 Research Article
Research Article
Determination of the Appropriate Metal/Cyanide Ratio for the Reaction of Gold and Cyanicidal Metals in Synthetic Solutions. Quantification of Free Cyanide by Colorimetry
Esmeralda Y García A1, Ramiro Escudero G1*, Rosa E Pérez S2, Martín Reyes P3 and Roberto Guerra G4
1Institute of Research in Metallurgy and Materials, Universidad Michoacana de San Nicolas de Hidalgo Santiago Tapia 403, C.P. 58000.Morelia, Michoacán, México
2Pharmacobiology Faculty, Universidad Michoacana de San Nicolas de Hidalgo Santiago Tapia 403, C.P. 58000.Morelia, Michoacán, México
3Academic Area of Earth Sciences and Materials, Universidad Autónoma del Estado de Hidalgo. Mineral de la Reforma 42186. Pachuca, Hidalgo, México
4Chemical Engineering faculty, Universidad Michoacana de San Nicolas de Hidalgo Santiago Tapia 403, C.P. 58000.Morelia, Michoacán, México
Ramiro Escudero G, Institute of Research in Metallurgy and Materials, Universidad Michoacana de San Nicolas de Hidalgo Santiago Tapia 403, C.P. 58000.Morelia, Michoacán, México
Received Date:September 19, 2024; Published Date:October 03, 2024
Abstract
Excessive addition of cyanide during the leaching of gold minerals causes passivation of the gold dissolution reaction, significantly decreasing the presence of gold species in the pregnant solution. Therefore, it is essential to determine the appropriate concentration of cyanide that will dissolve gold species and cyanide-like metals. This research proposes establishing the appropriate relationship, cyanicidal metals-cyanide, in synthetic solutions that allow the results to apply to the cyanidation of gold minerals. Synthetic solutions (unitary and binary) were prepared by mixing specific concentrations of NaCN, and Au, Cu, Fe, Mg, and Ca in deionized water; the Metal/CNadded ratio varied from 0.04 to 5, depending on the cyanicidal metal content in a mineral from the central region of Mexico. The free (unreacted) cyanide in the synthetic solutions was quantified by the volumetric method based on the color change of the problem solution upon adding the titration reagent AgNO3. The experimental results show that the Metal/CNadded ratio increases as the cyanide required to form the complex decreases, in the order: Ca → Mg → Fe → Cu → Au, which coincides with the thermodynamic calculations of the spontaneity of the reactions involved. The results propose a procedure to calculate the appropriate cyanide to ensure a mineral’s efficient dissolution of gold-bearing species.
Keywords:Cyanidation; Leaching; Cyanicides; Gold-bearing species; colorimetry
Introduction
Leaching of gold ores by cyanidation is a traditional technique that dissolves gold, forming the compound in solution Au(CN)2− . Due to its greater efficiency and economy, this technique is preferred over others, such as dissolution with thiourea, thiosulfate, thiocyanate, and aqua regia. However, if basic care is not observed, it is quite toxic when hydrocyanic acid is generated [1,2]. In practice, it is common to saturate the mineral-water-cyanide system with the cyanide reagent to ensure gold leaching after dissolving the cyanicidal elements present in the mineral; however, excessive saturation with cyanide can create a passivating layer on the gold particles, preventing contact with the cyanide and therefore inhibiting its dissolution or that of the gold-bearing species [2,3]. Consequently, it is essential to determine the appropriate amount of cyanide reagent that guarantees the dissolution first of the cyanicidal elements contained in the mineral and finally of the gold, considering that the added CN must be sufficient to dissolve the gold despite the CN being consumed first with the cyanicides. On the other hand, in practice, no procedure is applied to establish the concentration of cyanide needed to leach a given mass of gold ore; simply, a certain percentage of sodium or potassium cyanide is added to the mineral pulp (commonly 5% v/v), which represents a relatively high percentage, preventing the efficient dissolution of the gold species. This research aims to establish a procedure to determine the appropriate amount of cyanide reagent to be added to a mineral pulp. It starts with the chemical characterization of the head mineral, the thermodynamic analysis of the dissolution reactions of its cynical components, and finally, the mastery of the technique of quantification of free cyanide (unreacted cyanide) by volumetry. The reference mineral for this work comes from a deposit located in the central region of the state of Michoacán, Mexico.
Theory
The research on the dissolution of gold species mainly focuses on establishing the most appropriate technique to guarantee its technically and economically feasible dissolution [4-6]; there are relatively few reports on the aspects that inhibit the total dissolution of gold in minerals. According to Lin and Chen [7], as well as Bas and collaborators [8], in the presence of high concentrations of cyanide, a passivating layer is formed that prevents the dissolution of gold; they observed that the dissolution rate increases with cyanide concentrations of 0.1 mol/L and decreases when this concentration rises to 0.2 mol/L. The above is under normal conditions of 1 atmosphere of pressure and at room temperature. Ghobaldi et al. [9], from experimental results in the laboratory, established that the adequate amount of NaCN to maximize the dissolution and recovery of gold in a mineral is 700 grams per ton, although for this to occur, variables such as pulp density, pH, and particle size d80, must be established at specific values. It is not clear whether cyanide concentration is the predominant variable for optimal gold recovery when concluding that by establishing the pH from 10 to 11, gold dissolution decreases because OH- ions are adsorbed on the gold surface, preventing cyanide from penetrating the gold particles.
Procedure for quantifying free cyanide (CN-) by volumetry
The applied technique is based on the NMX-AA-058-SCFI-2001
standard, issued by the Mexican Ministry of Economy in 2001 [10],
and the ASTM D2036-09 standard [11]. The procedure consists of
the following steps:
a. 1.- Preparation of the Titrating Reagent (AgNO3) at 0.1
normal,
b. 2.- Preparation of the KI Reagent at 5% (v/v),
c. 3.- Preparation of the Solution with cyanide,
d. 6.- Titration with silver nitrate,
e. 7.- Calculation of the free cyanide in solution according to
the following expression:
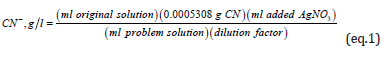
In the above equation, the constant 5.308 x 10-4 corresponds to the grams of cyanide that are associated with 1 ml of AgNO3; in other words, one milliliter of silver nitride reacts or identifies 5.308x10-4 grams of cyanide, according to the stoichiometric ratio of the reaction (1).
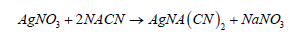
Experimental Methodology
Quantification of free cyanide by volumetric technique in NaCN solutions Materials and reagents
For the quantification of free cyanide by the volumetric technique, a 25 ml burette, 100 ml Erlenmeyer flasks, universal support, burette clamp, 500, 100, and 50 ml volumetric flasks, and a 100 ml beaker were used. The chemical reagents used were silver nitrate (AgNO3), sodium cyanide (NaCN), sodium hydroxide (NaOH), potassium iodide (KI), distilled water, Au, Ca, Mg, Cu, and Fe standards— all the above reagent grades. As mentioned in previous paragraphs, the volumetric quantification technique was applied according to NMX-AA-058-SCFI-2001, issued by the Mexican Ministry of Economy in 2001 [10], and the ASTM D2036-09 standard [11].
Quantification of free cyanide in synthetic solutions with metals
Five cyanicidal elements (Au, Ca, Mg, Cu, and Fe) were used to prepare synthetic solutions with known concentrations of these elements. The quantification was carried out in unitary and binary synthetic solutions; each determination was carried out in triplicate. In the experiments with unitary solutions, the concentration of metals was set at 50 ppm in 25 ml of deionized water; in the case of binary solutions, the concentration of gold was maintained at 50 ppm in 50 ml of deionized water, and the rest of the metals were varied from 200 to 300 ppm depending on their proportion according to the XRF analysis of a typical mineral from the south-central region of Mexico. The percentage of cyanide associated with metals varied from 0.002 to 0.13 (Metal/CNadded ratio from 0.04 to 5), depending on the cyanide concentration in the synthetic solutions.
Identification of CN-Metal complexes by UV-Vis technique
Each Metal-CN solution before free cyanide quantification was analyzed using the UV-Vis technique to identify the complex species formed for unitary and binary solutions. UV-Vis Spectroscopy equipment, Perkin Elmer brand, model Lambda BIO Series 24117, was used at 200 to 800 nm wavelengths.
Chemical characterization of gold ore
50 kilograms of mineral samples were collected at a point in the deposit in the southeastern Mexico region. The mineral rocks were pulverized in a disc mill, and the particle size was determined using the Coulter® LS100Q equipment. A representative sample of 100 grams with d80 of -75 μm was obtained by quartering. The mineral was elementally analyzed using the X-ray Fluorescence technique (XRF, S8 Tiger BRUKER®). Finally, the gold contained in the mineral sample was quantified using the atomic absorption spectroscopy technique (Perkin Elmer® PinAAcle 900H) after digesting the mineral in a microwave oven with aqua regia and hydrofluoric acid.
Results and Discussion
Results of chemical analysis by XRF
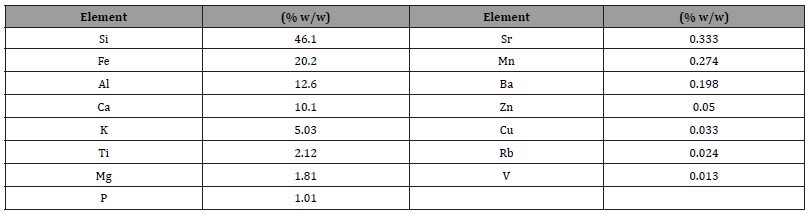
Table 1 shows the elemental analysis results by X-ray fluorescence performed on a gold mineral from central Mexico. It is important to mention that due to the sensitivity of the equipment and the technique, gold was not detected as it was present in units of parts per million (ppm) or grams per ton (gpt). Chemical analysis indicates that the ore contains 54 grams of gold per tonne (gpt).
Table 1:Elemental analysis of the mineral sample by X-ray fluorescence.
Order of cyanicides in the mineral
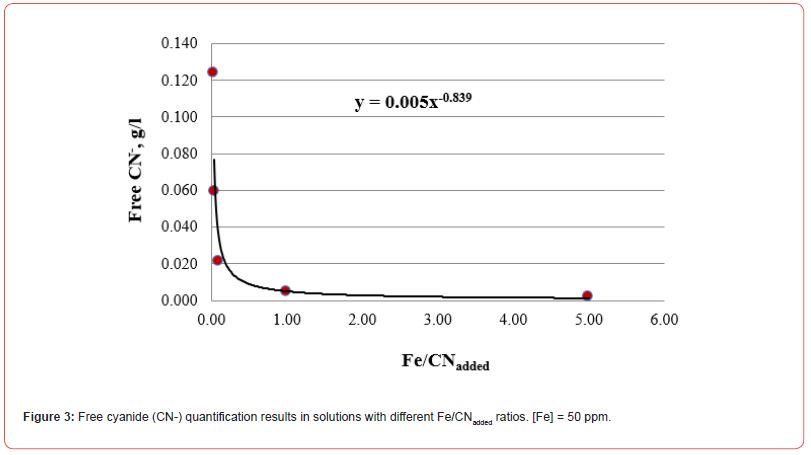
From the thermodynamic analysis of cyanidation reactions, the elements (from Table 1) most chemically similar to cyanide are established according to the value of the free energy of the reaction. The reactions involved are the following:
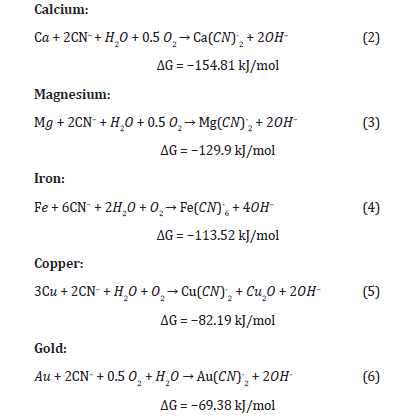
From the above, the order of affinity of the metals for cyanide is: Ca → Mg → Fe → Cu → Au. The first four elements react with CN⁻ before Au, so it is essential to add cyanide in a quantity that ensures the complete dissolution of the five cyanicidal metals.
Results of free CN- quantification by volumetry. Unit solutions
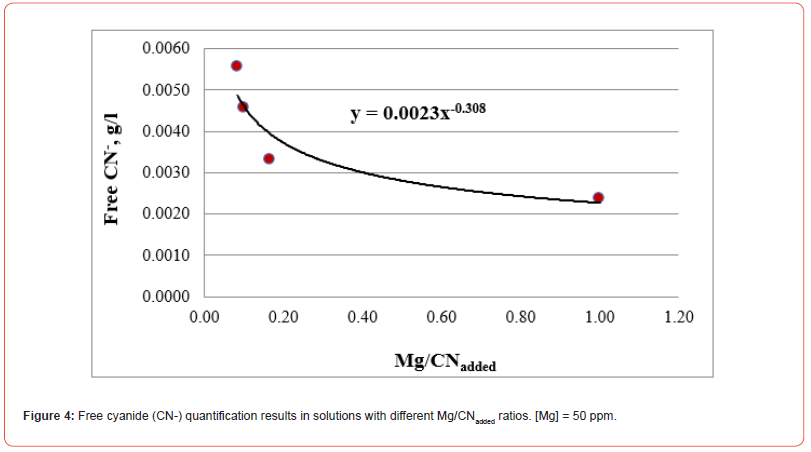
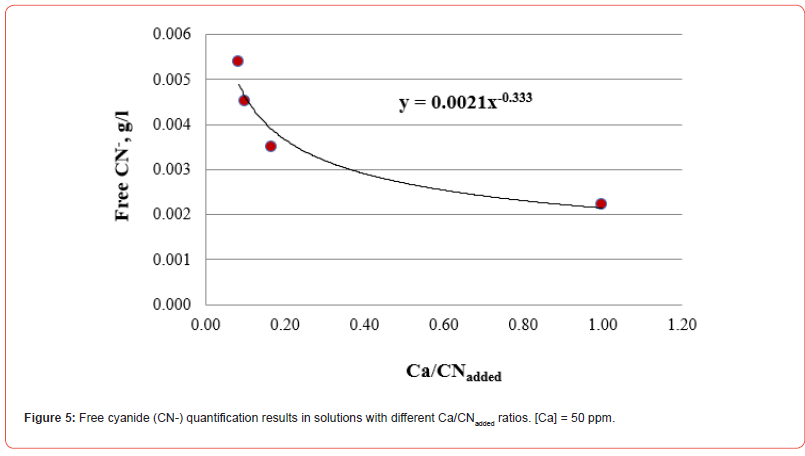
The results of free cyanide quantification by the volumetric technique to unitary solutions (deionized water + metal + CN) with known concentrations of metal (50 ppm) and cyanide are presented in Figures 1-5. The free CN- values are the average of three measurements.
The five figures above show that the amount of free cyanide decreases with the concentration of added cyanide (Metal/CNadded increases). Each figure includes the corresponding correlation equation; with this expression, it is possible to extrapolate the Metal/ CNadded ratio and establish at what value the free cyanide is practically zero (for example, CN- ≤ 0.0001 g/l) and that by subtracting the added cyanide, it results in the quantity of cyanide associated with the metal that forms the corresponding complex. Extrapolating for each system the Metal/CNadded ratio where the residual free cyanide in solution is considered equal to zero if free CN− is equal to or less than 0.0001 g/l, we find that this ratio increases with the cyanicidal character of the metal and agrees with the order of affinity to cyanide suggested by the value of free energy of the reaction, calculated for reactions (2) to (6), and shown in Table 2. From the table above, if the Metal/CNadded ratio increases, it means that less cyanide is required to form the Me(CN)x− complex, as the reaction is more spontaneous, such that the cyanicidal character of the metals agrees with the sequence of cyanide affinity suggested by the results of free energy of reaction for each metal. It is confirmed that the cyanicidal order is Ca → Mg → Fe → Cu → Au.
Table 2:Metal/CNadded in deionized water, and the free energies of reactions (2) to (6) of the cyanicidal metals.
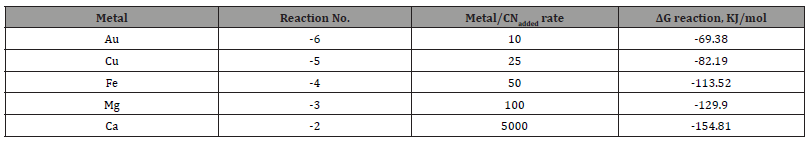
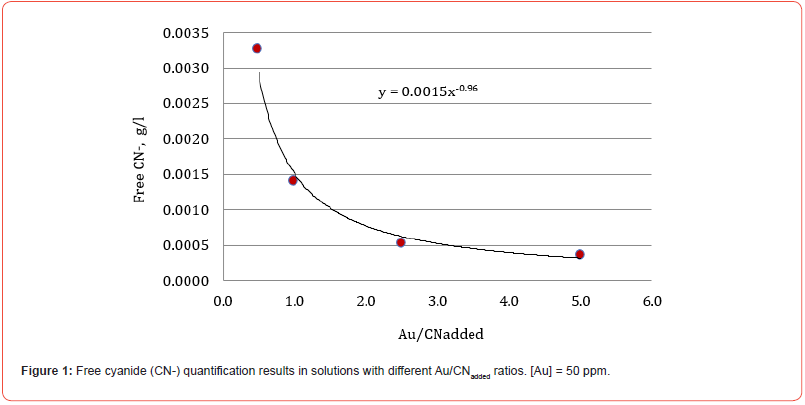
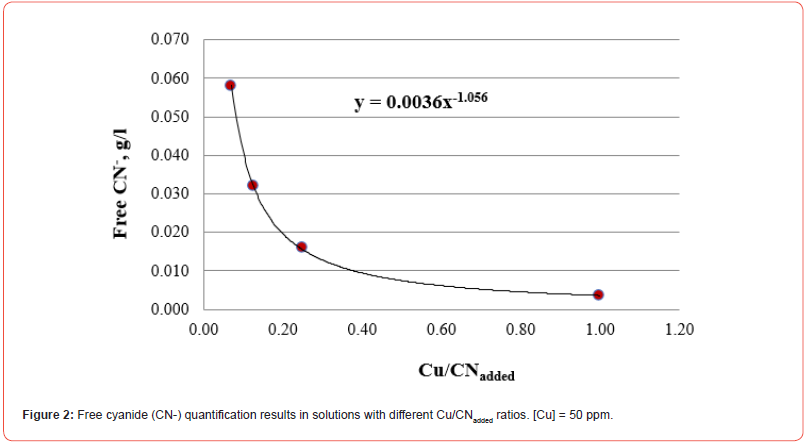
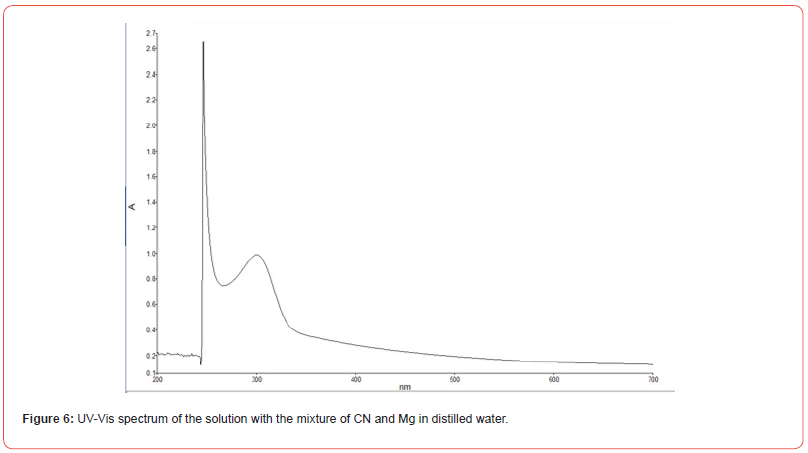
Identification of CN- Metal complexes by UV-Vis technique
The solutions analyzed by this technique show evidence of the formation of the Me(CN)x—complexes for the metals Au, Ca, Mg, and Cu, while for Iron, the species Fe(CN)6—was identified. Its characteristic position at the corresponding wavelengths coincides with those reported in the literature [12-17]. An example of the spectra obtained is shown in Figure 6, which corresponds to one of the solutions in Figure 4.
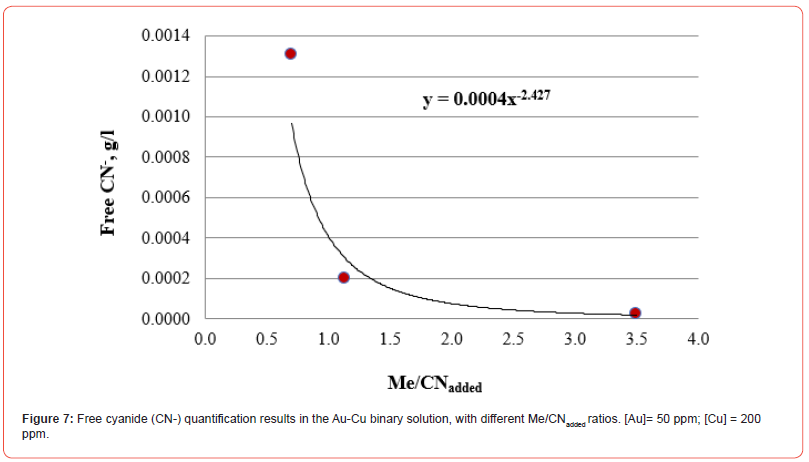
Results of free CN- quantification by volumetry. Binary solutions
The results of free cyanide quantification by the volumetric technique in binary solutions (deionized water + Au + metal + CN) with known concentrations of gold and other metals (concentration proportional to the content in the XRF analysis) are presented in Figures 7-10. The free CN- values are the average of three measurements.
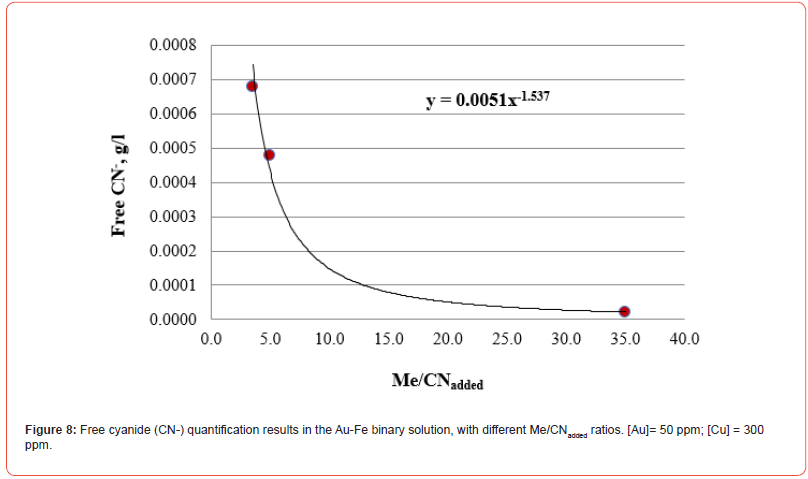
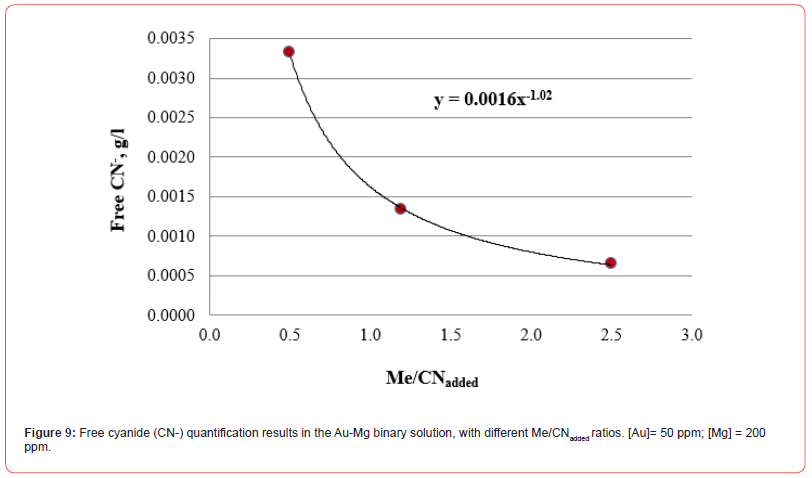
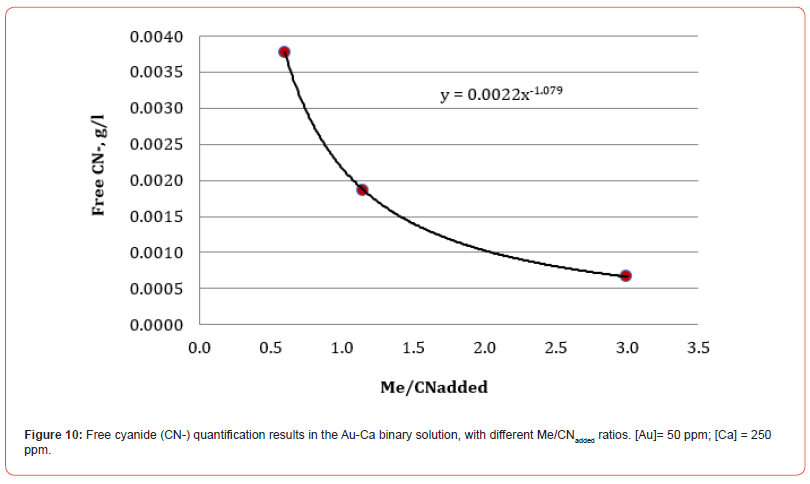
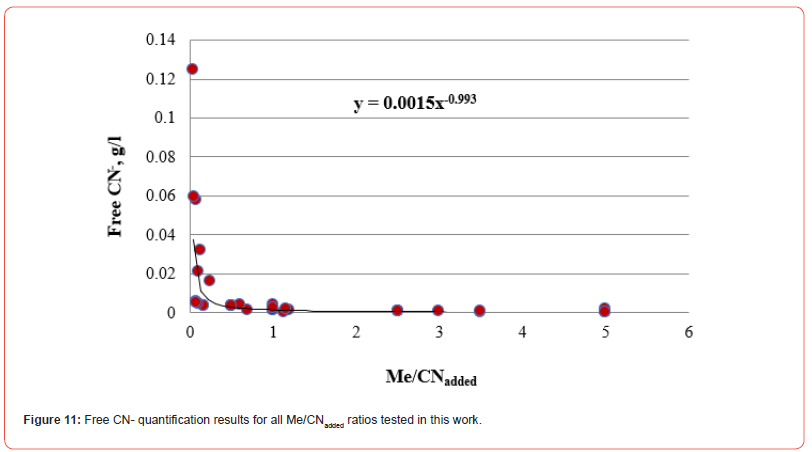
By including in a single figure all the Me/CNadded ratios for all solutions, unitary and binary, we obtain what is shown in Figure 11. Figure 11 shows that all the relationships fit reasonably to the same function, which allows establishing the concentration of free cyanide for any concentration of cyanicidal metal and the cyanide added to the aqueous system. By knowing the concentration of cyanicidal metals in the system, it is possible to determine the quantity of cyanide that must be added so that the free cyanide is less than or close to 0.001 g/l, which will help to avoid excess added cyanide, which can promote the passivation of the cyanidation reactions of the cyanicidal elements contained, for example, in a mineral.
Conclusion
From the experimental results of cyanidation of cyanicidal metals in unitary and binary synthetic solutions, the following conclusions are derived:
All the Me/CNadded ratios included in this research work are reasonably adjusted to the same function that allows establishing the g/l of free cyanide in the residual solution once the cyanide reacts with the cyanicidal metals contained in the system. This procedure avoids adding excess cyanide that inhibits the dissolution of cyanicidal species by promoting the passivation of the cyanidation reactions of the cyanicidal elements contained, for instance, in a mineral.
Acknowledgement
Esmeralda Y. García-Alcázar deeply thanks the UMSNH for the support received during her master’s studies and for carrying out this research work.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- JA Gasca Torres (2016) Carbón activado de carácter básico para recuperar oro de lixiviados cianurados. San Luis Potosí, S.L.P: IPICYT pp. 1-108.
- Yannopoulos JC (1991) The extractive metallurgy of gold. Van Nostrand Reinhold Editors pp. 1-281.
- EMyc Garcia Rosales (2021) Principios termodinámicos y cinéticos de la cianuración de oro y sus efectos en el proceso, Coahuila: Facultad de Ciencias Químicas, UAdeC.
- CE Elmore, RJ Brison, CW Keney (1987) New Technology for More Efficient Cyanidation of Gold Ores. Proceedings of the 26th CIM Annual Conference of Metallurgists 1(1): 295-307.
- F Nava Alonso, E Elorza Rodríguez, A Uribe Salas, y R Pérez Garibay (2007) Análisis químico de cianuro en el proceso de cianuración: revisión de los principales mé REVISTA DE METALURGIA 43(1): 20-28.
- Luis Mario Auquilla Arévalo, Clara Inés Damián Vélez, Juan Diego Espinoza Gárate (2019) Reducción de cianuro de agua residual proveniente de una empresa metalúrgica por tratamiento oxidativo con peróxido de hidró Tesis previo a la obtención del título de Ingeniero/a Químico/a. Cuenca, Ecuador.
- HK Lin, Chen X (2001) Electrochemical study of gold dissolution in cyanide solution. Minerals & Metallurgical Processing 18(3): 147-153.
- Ahmet Deniz Bas, Fariba Safizadeh, Wei Zhang, Edward Ghali, Yeonuk Choi (2015) Active and passive behaviors of gold in cyanide solutions. Trans. Nonferrous Met. Soc. China 25(10): 3442−3453.
- B Ghobadi, M Noaparast, SZ Shafaei, M Unesi (2014) Optimization of cyanidation parameters to increase the capacity of Aghdarre gold mill. JME Journal of Mining & Environment 5(2): 121-128.
- Norma NMX-AA-058-SCFI-2001. Diario Ofuicial de la Federació https://www.dof.gob.mx› nota_detalle_popup.
- ASTM Designation: D2036 − 09 (Reapproved 2022). Standard Test Methods for Cyanides in Water. US Department of defense. iTeh Standards. https://cdn.standards.iteh.ai
- Kurowa K, Masaki Y, Koga Y, Deming TJ (2013) Self-Assembly of Discrete Metal Complexes in Aqueous Solution via Block Copolypeptide Amphiphiles. International Journal of Molecular Sciences 14(1): 2022-2035.
- Manal A, Rawashdeh Omary, Mohammad A, Omary, Howard H Patterson (2000) Oligomerization of Au(CN)2- and Ag(CN)2- Ions in Solution via Ground-State Aurophilic and Argentophilic. J Am Chem Soc Vol 122(42): 10371-10380.
- Monica CV, Avila Y, Mojica R (2023) Atypical Metal‐Center Coordination in Two Cyanide‐Based Coordination Polymers. ChemistrySelect 8(32): e202302901-e202302908.
- Gabler N, Hartley J, Frisch G (2017) Voltammetric and spectroscopic study of ferrocene and hexacyanoferrate and the suitability of their redox couples as internal standards in ionic liquids. Physical Chemistry Chemical Physics 19(42): 28841-28852.
- Ke Ching Bin, Lu Te Ling, Chen Jian Lian (2018) Capacitively Coupled Plasma Discharge of Ionic Liquid Solutions to Synthesize Carbon Dots as Fluorescent Sensors. Nanomaterials 8(6): 372-380.
- Kianoush Barani, Javad Torkashvand, Hossein Rouhbakhsh (2023) Characterization of Gold and Copper Complexes in Cyanide-Glycine System by Uv-Vis Spectroscopy. Journal of Chemical Technology and Metallurgy 58(5): 906-912.
-
Esmeralda Y García A, Ramiro Escudero G*, Rosa E Pérez S, Martín Reyes P and Roberto Guerra G. Determination of the Appropriate Metal/Cyanide Ratio for the Reaction of Gold and Cyanicidal Metals in Synthetic Solutions. Quantification of Free Cyanide by Colorimetry. Adv in Mining & Mineral Eng. 1(3): 2024. AMME.MS.ID.000512.
-
Cyanidation; Leaching; Cyanicides; Gold-bearing species; colorimetry
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






