- Lolodorf high-K syenites: [59]). The MgO (≤ 1.27 wt.%), Cr (≤ 45.62 ppm), and Ni (≤ 19.92 ppm) in Mbanga syenites are largely below the values in syenites formed from a mantle source magma presented in Zhu et al. [88]; but dominantly above the values MgO (≤ 0.97 wt.%), Cr (<2.0 ppm), and Ni (≤1.74 ppm) in Mindif syenites, suggested to have formed from crustal source magma (cf. [82]. They were probably cooled through feldspar fractionation mechanism from magmas (enriched in some LILE and HFSE) originated from partial fusion of preexisting igneous or sedimentary protolith, as their plots fell in I-S field in Figure 11, and within the volcanic arc setting (see Figure 12). The U contents (>133 ppm) in the syenitic rocks show a positive anomaly, as an U enrichment in most granitoids ranges from 3 to 4 ppm, with just specific granitic rocks having high U concentrations permitting the crystallization U ore minerals to form deposits (c.f [29]). This therefore support their classification as U-enriched alkaline syenites different to U-depleted alkaline syenites and high-K syenites respectively, cropping in Mindif (Far North of Cameroon part of the Pan-African Mobile Belt) and Doum- Lolodorf in the south of Cameroon (Nyong Unit part of the Archean to Paleoproterozoic Ntem Complex or Congo Craton).
Characteristics and formation of the uranium ores
The geochemical and mineralogical data for the analyzed U-bearing ores shows some difference 605 in U abundances and U-ore minerals. The U contents (133-446.4 ppm) in the studied ores are less than the U (465 ppm: [90]) found in low grade U ore from Saghand Mine in Iran; which therefore classify them as low grade uranium ores. The difference within the studied ores is visible for other quantified elements (e.g., Pb, Zr, and V) and minerals. The variation with relatively high Pb values (> 109 ppm) in (alkaline syenite: MGA03, MGA04, and MGA05) can be due to presence of daughter Pb resulted from radioactive decaying of U in those ores as these ore samples have the highest U contents (>316 ppm). The highest Zr contents (>215 ppm) might be due to the presence zircon, as this mineral is found in those rocks. The relatively high V contents (> 118 ppm) in MGA04, and MGA05 can be due to presence of U-V-bearing ore mineral (notably carnotite) found in all samples. The highest U content ≥ 415 ppm were found in alkaline syenite (MGA04 and MGA05) and the lowest value 133.53 ppm in MGA02 (alkaline quartz syenite). Intermidiate and close contents 341.88 ppm and 316.8 ppm were respectively found MGA01, and MGA03. With this difference in U abundance, three groups of U-bearing ores can therefore be distinguished: a. Relatively high U-V-Zr-Pb-ores; b. Relatively low U- Pb-Zr-V-ores; and c. Very low U-Pb-Zr-V-ore.
These three distinguished groups show difference in U mineralogical features, and other significant minerals such as chlorite, calcite, magnetite, ilmenite, and albite. Within the U-ore mineral suites, torbernite was exclusively found in MGA03 (alkaline syenite) of the group 2 U-ore, classified as relatively low U-ore. This mineral is associated to trace of uraninite (UO2), autunite (Ca(UO2)2(- PO4)2, 10H2O), and U-rich titanium ore mineral, but lack carnotite (K2(UO2)2(VO4)2.1-3H2O) and uranophane (UO2)6(SO4)2(OH)6- 10(H2O), coffinite (U(SiO4)1-8(OH)4x, chlorite, calcite, and ilmenite which were found in the other samples. The absence of those minerals might due to their non- crystallization in this ore during its formation. The MGA03 can be classified as torbernite- bearing relatively low U-ore; whereas, the other ores can be classified as torbernite-barren relatively high to very low U-ores. The uranium mineralogical composition in all the studied ores made up of uraninite, uranophane, coffinite, carnotite, and/or torternite is in some case different to the Kitongo brecciated albitized U-ores [3] as the Kitongo ores do not enclose uranophane, carnotite, and torternite but enclose uraninite, coffinite, U-Zr-Si, and U-Fe-Si phases, with the two last ore phases not found in the Mbanga U-ores. This difference is also that of some other ore minerals and gangue minerals notably, the presence of hematite, aegirine, riebeckite, apatite in the Kitongo ores, but, their absence in the studied U-ores which also enclose chlorite, biotite, and ilmenite not found in the Kitongo ores.
They present some similarities in gangue minerals precisely the presence of calcite in both ores. For Kouske et al. [3] the presence of calcite in the Kitongo ores was probably resulted from the combination of Ca derived from albitization of magmatic plagioclase or the retrogression of Ca-rich amphibole. It is possible that, albitization of magmatic plagioclase was the source of calcite found in the studied ores, although supporting information is lacking. Mineral such as chlorite was found associated with U ore mineral in the Huangsha uranium mine in China [30]. This chlorite was originated from choritization of biotite, from metasomatism of feldspar with Fe-Mg hydrothermal fluids, or associated with vein-like and disseminated pitchblende accompanied by zircon, uranothorite, sericite or illite/ hydromica, pyrite, and rare earth minerals, formed by filling and precipitation of ore- forming fluid at the main metallogenic stage [30].
In the Mbanga U ores, chlorite is closely associated with coffinite in albite groundmass; which therefore show some difference in ore mineral association, and probably, the difference in ore forming process. The formation mechanism of the Mbanga U ore minerals occurrences can not be clearly elucidated at this stage as data on mineral chemistry precisely those of U ore minerals are lacking. The presence of OH, H2O, and OH-H2O-bearing uranium ore minerals (autunite, carnotite, uranophane, and coffinite) suggests that the Mbanga U ores were formed from precipitation of U- rich hydrothermal fluids with part of these fluids being Pb-V-Zr enriched than others. The fluids enclose abundant phosphate and calcium, source of the precipitation of the U-P-Ca ore (autunite). The presence Sibearing U-ore mineral (coffinite) might suggests the Si-enrichment of the hydrothermal fuids during the period when this mineral formed. The testimony of substential S content in the fluid is the presence uranophane which is a U-S-bearing mineral. In Pownceby and Johnson (2013), uraninite encloses (50-80 % of U), carnotite (50-55 % of U), autunite (up to 60 % of U), and coffinite (40 % of U). The presence of all these U-bearing minerals clearing show U-en richment in the precipitated hydrothermal fluids.
Radiometry
The measured radiometric and obtained U3O8 data for the Mbanga U-bearing syentic rocks help to differentiate and classify the ores. The measured radiometric data (6600-3542 c/s) are 667 extremely variable, showing a difference in radiometric response for each of the sampled ores. This variation is observed within ore samples from the same rock type. For example, within 669 samples from alkaline syenites, the highest value 6600 c/s was recorded in MGA04, and the 670 lowest 3542 c/s, in MGA03. This difference lead to distinguish two sub-group of ore within the alkaline syentes:
a. The relatively high radiometric ore (with U radiometry exceeding 5400 c/s), and
b. Relatively low radiometric ore (with U radiometry 3542 c/s).
Other relatively high U radiometries were recorded in MGA05 (alkaline syenites), and in MGA01 (transitional alkaline syenite) which can lead their classification within relatively high U radiometric ores group. The lowest radiometric value 3542 c/s is greater than the value ≤1300 c/s in altered part of Razgah U-bearing nepheline syenite in the East Azerbaijan [6]. The U content (36.6 ppm) in the Razgah U-bearing nepheline syenite is less than the values (U≥133 to up to 446.4 ppm) in the studied Mbanga syentic rocks, which shows that, the studied syenites are U highly enriched than the Razgah U-bearing nepheline syenite. The transformed radiometric values to that of triuranium octaoxide (U3O8) ranging from 0.33 to 0.59 wt.%, show correlations. Samples with a high radiometric value also have a the highest U3O8 values; same for the one with low radiometric value whose U3O8 value is low. For AIEA/ OCDE [91], U3O8 values for world U reserves range from 0.1 to 27 wt.%; with a recovery proportion of ≥ 0.5 wt.%. The U3O8 values in two of the studied samples (MGA01: 0.54 wt.%, and MGA04: 0.59 wt.%) are above the minimum recovery proportion (0.5 wt.%) presented in AIEA/OCDE [91]; which therefore makes rocks from were the two samples were collected interesting.
U extraction from the ores
The H2SO4 acid leaching of U found from an ore is influenced by many parameters such as the nature and composition of the U ore minerals and gangue minerals, temperature during leaching process, effect of used H2SO4 acid concentration, effect of S/L ratio, and effect of stirring speed [44,46,92]. Three parameters such as the effect of oxidant and ions from gangue minerals, effect of leaching temperature, and leaching time were examined and discussed.
Effect of oxidant and ions from gangue and other ore minerals
The dissolution of U ore minerals and associated gangue minerals liberates strange substances whose presence can negatively or positively impact the U extracting process; either by reducing the acid concentration (acid consumption), and influence oxidation- dissolution of an ore [92-95]. The acid consumption during the U extraction does not depend on the U content in the ore, but it is controlled by the gangue constituents. The studied samples are composed of primary U-ore mineral (uraninite), and secondary U ore minerals (hydro-oxides: carnotite, uranophane, autunite, coffinite, and torternite) which are easily leached than other U-bearing minerals (such as xenotime, zircon, monazite, apatite, and titanite: [29]) resistant to leaching. These minerals were dominantly in their reduced form which facilitate the lixiviation of U with the addition of an oxidant such as manganese dioxide (which aid to transform U4+ present in uraninite to U6+: [96]). Gangue minerals made up of quartz, feldspars, biotite, amphibole, chlorite, calcite, ilmenite, and magnetite did not negatively influence the U extraction process. The presence of calcite (the main acid consumer: [92]) and iron ore minerals (magnetite and ilmenite) probably increase the U extraction by reducing the concentration of acid. The presence of Fe3+ in particular, increases the U oxidation potential; this ion is currently used to oxidate U during commercial leaching processes [97]. For Mattus and Torma [98] the presence of Fe3+ in a solution during H2SO3 acid U leaching oxidizes the uranium while the oxidant reagent oxidizes ferrous ion to ferric ion.
Effect of leaching temperature
The increase in leaching temperature emproves the U extraction [46,99,101]. From studies in IAEA [100], 16 % of U was extracted at 8oC leaching temperature, and 90% of U extraction when the temperature was 26oC. Rasah et al. [46] notice a change in U extraction from 25 to 90oC; with an increase to up to 98.5% when the leaching temperature is up to 80oC, and stable, with the slight increase of temperature to 90oC. Eligwe et al. [99] and Roshani and Mirjalili [101] noticed an increase in U extraction with the increase in temperature with high acid consumption. They related this to the increase in temperature used to dissolve the gangue minerals. The maximum temperatures for U extraction from the studied Mbanga ores were recorded at 40 to 60oC. The increase in temperature at 40oC optimized the U leaching from their bearing main ore minerals. This temperature is lower that of Rashad et al. [46] (80oC) but higher than that of IAEA [100] (26oC) for a maxium U leaching.
Effect of H2SO4 acid concentration
Eligwe et al. [99] and Rashad et al. [46] noticed an increase in U extraction with the increase in H2SO4 concentration up to a limit which the increase in acid concentration do not increase the U extraction. Experimental work carried out on a synthetic uranium ore mineral by Laxen [97] (leaching temperature: 35oC and H2SO4 concentration: 3 to 40 g/l) also shows the increase in U leaching with the increase in acid concentration. Jansen van Rensburg [102] reported that at these following condition (leaching temperature: 35°C and H2SO4 concentration: 4 to 5.2 g/l) with a maximum H2SO4 concentration of 1.2 g/l, U extraction decreases to 1.1 %, and negative, when the acid concentration is above 1.5 g/l. The oxidation of U found in the studied Mbanga uranium ores was done by protons found in the solution of H2SO4 acid, the presence of oxidizing agents from dissolution of gangue minerals, and the use of oxidant during the leaching process. In this case, insoluble U4+ found in UO2 is then oxidized to U6+ that whole react with H2SO4 acid to form a solution of sulfate acid enclosing a variety of metallic ions such as that of U, Fe, Al, Cu, Ca, Mg, Mo, V, Se… from oxidation-dissolution of both ore and gangue minerals. The extraction of U from Mbanga ores is within 80 to 90% whereas the concentrations of other metallic ions varying from one sample to another, range from 0.02 to 0.93%. This proportion (80-90%) extracted U, is greater than the 55 % U extraction recorded by Rashad et al. [46] during the H2SO3 acid leaching of Wadi Nasib U ore, South western Sinai, Egypt. It is close to the optimum H2SO3 acid leaching efficiency 83% presented by Hamza et al. [103] when recovery U from taillings.
Conclusions
The studied Mbanga syentic rocks are U-bearing alkaline (syenite, quartz syenite, high-K 751 syenite), mainly metaluminous formed from different magmatic melts originated from partial fusion of crustal rocks. The associated uranium ores are low grade ores suggested to have formed from crystallization of magmatic melts with influences of hydrothermal fluids which lead to the precipitation of OH, H2O, and OH-H2O-bearing uranium ore minerals and some gangue miinerals (e.g., chlorite). The found uranium ores formed two sub-groups: relatively high radiometric, and relatively low radiometric ores with part of the ores being of interest. The ores were easily H2SO3 acid leached with the aid of oxidant and the presence of ion from iron ore minerals, and, calcite within the gangue minerals suites. The maximum leaching temperatures (40- 60oC), less than those of some compared low grade uranium ores; place the processed ores within average temperature leached ores. The uranium recovery rate (80-90 %), more than the recovery rates of some referenced ores, places these ores within easily processed ores.
Acknowledgements
The authors extern their gratitude to Institute of Geology and Mining Research in Yaoundé (Cameroon) for providing the samples preparation laboratory facilities. Special thanks to two anonymous reviewers whose useful comments helped to improve the quality of this manuscript.
Conflict of Interest
There is no financial or other relationship with people or organizations that may inappropriately influence this work.
Funding
This work was partly funded by the Institut of Geological and Mining Research (IGMR), Yaoundé (Cameroon) by providing samples preparation laboratory facilities. The other part was funded by the authors.
Data Availability
The datasets generated during the current study are available from the corresponding author (Dr. KANOUO Sylvestre Nguo) on reasonable request.
References
- Alexandre P, Kyser K, Jiricka D (2009) Critical geochemical and mineralogical factors for the formation of unconformity related uranium deposits: comparison between barren and mineralized systems in the Athabasca Basin, Canada. Econ Geol 104(3): 413–435.
- Kouske AP, Cheo ES, Ghogomu RT, Ngako V (2012) Na-metasomatism and uranium mineralization during a two-stage albitization at Kitongo, northern Cameroon: structural and geochemical evidence. Int J Geoscie 3(1): 258–279.
- Kouske PA, Gerard M, Etame J, Kanouo SN, Tchouatcha SM, et al. (2022) Paragenesis, mineral chemical and microtextural studies of uranium bearing minerals in the brecciated albitites U-ores from the Kitongo shear zone, Poli region, northern Cameroon. Int J Earth Scie 111(1): 1413-1426.
- Padhi AK, Mukherjee MK, Tripathi BK, Pande D, Bisht BS, et al. (2023) Polymetallic Uranium Mineralisation in Rohil, Rajasthan, Western India: Insights from Mode of Occurrences, Structural Controls, Alteration Geochemistry and Exploration. Minerals 13(4): 555-580.
- Saric RM, Stojanovic M, Babic M (2008) Uranium in plant species grown on natural barren soil. J Plant Nutrition 18(7): 1509-1518.
- Seyyedbeygloo MAS, Ahadi M, Lotfi M, Hezareh RM, Ziazarifi A (2010) The investigation of Razgah nepheline syenite as a suitable source rock for sedimentary uranium mineralization in East of Azerbaijan, Iran. 10th Int Multidisciplinary Scie Geoconfrence SGEM 20-26 pp. 561-568.
- Mustoe EG (2020) Uranium mineralization of fossil wood. Geoscie 10(4): 133-158.
- Zhang B, Wang X, Zhou J, Han Z, Liu W, et al. (2020) Regional geochemical survey of concealed sandstone-type uranium deposits using fine-grained soil and ground water in the Erlian basin, north-east China. J Geoch Expl 216(5): 106573-106585.
- Bai H, Wang W, Lu Q, Wang W, Feng S, et al. (2022) Geological Characteristics and Control Mechanism of Uranium Enrichment in Coal-Bearing Strata in the Yili Basin, Northwest China: Implications for Resource Development and Environmental Protection. ACS Omega 7(6): 5453−5470.
- Liu Z, Peng S, Qin M, Huang S, Geng Y, et al. (2022) Constraints on Sandstone-Type Uranium Deposits by the Tectonic Uplift and Denudation Process in the Eastern Junggar Basin, Northwest China: Evidence from Apatite Fission Track and Detrital Zircon U-Pb Ages. Minerals 12(7): 905-915.
- Havelcová M, Sýkorová I, René M, Mizera J, Coubal M, et al. (2022) Geology and petrography of uraniferous bitumens in Permo-Carboniferous sediments (Vrchlabí, Czech Republic). Minerals 12(5): 544-553.
- Slukovskii Z (2023) Uranium in Lake Sediments of Humid Zone: A Case Study in the Southeast Fennoscandia (Karelia, Russia). Water 15(7): 1360-1376.
- (2009) International Atomic Energy Agency. World distribution of uranium deposits (UDEPO) with uranium deposit classification, IAEA, TECDOC – 1621, Vienna.
- Gregory WR (2016) Uranium: Geology and Applications. Circular No. 46 pp. 1-36.
- Lecomte A, Cathelineau M, Michels R, Peiffert C, Brouand M (2017) Uranium mineralization in the Alum Shale Formation (Sweden): Evolution of a U-rich marine black shale from sedimentation to metamorphism. Ore Geol Rev 88(1): 71-98.
- Vakanjac B, Rutherford N, Vakanjac RV, Đumic T, Miloševic, DS (2020) Distribution of Uranium and Rare Elements in Radioactive Phosphate-Bearing Anomalies in Southeast Mongolia. Minerals 10(4): 307-325.
- Cuney M, Mercadier J, Bonneti C (2022) Classification of sandstone-related uranium deposits. J Earth Scie 33(2): 236-256.
- Walton Day K, Blake J, Seal RR, Gallegos TJ, Dupree J, et al. (2022) Geoenvironmental Model for Roll-Type Uranium Deposits in the Texas Gulf Coast. Minerals 12(6): 780-814.
- Jerden Jr LJ, Sinha KA, Zelazny L (2003) Natural immobilization of uranium by phosphate mineralization in an oxidizing saprolite-soil profile: chemical weathering of Coll Hill uranium deposit, Virginia. Chem Geol 199(1): 129-157.
- Tzortzis M, Haralabos T, Christofides S, Christodoulides G (2003) Gamma radiation measurements, and dose rates in commercially used natural tiling rocks (granites). J Environ Radioact 70(3): 223-235.
- Jerden Jr LJ, Sinha KA (2006) Geochemical coupling of uranium and phosphorous in soils overlying unmined uranium deposit: Coles hill, Virginia. J Geochem Explor 91(1-3): 56-70.
- Saïdou, Tokonami S, Elé Abiama P (2016) Natural Radiation Survey in the Uranium and Thorium Bearing Regions of Cameroon. Radiat Environ Med 5(1): 53–58.
- Tifang AJ, Suh EC, Ndimeh NE, Desire1 AZ, Yannah M (2017) Preliminary survey for uranium in Cretaceous sandstones of Bonako-Sole areas, Ngondo Complex, Southwestern Cameroon. Earth Scie 6(1): 1-9.
- Nkoulou II NJE, Feutseu TS, Bineng GS, Manga A, Tchuente SYF, et al. (2018) Natural radioactivity measurements in soil, external dose and radiological hazard assessment in the uranium and thorium bearing region of Lolodorf, Cameroon. Radioisotopes 67(9): 435–446.
- Bineng SG, Saïdou, Hosoda M, Siaka TYF, Akata N, et al. (2020) External radiation exposure to the public using car-borne survey method in the uranium and thorium bearing Region of Lolodorf, Cameroon. Radiat Env Medicine 9(1): 13–20.
- Heikal STM, Abu El Ela MA, Shereif SA, Azer KM, Masoud EA (2022) Geochemical and Spectrometric Characteristics of Natural Radioactivity Levels (238U, 232Th, 40K) of monzo- syenogranites from Wadi El-Nabi' Area, Egyptian Nubian Sield. Arab J Nucl Scie Appl 55(4): 109-131.
- Liu X, Wu J, Pan J, Zhu M (2014) Granite-related Hypothermal Uranium Mineralization in South China. URAM2014,23 to 27 June 2014, Vienna, Austria pp. 1-31.
- Qiu FL, Wu Y, Wang Q, Wu LF, He ZB, et al (2022) Metallogenic mechanism of typical carbonate-hosted uranium deposits in Guizhou (China). Minerals 12(5): 585-609.
- Cuney M (2014) Felsic magmatism and uranium deposits. Bull Soc géol France 185(2): 75-92.
- Wu D, Pan J, Xia F, Huang G, Lai J (2019) The Mineral Chemistry of Chlorites and Its Relationship with Uranium Mineralization from Huangsha Uranium Mining Area in the Middle 892 Nanling Range, SE China. Minerals 9(3): 199-222.
- Kanouo SN, Yongue FR, Ekomane E, Njonfang E, Ma C, et al. (2015) U-Pb ages for zircon grains from Nsanaragati Alluvial Gem Placers: Its correlation to the source rocks. Resour Geol 65(2): 103-121.
- Kanouo SN, Ngueutchoua G, Kouske PA, Yongue, FR, Venkatesh AS (2018) Trace element and U-Pb core age for zircons from western Meiganga gold placer, Cameroon: Their genesis and Archean-Proterozoic sources. Minerals 8(5): 194-222.
- Kanouo SN, Kouské PA, Ngueutchoua G, Venkatesh AS, Sahoo RP, et al. (2021) Eoarchean to Neoproterozoic detrital zircons from the south of Meiganga gold-bearing sediments (Adamawa, Cameroon): Their closeness with rocks of the PanAfrican Cameroon Mobile Belt and Congo Craton. Minerals 11(7): 1-32.
- Ruzicka V (1993) Vein uranium deposits. Ore Geol Rev 8(3-4): 247-276.
- Alexandre P, Kyser K, Layton Matthews D, Beyer RS, Hiatt EE, et al. (2015) Formation of the enigmatic Matoush uranium deposit in the Paleoprotozoic Otish Basin, Quebec, Canada. Miner Deposita 50(7): 825-845.
- Bonnetti C, Liu X, Li G, Mercadier J, Cuney M, et al. (2017) New insights for the genesis of granite-related vein-type uranium deposits in Xiazhuang and Zhuguang ore fields, SE China. Conference paper: The 14th SGA Biennial Meeting, Québec city, Canada.
- Li L, Wang Z, Xu D (2021) Relationship between Uranium Minerals and Pyrite and Its Genetic Significance in the Mianhuakeng Deposit, Northern Guangdong Province. Minerals 11(1): 73-87.
- Goyal N, Basu H, Sarbajna C, Saxena A, Nayak RA, et al. (2022) Hydrothermal Uranium Mineralization in Umra, Udaipur District, Rajasthan, India: Inferences from Petrography, Mineral Chemistry and Sulfur Isotopic Studies. Journal Geological Society of India 98(8): 1058-1067.
- Dickinson KA, Duval JS (1977) South-Texas Uranium: Geologic Controls, Exploration Techniques, and Potential. In: Campbell, M. D., ed., Geology of Alternate Energy Resources. Houston Geological Society, Houston pp. 45-66.
- King I (2014) Uranium ore processing. Application of United Nations Framework Classification – 2009 (UNFC-2009) for Uranium Resources pp. 36-45.
- (1993) International Atomic Energy Agency. Uranium extraction technology. IAEA Vienna, Technical Reports Series no pp. 359-405.
- Junior GO (1993) Bacterial leaching of uranium ore from Figueira-PR, Brazil, at laboratory and pilot scale. FEMS Microbiology Reviews 11(1-3): 237-242.
- Guettaf H, Becis A, Ferhat K, Hanou K, Bouchiha D, et al. (2009) Concentration–purification of uranium from an acid leaching solution. Physics Procedia 2(3): 765-771.
- Kiegiel K, Abramowska A, Biełuszka P, Kołtuniewicz ZP, Wołkowicz S (2016) Solvent extraction of uranium from leach solutions obtained in processing of Polish low-grade ores. J Radioanal Nucl Chem 311(1): 589-598.
- Danko B, Dybczyński SR, Samczyński Z, Gajda D, Koniecko HI, et al. (2017) Ion exchange investigation for recovery of uranium from acidic pregnant leach solutions. Nukleonika 62(3): 213-221.
- Rashad MM, Mohamed AS, EL sheikha ME, Miraa EH, Abd El Wahaba MG, et al. (2020) Kinetics of uranium leaching process using sulfuric acid for Wadi Nasib ore, South western Sinai, Egypt. Aswan University J Env Studies (AUJES) 1(2): 171-182.
- De Trey M, Lene G (1983) Iurep orientation phase report. Cameroon pp. 162-180.
- Saïdou, Bochud FO, Baechler S, Kwato NMG, Ngachin M, et al. (2011) Natural radioactivity measurements and dose calculations to the public: case of the uranium-bearing region of Poli in Cameroon. Radiat Meas 46(2): 254-260.
- Saïdou, Abdourahimi, Tchuente Siaka YF, Bouba O (2014) Indoor radon measurements in the uranium regions of Poli and Lolodorf, Cameroon. J Env Radioact 136(1): 36–40.
- Kouske AP (2013) Uranium occurrences associated with sodium metasomatism within the Pan-African mobile zone - the Kitongo, Salaki and Mayo Nielse uranium mineralizations in the Poli region, northern Cameroon: structural, petrographic and geochemical investigations. PhD Thesis, University of Yaoundé I (Cameroon) pp. 300-350.
- Penaye J, Toteu SF, Tchameni R, Van Schmus WR, Tchakounté J, et al. (2004) The 2.1Ga West Central African Belt in Cameroon: extension and evolution. J African Earth Scie 39(3-5): 159–164.
- Lasserre M, Soba D (1976) Age Libérien des granodiorites et des gneiss à pyroxènes du Cameroun Mé Bull BRGM 2(4): 17–32.
- Toteu SF, Van Schmus WR, Penaye J, Nyobé JB (1994) U–Pb and Sm–Nd evidence for Eburnean and Pan-African high-grade metamorphism in cratonic rocks of southern Cameroon. Precambrian Res 108(3-4): 45–73.
- Toteu FS, Van Schmus WR, Penaye J, Michard A (2001) New U–Pb and Sm–Nd data from north-central Cameroon and its bearing on the Pre-Pan African history of Central Africa. Precambrian Res 108(1-2): 45–73.
- Lerouge C, Cocherie A, Toteu FS, Penaye J, Mile PJ, et al. (2006) Shrimp U-Pb zircon age evidence for Paleoproterozoic sedimentation and 2.05 Ga syntectonic plutonism in the Nyong Group, South-Western Cameroon: Consequences for the Eburnean-Transamazonian belt of NE Brazil and Central Africa. J Afr Earth Scie 44(4-5): 413–427.
- Kornprobst J, Cantagrel JM, Fabries J, Lasserre M, Rollet M, et al. (1976) Existence in Cameroon of a Pan-African or older alkaline magmatism, the nepheline syenite with mboziite of Nkonglong; comparison with the known alkaline rocks of the same region. Bull Soci Geol France 18(5): 1295–1305.
- Edimo A (1985) Le massif syénitique d'Akongo-Lolodorf. Interprétation des anomalies radiomé Comparaison avec l'arc syénitique Mont des Eléphants-Rocher du Loup. Thèse 3 ème cycle, Univ. Orléans, France.
- Nédélec A, Minyem D, Barbey P (1993) High-P-high-T anatexis of Archean tonalitic grey gneisses: The Eseka migmatites, Cameroon. Precambrian Res 62(3): 191–205.
- Tchameni R, Mezger K, Nsifa EN, Pouclet A (2001) Crustal origin of Early Proterozoic syenites in the Congo Craton (Ntem Complex), South Cameroon. Lithos 57(1): 23-42.
- Ganno S, Moudioh C, Nzina Nchare A, Kouankap NDG, Nzenti PJ (2010) Geochemical fingerprint and iron ore potential of the siliceous itabirite from Palaeoproterozoic Nyong Series, Zambi Area, Southwestern Cameroon. Resour Geol 66(1): 71-80.
- Ebah Abeng AS, Ndjigui PD, Beyann AA, Teutsong T, Bilong P (2012) Geochemistry of pyroxénites, amphibolites and their weathered products in the Nyong unit, SW Cameroon (NW border of Congo craton): implication for Au-PGE exploration. Journal of Geochemical Exploration 114(1): 1-19.
- Ndong BF, Ntomba MS, Okia BD, Mvondo OJ (2013) Deformation and metamorphism in the central part of the Ngovayang Massif (South Cameroon). Int J Geol 1(7): 32-37.
- Ndong BF, Sobdjou KC, Mero Y, Ntomba MS, Nzenti JP, Mvondo OJ (2016) Origin and tectonic framework of the Ngovayang Iron Massifs, South Cameroon. Scie Res 4(1): 11-20.
- Ndema MJL, Ngnotue T, Ngo NCD, Nzenti JP, Cheo SE (2014) Origin and evolution of the formation of the Cameroon Nyong Series in the western border of the Congo Craton. J Geoscie Geomatics 2(2): 62-75.
- Ako AT, Vishiti A, Suh EC, Kedia CA, Omang OB (2017) Geological models of Platinum Group Elements (PGE) depletion in metamorphosed ultramafic rocks of the Nyong Series, Southeast Cameroon. Int J Mining Scie 3(4): 52-63.
- Chombong NN, Suh CE, Ilouga CDC (2013) New detrital zircon U-Pb ages from BIF- related metasediments in the Ntem Complex (Congo craton) of southern Cameroon, West Africa. Nat Scie 5(7): 835–847.
- Chombong NN, Suh EC, Lehmann B, Vishiti A, Ilouga CD, et al. (2017) Host rock geochemistry, texture and chemical composition of magnetite in iron ore in the Neoarchaean Nyong unit in southern Cameroon. Appl Earth Scie 126(3): 1-17.
- Kouankap NGD, Njiosseu TEL, Takodjou WJD, Kamguia WB, Afahnwie NA, et al. (2018) Petro-structural characterization of Bonguen Area, Nyong Series, Cameroon: Insight into the Northern Extension of Kribi-Campo Shear Zone. Earth Sciences 7(5): 236-241.
- Fuanya C, Bolarinwa TA, Kankeu B, Yongue FR, Tangko TE, et al. (2019) Geochemical characteristics and petrogenesis of basic rocks in the Ako’ozam–Njabilobe area, Southwestern Cameroon: implications for Au genesis. SN Applied Scie 1(8): 904-910.
- Aye BA, Sababa E, Ndjigui PD (2017) Geochemistry of S, Cu, Ni, Cr and Au-PG from in the garnet amphibolites the Akom II area in the Archaean Congo Craton, Southern Cameroon. Geochemistry 77(1): 81–93.
- Aye AB, Tamfuh AP, Tata E (2021) Geochemistry of Amphibolites in Akom II, Nyong Series, North Western Border of the Congo Craton, South Cameroon. Int J Advan Geosci 9(1): 33-41.
- Tsama MG, Ekollo KNS, Tchouffa BB, Ndong BF (2021) Geochemistry and SM-ND dating of amphibolites of Eseka greenstones belt (Nyong Complex South Cameroun): highlighting of distensive episodes in compressive orogenic context. International Scientific Conference Geobalcanica.
- Ottou MJE, Ntomba MS, Ndong BF, Akame MJ, Owona S, et al. (2014) Géomorphologie structurale et risque naturel dans une portion de zone mobile du complexe du Nyong au SW Cameroun: cas de la région Lolodorf-Mvengue. Afr Scie 10(4): 288-298.
- Ottou MJE, Ntomba MS, Ntieche B, Takamte MCR, Bisse BS, et al. (2022) Petrology of the meta‑mafic rocks from the Lolodorf area, Nyong complex (Southwest Cameroon): implication for the origin and emplacement conditions SN Applied Scie 4(8): 216-220.
- Nsifa EN, Tchameni R, Nédélec A, Siqueira R, Pouclet A, et al. (2013) Structure and petrology of Pan-African nepheline syenites from the South West Cameroon; Implications for their emplacement mode, petrogenesis and geodynamic significance. J Afr Earth Scie 87(7): 44- 58.
- Tchameni R (1997) Géochemie et géochronologie des formations de l'Archéen et de Paléoprotérozoique du Sud Cameroun (Groupe du Ntem, Craton du Congo). Thèse de Doctorat de l'Université d'Orléans pp. 395-400.
- Cox GK, Bell DJ Pankhurst JR (1979) The Interpretation of Igneous Rocks. George Allen & Unwin, London.
- De la Roche H, Leterrier J, Grand Claude P, Marchal M (1980) A classification of volcanic and plutonic using R1R2-diagrams and major element analyses-its relationship with current nomenclature. Chem Geol 29(1-4): 183–210.
- Sun S, McDonough FW (1989) Chemical and Isotopic Systematics of Oceanic Basalts, Implications for Mantle Compositions and Processes. Saunders AD and Norry MJ (Eds.) Magmatism in the Ocean Basins Geol Soc Spec Publ 42(1): 313-345.
- Peccerillo A, Taylor RS (1976) Geochemistry of Eocene Calc-Alkaline Volcanic Rocks from the Kastamonu Area, Northern Turkey. Contributions to Mineral Petrol 58(3): 63-71.
- Shand SJ (1943) Eruptive rocks their genesis, composition, classification, and their Relation to Ore-Deposit with a chapter on Meteorite, John Wiley & Sons., New York pp. 444-489.
- Kanouo SN, Wang L, Kouske PA, Yomeun BS, Basua AAE (2019) Petro-Geochemistry, Genesis and Economic Aspect of Syenitic and Mafic Rocks in Mindif Complex, Far North Cameroon, Central Africa. Int J of Geoscie 10(12): 1081-1114.
- Wahen BJ, Currie LK, Chappell WB (1987) A-type granites: geochemical characteristics, discrimition and petrogenesis. Contrib Mineral Petrol 95(4): 407-419.
- Lubala RT, Frick C, Rogers JH, Walraven F (1994) Petrogenesis of syenites and granites of the Schiel Alkaline Complex Northern Transvaal, South Africa. The J Geol 102(3): 307-316.
- Heilimo E, Mikkola P, Huhma H, Halla J (2017) Alkaline rich quartz syenite intrusions of the Western Karelia Subprovince. Geol Soci Lon Spec Publ 449(1): 61-88.
- Rosa P, Ruberti E (2018) Nepheline syenites to syenites and granitic rocks of the Itatiaia Alkaline Massif, Southeastern Brazil: New geological insights into a migration Ring Complex. Brazilian J Geol 48(2): 347-372.
- Walkins RT, Le Roex AP (1991) Petrology and structure of syenite intrusions of the Okenyenya Igneous Complex. Communications of the Geological Survey of Namibia 7(8): 59- 75.
- Zhu SY, Yang HJ, Sun FJ, Zhang, HJ, Wu YF (2015) Petrogenesis of coeval silica-saturated and silica-undersaturated alkaline rocks: Mineralogical and geochemical evidence from the Saima alkaline complex, NE China. J Asian Earth Scie 117(1): 184-207.
- Pearce JA, Harris NBW, Tindle AG (1984) Trace Element Discrimination Diagrams for the Tectonic Interpretation of Granitic Rocks. J Petrol 25(4): 956-983.
- Mahmoud A, Abbas SS, Hasan TZ, Ali V (2011) Bioleaching of Low Grade Uranium Ore of Saghand Mine. Iran J Chem Chem Eng 30(4): 71-79.
- (2006) International Atomic Energy Agency. Rapport d’ensemble sur la technologie nucléaire 2006. no 6098, OCDE, Paris.
- Siedel CD (1981) Extracting uranium from its ores. IAEA Bull 23(2): 24-28.
- Ramadevi G, Sreenivas T, Navale AS, Padmanabhan NPH (2012) Solvent extraction of uranium from lean grade acidic liquor with alamine 336 reagent. Journal of Radioanalytical and Nuclear Chemistry 294(1): 13-18.
- Tamrakar PK, Prajapati D, Sarangi AK (2010) Etude des caractéristiques de lixiviation à basse minerai d'uranium de qualité de la mine de Bagjata pp. 666–672.
- Venter R, Boylett M (2009) The evaluation of various oxidants used in acid leaching of uranium. Hydrometallurgy Conference. Southern African Institute of Mining and Metallurgy, South Africa pp. 445-456.
- Umanskii BA, Klyushnikov MA (2013) Bioleaching of low grade uranium ore containing pyrite using ferrooxidans and A. thiooxidans. J Radioanal Nucl Chem 295(1): 151–156.
- Laxen PA (1971) Dissolution of Uranium Dioxide as an Electron Transfer Reaction, The Recovery of Uranium: Proceedings of a Symposium. International Atomic Energy Agency, Vienna.
- Mattus AJ, Torma AE (1980) Uranium extraction from a lowgrade ore by acidic leachants. Metall 34(1): 33–36.
- Eligwe C, Torma A, Devries F (1982) Leaching of uranium ores with the H2O2 - Na2SO4 -H2SO4 system. Hydrometallurgy pp. 83-95.
- International Atomic Energy Agency, (1993) Uranium Extraction Technology. Technical report series no 359.
- Roshani M, Mirjalili K (2009) Studies on the leaching of an arsenic–uranium ore. Hydrometallurgy 98(3-4): 304–307.
- Jansen van Rensburg N (2014) A Value Based Approach to Leach Optimization at Rössing Uranium Limited (Master’s Thesis), University of Cape Town pp. 181-190.
- Hamza FM, El Aassy EI, Guibal E (2019) Integrated treatment of tailing material for the selective recovery of uranium, rare earth elements and heavy metals. Minerals Eng 133(1):138-148.
-
Raoul Pierre Fodjo, Nguo Sylvestre Kanouo*, Francois Bidzang Ndong and Emmanuel Archelaus Afanga Basua. Characterization and Processing of U-Bearing Syenitic Rocks from Mbanga Massif, South Region, Cameroon: Insight from Geochemistry,Mineralogy, Radiometry, and H2SO4 Acid Leaching. Adv in Mining & Mineral Eng. 1(3): 2024. AMME.MS.ID.000514.
-
Cameroon; Mbanga massif; syenitic rock; uranium ore; characteristics; H2SO4 acid leaching process
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






