 Review Article
Review Article
Football Sport Injuries and the Temporomandibular Complex
Bradley Eli1, Vincent Fricton2, Linda Sangalli2, Dilip Dudhat3 and James Fricton4*
1Orofacial Therapeutics, CA. Director, Facial Pain Specialists, Encinitas, CA
2College of Dental Medicine - Illinois, Midwestern University, IL
3Advanced Dental Esthetics, PA
4Department of Dentistry, Medicine, and Public Health, University of Minnesota, MN
James Fricton, Department of Dentistry, Medicine, and Public Health, University of Minnesota, MN, USA
Received Date: November 26, 2024; Published Date: December 10, 2024
Football and other sport players have a high prevalence of temporomandibular disorders (TMD), headaches, and orofacial pain. There is also a relationship between concussion injuries and TMD. We hypothesize that these conditions can be related to repetitive trauma to the temporomandibular muscle and joint (TMJ) complex during football and other contact sports. We outline how helmet impacts can transmit force via the chinstrap to adversely affect vital TMJ structures. We relay how this repetitive trauma can trigger the phenomenon of reactive neuromuscular tension. When sustained, this protective response can instigate and perpetuate chronic pain via a “pain cycle”. We explain how sustained neuromuscular tension in the TMJ region may be associated with chronic headache and neck pain via central sensitization. Finally, we outline a self-directed treatment approach that can be used to relieve trauma-related neuromuscular tension and help prevent chronic pain.
Introduction
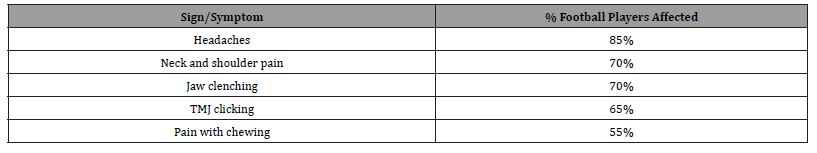
Pain in the temporomandibular muscle and joint (TMJ) structures may represent a silent epidemic among American football and other sports players. This is not surprising given the high exposure to low- and medium-level trauma during a typical game. Some studies record that there are greater than 1400 football helmet impacts per player in a typical season [1]. This frequent number of traumatic impacts to the TMJ structures justifies a thoughtful investigation of the contribution of repetitive TMJ trauma to the development of chronic headaches, jaw and orofacial pain in football players. Indeed, several clinical studies report a high prevalence of head, neck, and TMD pain in American football players (Table 1) [2,3].
Table 1: Common TMJ-related signs and symptoms seen in active American football players [2-3].
Athletes in general, especially those playing contact sports such as football and hockey, have a higher prevalence of TMJrelated disorders than do non-athletes [2-4]. The primary reason consists in the higher risk of macrotrauma to the TMJ complex (the joint space, articular disc, ligaments, tendons, muscles, and neurovascular structures of the TMJ), which consists in one of the multifactorial etiology and pathogenesis of temporomandibular disorders (TMD) and are strongly associated with the development of chronic jaw pain [5].
In general, head, neck, and jaw pain are associated by a bidirectional relationship. For example, in a large representative cohort sample of US adults, 53% of those with TMD pain also experienced severe headaches or migraines while 54% of those with neck pain also had headaches [6-8]. The duration of these pain conditions also increases the risk of developing co-morbid pain [9]. The overlapping between these co-morbidities resides in the common neuroanatomy (i.e. trigeminovascular system and trigeminocervical complex) [10] and in a shared pathogenesis involving peripheral and central sensitization, a known accepted model for developing chronic pain [11].
In this review, the association between repetitive trauma from football and sports injuries to the TMJ complex and the development of chronic TMD, orofacial, head, and neck pain reported in American football athletes will be explored. First, we will discuss emerging evidence of the relationship between concussions and TMD pain. We will also describe how American football helmets may impact key anatomical structures in the TMJ complex when subjected to macrotrauma. We will also provide an overview on how repetitive trauma may trigger reflexive neuromuscular tension in the TMJ region, thus initiating and/or perpetuating pain and the pain cycle. We will discuss how masticatory neuromuscular tension is related to TMD pain, headaches and neck pain. Finally, we will conclude by proposing a self-care patient-oriented conservative treatment paradigm aimed at preventing chronic pain.
Discussion
Concussions and TMJ Pain. There is mounting concern over the emerging incidence of football concussions and their long-term risks. Football, being a contact sport with high-impact collisions, poses a significant risk for players to experience concussions (mild traumatic brain injuries, or mTBIs). For incidence estimates of concussions, one study found that 3% of youth players, 7% of high school players, and 5% of college football players will have at least one concussion per season [12]. As awareness about the potential long-term consequences of concussions has increased, there has been a heightened focus on player safety and concussion management protocols in football. Efforts are being made to improve concussion recognition, prevention, and treatment strategies in order to better protect the health and well-being of athletes.
Emerging research is showing that TMD symptoms are highly prevalent in those seeking care for prolonged post concussive symptoms. A cross-sectional recent study by Karpuz and colleagues (2023) compared patients with a history of traumatic brain injury (TBI) who were seeking care for prolonged symptoms to the general population investigating the presence of TMD using a Fonseca questionnaire [13]. The study found that 80% of TBI patients screened positive for TMD symptoms, and the prevalence increased to 94% when subgroup analysis was performed of TBI patients with headaches. In addition, on the physical exam, the TBI group displayed a statistically significant decrease in the range of motion and masticatory muscle pressure pain thresholds. This study further elaborated on the longstanding suspicion among Orofacial Pain Specialists that there is a strong correlation between sports-related concussions and TMD. This connection is especially significant in the context of football, where the repetitive trauma to the TMJ and the risk of concussions often coincide. The data reinforces the need for heightened awareness and comprehensive assessment of both concussions and TMD in football and other contact sports players, as these conditions can have a cumulative impact on an individual’s overall well-being.
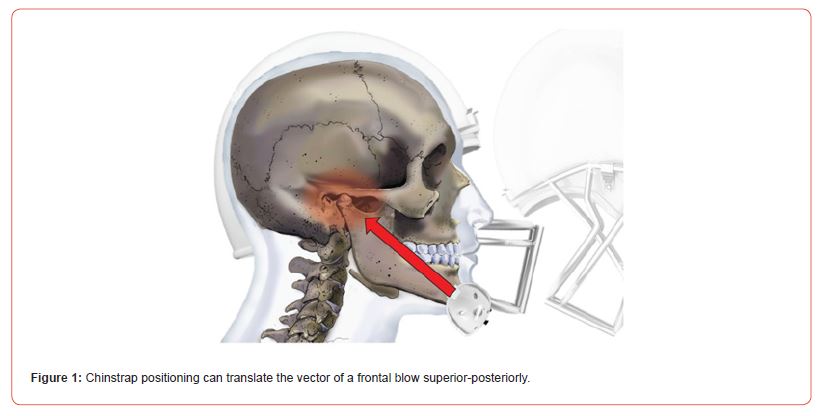
Football Helmets and TMJ Trauma: Helmeted sports transmit force through the mandible via the chinstrap. The amount of force from frontal blows to a football helmet through the mandible is between 600-800 Newtons, depending on the speed of impact [14]. Importantly, the chinstrap on the helmet can change the vector of force such that it travels directly through the TMJ apparatus, depending on the angle of impact (Figure 1).
Figure 1:Chinstrap positioning can translate the vector of a frontal blow superior-posteriorly.
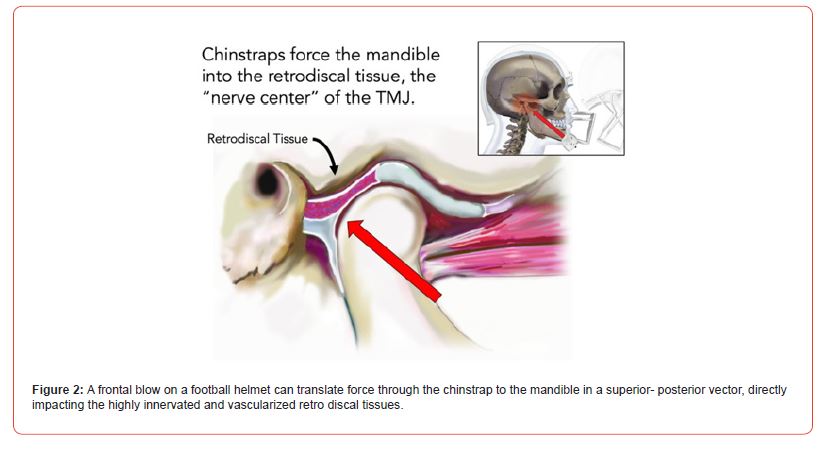
To better appreciate its significance, it is important to connect this concept within the context of TMJ anatomy. The highly innervated and vascularized portion of the TM joint is called the retro discal tissue, the posterior part of the articular disc in the glenoid fossa (Figure 2). Interestingly, histologic studies in animal models have shown the presence of calcitonin gene related protein (CGRP) in nerve bundles located in the retro discal tissue [15]. CGRP has been identified as one of the key intermediary molecules in the process of developing chronic pain [16]. The retro discal tissue may be intended as the “nerve center” of the TMJ complex and exhibits high intensity signal by magnetic resonance image (MRI) studies in subjects with acute TMJ pain [17]. The vector of force from a chinstrap to the mandible pushes the condylar head directly towards the retro discal tissue [18].
Figure 2:A frontal blow on a football helmet can translate force through the chinstrap to the mandible in a superior- posterior vector, directly impacting the highly innervated and vascularized retro discal tissues.
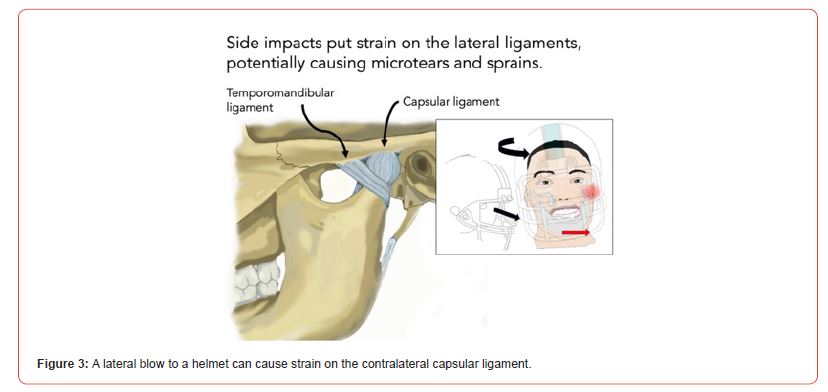
Helmeted sports have also an effect on the mandible in cases of lateral blows. The transmitted force typically falls in the range of 100-180 Newtons. Despite a lower force magnitude, lateral blows are directed against the contralateral capsular ligament, which itself is a highly innervated structure (Figure 3) [19].
Figure 3:A lateral blow to a helmet can cause strain on the contralateral capsular ligament.
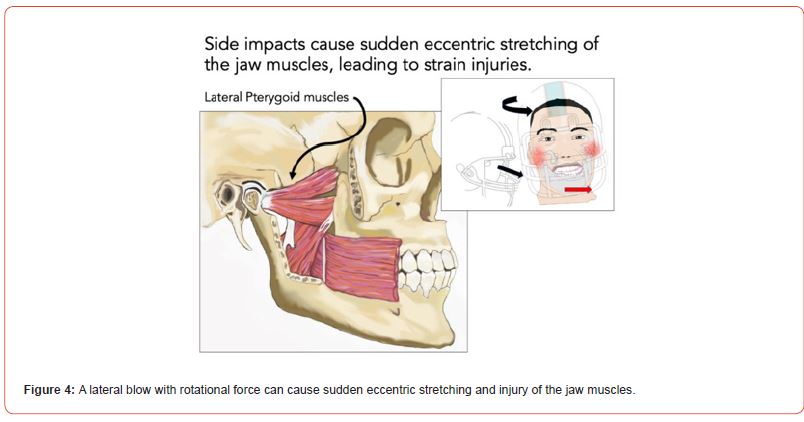
Furthermore, lateral blows may cause sudden eccentric stretching of the masticatory muscles and the TMJ ligaments (Figure 4). As a result of peripheral sensory input, neuroinflammatory mediators can be released in the trigeminal ganglion [20]. Eccentric stretching is also known to accelerate pain sensitivity after lowlevel clenching activity [21], so that lateral blows are as concerning as frontal blows. When we extrapolate these traumatic events to a typical football season with an estimated 1400 helmet impacts [1], repeated TMJ injury and TMD symptoms emerge.
Figure 4:A lateral blow with rotational force can cause sudden eccentric stretching and injury of the jaw muscles.
Centrally Mediated Neuromuscular Tension: The key element that links repetitive microtrauma to chronic pain is posttraumatic neuromuscular tension, typically manifested as muscle guarding or “protective co-contraction”. In orthopedic injuries, muscle guarding is an involuntary protective mechanism where the muscles tense at submaximal levels to immobilize the joint and prevent further injury. Post-traumatic muscle guarding is associated with muscle pain, stiffness, myofascial pain, and muscle spasm [22]. Experimental evidence shows that such increased muscle tone and reactivity can be a centrally mediated response to pain [23]. Similarly, the same can occur in the orofacial region in response to TMJ trauma and masticatory muscle strain, a condition that has been referred to as Jaw and Muscle Sprain/Strain (JAMSS) [24].
While muscle guarding starts as a protective mechanism, it may become itself a source and accelerant of persistent pain. For instance, in orthopedic sprain injuries studies, muscle guarding is experimentally defined as an increase in the muscle tone to 30% of its resting electromyographic (EMG) recordings [20]. If protective guarding in JAMSS injuries approximates this magnitude, it may negatively affect TMJ muscle physiology. To the best of our knowledge, to date there are no published studies of EMG tone after JAMSS injuries.
Muscle guarding and voluntary clenching are different phenomenon, but studies on voluntary clenching may provide perspective on the physiologic impact of the sustained submaximal contraction in the guarding response. For example, studies have shown that experimental clenching exercises may increase pain, fatigue, and stress scores [25] and may result in tender muscles and hyperalgesia [26]. In one study, normal subjects performing six clenching exercise for 5 minutes at 20% maximal force had shortterm increases in pain, fatigue, and mental stress [27]. Similar findings were observed in healthy males after 60-min clenching activity at 10% of maximal voluntary contraction [28]. An increase in fatigue and pain secondary to low-grade jaw clenching has been demonstrated in experimental conditions, but also in subjects exhibiting daytime parafunctional habits. For example, the intensity of daytime clenching has been observed to be increased in subjects with higher trait anxiety features [29]. Similarly, studies have shown that subjects with masticatory muscle pain have greater intensity and frequency of daytime jaw clenching compared to healthy individuals [30], when tested with EMG.
Reactive neuromuscular tension after injury may result in tissue hypoxia and develop myofascial tender trigger points. Experimental jaw clenching has been shown to disrupt muscle tissue oxygenation, thereby promoting muscle fatigue and myofascial pain [31]. This tissue hypoxia can drive muscular energy metabolism towards anaerobic glycolysis, which is less efficient and produces lactic acid and other metabolic byproducts. Tissue ischemia from jaw clenching can also increase levels of proinflammatory cytokines in muscle tissues, developing peripheral sensitization at motor endplates and myofascial pain [32]. This includes CGRP, whose release can be stimulated peripherally by lactic acid induced low-Ph environments [33]. We hypothesize that sustained post-traumatic submaximal neuromuscular tension may have the same physiologic effect.
Clenching and the pain cycle: Clenching of the teeth contributes to our understanding of why microtrauma to the jaw frequently leads to TMD development [34]. Once peripheral muscle inflammation is established, it triggers the process of peripheral sensitization of the myofascial structures and central sensitization, where pain thresholds and responses to normal stimuli become impaired. CGRP plays a key role in this process. As a result, this can lead to vicious cycle of sustain pain, referred to as “the pain cycle.”
When chronic pain persists, mechanisms such as peripheral sensitization induce neuroplastic changes in central pain processing centers, such that pain signaling becomes independent of peripheral sensory input. This is called “central sensitization”, and it is the hallmark of chronic pain syndromes. Central sensitization itself will then cause increased resting muscle tone, myofascial tenderness, regional and referred pain [35].
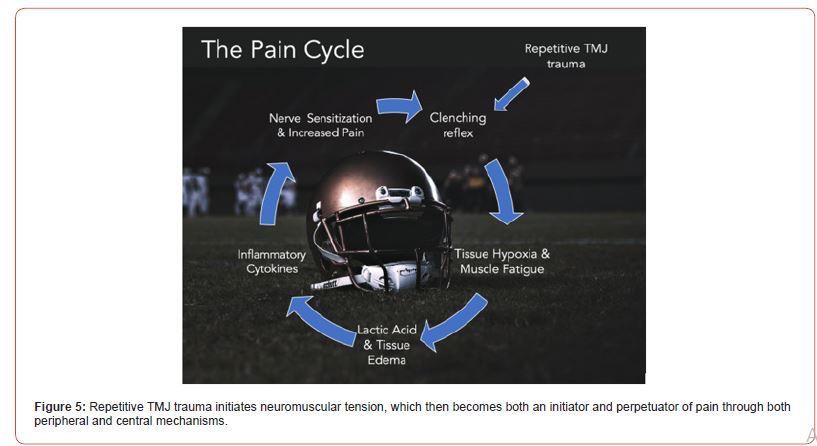
Here is a hypothetical sequence on how pain cycle develops and continues (Figure 5):
1. Significant macrotrauma or repetitive low-level microtrauma to the TMJ starts the cycle.
2. Reactive guarding and jaw clenching arise as an involuntary protective response by the masticatory jaw muscles.
3. Constant masticatory muscle tension leads to hypoxia and muscle fatigue.
4. Hypoxia drives the muscular energy metabolism towards anaerobic glycolysis which produces lactic acid and other metabolic byproducts and tissue edema.
5. The acidic environment increases the levels of proinflammatory cytokines in muscle tissues, further contributing to tissue edema.
6. Inflammatory cytokines, including CGRP, lead to peripheral nerve hypersensitivity. This peripheral sensitization is accompanied by a decreased pain threshold associated with myofascial trigger points.
7. Peripheral sensitization and increased pain levels then trigger more guarding, jaw clenching in the jaw and myofascial pain.
8. Decreases in inhibition of peripheral input to the central nervous systems leads to increase in central sensitization which causes the pain cycle to continue.
Figure 5:Repetitive TMJ trauma initiates neuromuscular tension, which then becomes both an initiator and perpetuator of pain through both peripheral and central mechanisms.
This line of reasoning is supported by the clinical association between neuromuscular tension and chronic TMD. For instance, when clenching behavior is reported both at night and at daytime, an individual is 40% more likely to have chronic TMD pain [36]. Further, according to a recent metanalysis, the resting activity of the temporalis and masseter muscles in those with TMD was suggested to be higher than controls, and range of motion and bite force are impaired [37]. This baseline increase in masticatory muscle activity is also seen in chronic headache conditions, such as tension type headaches [38]. In summary, neuromuscular tension can both initiate and sustain chronic pain, initially through peripheral sensitization and ultimately through central sensitization.
Neuromuscular Tension and Headaches: There appears to be a strong reciprocal relationship between clenching and headache [39]. For instance, clenching at night leads to significantly higher levels of orofacial pain and headache compared to controls [40]. Those who clench during the day are more than 2 times more likely to experience headache than controls [39]. When considering tension type headaches, brief experimental clenching exercises can induce a headache in around 70% of patients [41] and the presence of habitual awake clenching increases this likelihood by more than 5 times [42].
The anatomical connection between orofacial pain, headache, and neck pain is the trigeminovascular system and the trigeminocervical complex. This is particularly important for football players, as 85% report having headaches associated with playing the sport [3]. As noted above, when trauma-induced clenching triggers peripheral sensitization, it can lead to symptoms throughout the entire trigeminal system. Further, the entire muscular complex of the TMJ can be affected when one aspect of the apparatus is injured. For instance, if an irritant is injected into the temporalis tendon, the mechanical sensitivity of the surrounding masticatory muscles increases, as does the reporting of headache frequency [43]. Relief from orofacial neuromuscular tension can often reduce headache symptoms. For instance, therapies such as physical therapy addressing the jaw muscles and TMJ [44], or behavioral intervention focused on clenching awareness by relaxing the jaw while keeping the teeth apart can reduce headache intensity and frequency [45] in those with comorbid TMD and headache [46].
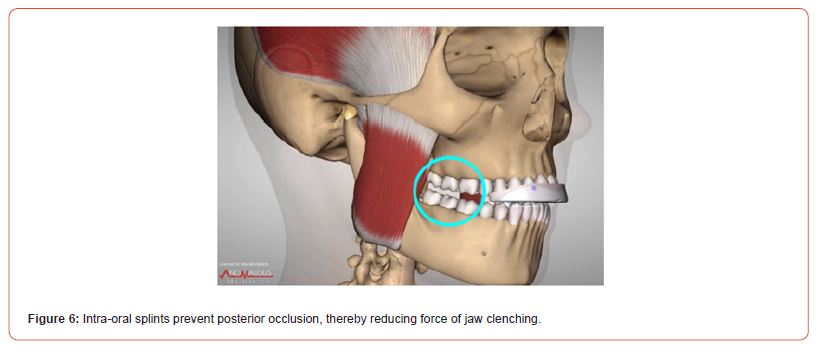
Intra-oral TMD splints: Intra-oral splints for TMD can also help reduce the forces of clenching. Even if occlusal appliances are normally recommended as nighttime treatment, football and other sports players may benefit from an oral splint used during their performance. It is known that during high-intensity performance the clenching activity is involuntary and sustained. A design of oral splint that may suit football and other sports players if they have a jaw injury includes the anterior bite plane (ABP) splint (Figure 6). When wearing this type of oral splint, only the anterior teeth are in contact. Thus, as the molars are the primary force transmission region of the jaw, neuromuscular tension and clenching force are effectively diminished [47]. Indeed, the anterior bite plane splint has an inhibitory effect on masseter muscle EMG activity according to clinical studies [48]. Additional encouraging results derived from a randomized clinical study that showed a reduction in headache when an anterior split was used in patients with chronic migraine, thus suggesting that these subjects may exhibit nocturnal clenching as contributing factor to their headache [49]. Nevertheless, future studies with larger sample size are advocated to replicate these findings.
Figure 6:Intra-oral splints prevent posterior occlusion, thereby reducing force of jaw clenching.
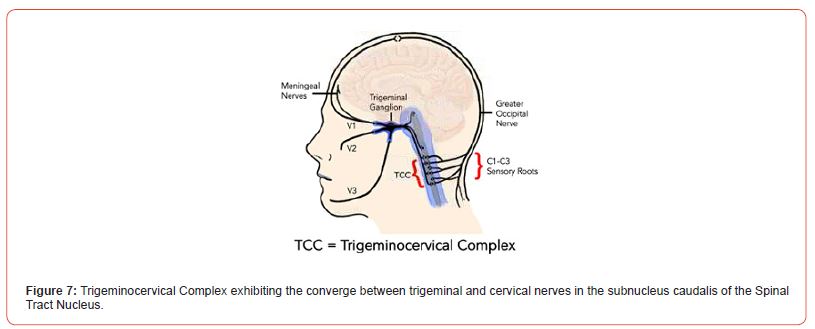
Clenching and Neck Pain: There is also a strong association between neck pain and TMD, where up to 70% of those with neck pain will have associated TMD symptoms [50]. In fact, neck pain severity will increase in tandem with TMD severity [51]. In people with TMD, there tends to be lower endurance in neck muscles, reduced neck mobility, and increased neck disability [52]. There are two complementary explanations for this connection. This first can be explained by trigeminocervical convergence and the phenomenon of referred pain. Convergence is when nerves from different areas converge in the same neuroanatomic space such that pain signals get mixed or confused [53]. In this case, convergence of trigeminal and cervical nerves occurs in the trigeminocervical complex (TCC) (Figure 7) in the brain stem [10]. From a clinical standpoint, this means that these subjects will report pain in the jaw or in the head (i.e., site of the pain), where instead the primary source of pain is within the neck muscles.
Figure 7:Trigeminocervical Complex exhibiting the converge between trigeminal and cervical nerves in the subnucleus caudalis of the Spinal Tract Nucleus.
However, convergence cannot explain how pain in the two related areas becomes independent. In this regard, the process of central sensitization is most likely the explanation. When strong or persistent sensory pain signals come from the head, neck, or jaw, they will stimulate microglial cells in the TCC, with subsequent release of CGRP [54]. This facilitates a neuroplastic response in higher cortical regions, such that the pain becomes chronic and independent of the initial stimulus. This helps understand the high comorbidity of chronic headaches, neck pain, and TMD [55,56].
Treatment Considerations. There are specific conservative measures that football players can take to prevent and address various types of TMD and orofacial pain. These self-care conservative measures can be adopted during or after games where particularly severe contact impacts occurred or when any orofacial pain symptoms are present.
• Oral TMD splint therapy: As mentioned above, the use of an intra-oral TMD splint, such as an ABP in the setting of acute injury can prevent microtrauma and acute injury by relaxing clenching activity. This in turn can facilitate general neuromuscular relaxation in the orofacial region [46].
• Cryotherapy: Icing the head, neck, and jaw region in the acute setting is a standard orthopedic therapy as it can decrease swelling, reduce nerve signal tracking, and provide comfort to muscles [57]. The inhibition of noxious C-fibers in favor of thermal A-delta fibers has also been hypothesized as a mechanism of action.
• Physical therapy: There are simple jaw physical therapy exercises that can be used to reduce orofacial tension and clenching activity. Gentle jaw stretching to maximum range of motion with tongue blades or knuckles between the incise teeth and postural exercise keeping the teeth apart, with the tongue relaxed is at the basis of every behavioral training program [42].
• Behavioral Therapy: Simple behavioral interventions are easy to learn and provide a key ingredient in holistic pain treatment. Two evidence-based techniques that are simple to learn include dispositional mindfulness [58] and deep breathing exercises [59].
• Jaw Rest: Jaw rest is a practical method of decreasing neuromuscular tension. This consists predominantly of dietary restrictions, such as avoiding hard and chewy foods in favor of a soft diet. Parafunctional habits such as chewing gum or biting nails should also be avoided. Jaw movements should be reduced to the pain-free limit.
• Over the Counter (OTC) analgesics: The use of a short-cycle of OTC analgesic medicines like ibuprofen or acetaminophen taken clock-regulated can help decrease overall levels of pain and reduce peripheral sensitivity and hyperalgesia [60,61].
• Muscle Trigger Point Injections: To reduce peripheral and central sensitization of myofascial trigger points, injections with local anesthetic or botulinum toxin with associated needling of the tender myofascial trigger point has also shown to decrease overall levels of pain level and muscle function long term [62-67].
Conclusion
The current review presented a framework for understanding the impact of repetitive low-level trauma and macrotrauma to the TMJ complex in American football and other impact sports that contribute the highly prevalent head, neck, and jaw complaints of its players. This is particularly important given the emerging evidence of the association between prolonged post-concussive symptoms and TMD symptoms. We also presented the mechanism for these injuries with findings that suggest reactive neuromuscular tension can initiate the pain cycle, and how both processes relate to peripheral and central sensitization and development of chronic pain. Finally, we relayed simple, conservative measures that can mitigate trauma-related orofacial pain. By raising awareness on the etiology and pathogenesis of these mechanisms, we hope to contribute to mitigate the high prevalence of TMD and orofacial pain in this subset of competitive athletes.
Acknowledgement
None.
Conflict of Interest
None.
References
- J Cournoyer, A Post, P Rousseau, B Hoshizaki (2016) The Ability of American Football Helmets to Manage Linear Acceleration with Repeated High-Energy Impacts. J Athl Train 51(3): 258-263.
- Vito Crincoli, Corrado De Biase, Angela Pia Cazzolla, Alessandra Campobasso, Mario Dioguardi, et al. (2022) Effects of Contact Sports on Temporomandibular Disorders: An Observational Study. Dent. J 10(10): 180.
- RE Sallis, K Jones (2000) Prevalence of headaches in football players. Med. Sci. Sports Exerc 32(11): 1820-1824.
- HC Freiwald, NP Schwarzbach, A Wolowski (2021) Effects of competitive sports on temporomandibular dysfunction: a literature review. Clin. Oral Investig 25(1): 55-65.
- Sonia Sharma, Jean Wactawski-Wende, Michael J LaMonte, Jiwei Zhao, Gary D Slade, et al. (2019) Incident injury is strongly associated with subsequent incident temporomandibular disorder: results from the OPPERA study. Pain 160(7): 1551-1561.
- O Plesh, S Adams, S Gansky (2011) Temporomandibular Joint and Muscle Disorder (TMJMD) - type pain and Co- morbid Pains in a National US Sample. J. Orofac. Pain 25(3): 190-198.
- J Botros, M Gornitsky, F Samim, Z der Khatchadourian, AM Velly (2022) Back and neck pain: A comparison between acute and chronic pain-related Temporomandibular Disorders. Can. J. Pain 6(1): 112-120.
- IE Tchivileva, R Ohrbach, RB Fillingim, JD Greenspan, W Maixner, et al. (2017) Temporal change in headache and its contribution to risk of developing cirst-onset TMD in the OPPERA study. Pain 158(1): 120-129.
- TT Nguyen, P Vanichanon, K Bhalang, S Vongthongsri (2019) Pain Duration and Intensity Are Related to Coexisting Pain and Comorbidities Present in Temporomandibular Disorder Pain Patients. Oral Facial Pain Headache 33(2): 205-212.
- R Castien, W De Hertogh (2019) A Neuroscience Perspective of Physical Treatment of Headache and Neck Pain. Front. Neurol 10: 276.
- (2023) E National Academies of Sciences et al., State of the Science on TMDs. National Academies Press (US) 2020.
- TP Dompier, Zachary Y Kerr, Stephen W Marshall, Brian Hainline, Erin M Snook, et al. (2015) Incidence of Concussion During Practice and Games in Youth, High School, and Collegiate American Football Players. JAMA Pediatr 169(7): 659-665.
- S Karpuz, R Yılmaz, H Yılmaz (2023) Evaluation of temporomandibular joint dysfunction in traumatic brain injury patients. J. Oral Rehabil 50(6): 476-481.
- S Rowson, DE McNeely, SM Duma (2008) Force transmission to the mandible by chin straps during head impacts in football. Biomed. Sci. Instrum 44: 195-200.
- Y Haeuchi, K Matsumoto, H Ichikawa, S Maeda (1999) Immunohistochemical demonstration of neuropeptides in the articular disk of the human temporomandibular joint. Cells Tissues Organs 164(4): 205-211.
- K Messlinger (2018) The big CGRP clood-sources, sinks and signalling sites in the trigeminovascular system. J. Headache Pain 19(1): 22.
- M Kuroda, M Otonari-Yamamoto, T Sano, M Fujikura, M Wakoh (2015) Diagnosis of retrodiscal tissue in painful temporomandibular joint (TMJ) by cluid-attenuated inversion recovery (FLAIR) signal intensity. Cranio J. Craniomandib. Pract 33(4): 271-275.
- (2015) UFO Themes 29: Temporomandibular Joint Imaging. Pocket Dentistry.
- A Sava, M Scutariu (2012) Functional anatomy of the temporo-mandibular joint (II). Rev. Med. Chir. Soc. Med. Nat. Iasi 116(4): 1213-1217.
- D Dessem, R Ambalavanar, M Evancho, A Moutanni, C Yallampalli, et al. (2010) Eccentric muscle contraction and stretching evoke mechanical hyperalgesia and modulate CGRP and P2X (3) expression in a functionally relevant manner. 149(2): 284-295.
- Y Tanabe (2021) Pain sensitivity after low-level clenching is incluenced by preloading eccentric exercise. Odontology 109(1): 29-40.
- S Hanlon, J Caccese, CA Knight, C Buz Swanik, TW Kaminski (2016) Examining Ankle-Joint Laxity Using 2 Knee Positions and With Simulated Muscle Guarding. J. Athl. Train 51(2): 111-117.
- JJ Stefanik, L Frey-Law, N A Segal, J Niu, C E Lewis, et al. (2020) The Relation of Peripheral and Central Sensitization to Muscle Co-contraction: The MOST Study. Osteoarthritis Cartilage 28(9): 1214-1219.
- J Fricton, B Eli, A Gupta, N Johnson (2016) Preventing chronic pain after acute jaw sprain or strain. J Am Dent Assoc 147(12): 979-986.
- Simple Futarmal Kothari, Meike Visser, Kimberley Timmerman, Lene Baad-Hansen, Michail Koutris, et al. (2021) Painful and non-painful symptoms evoked by experimental bracing and thrusting of the mandible in healthy individuals. J. Oral Rehabil 48(9): 1004-1012.
- A Dawson, T List, M Ernberg, P Svensson (2012) Assessment of proprioceptive allodynia after tooth-clenching exercises. J. Orofac. Pain 26(1): 39-48
- Tomoko Ikoma, Karina Haugaard Bendixen, Taro Arima, Andreas Dawson, Taihiko Yamaguchi, et al. (2018) Effects of Low-Intensity Contractions of Different Craniofacial Muscles in Healthy Participants-An Experimental Cross-Over Study. Headache 58(4): 559-569.
- P Svensson, A Burgaard, S Schlosser (2001) Fatigue and pain in human jaw muscles during a sustained, low- intensity clenching task. Arch. Oral Biol 46(8): 773-777.
- M Rofaeel, JCF Chow, I Ciofci (2021) The intensity of awake bruxism episodes is increased in individuals with high trait anxiety. Clin. Oral Investig 25(5): 3197-3206.
- I Ciofci, D Landino, V Donnarumma, T Castroclorio, F Lobbezoo, et al. (2017) Frequency of daytime tooth clenching episodes in individuals affected by masticatory muscle pain and pain-free controls during standardized ability tasks. Clin. Oral Investig 21(4): 1139-1148.
- Arata Tsutsui, Kazunori Nakajima, Takahiro Sakaue, Shinji Togo, Yoshiaki Matsuda, et al. (2022) Jaw-Clenching Intensity Effects on Masseter Oxygen Dynamics and Fatigue: A NIRS Oximetry Study. Adv. Exp. Med. Biol 1395: 435-441.
- S Louca Jounger, N Christidis, P Svensson, T List, M Ernberg (2017) Increased levels of intramuscular cytokines in patients with jaw muscle pain. J. Headache Pain 18(1): 30.
- X Wang, RR Fiscus (1997) Lactic acid potentiates bradykinin- and low-pH-induced release of CGRP from rat spinal cord slices. Am. J. Physiol.-Endocrinol. Metab 273(1): E92-E98.
- TB Just, SJ Schwulst (2021) Temporomandibular disorders and traumatic brain injury: Two sides of the same coin. Adv. Oral Maxillofac. Surg 4: 100193.
- V Evans, RGL Koh, FCK Duarte, L Linde, M Amiri, et al. (2021) A randomized double blinded placebo-controlled study to evaluate motor unit abnormalities after experimentally induced sensitization using capsaicin. Sci. Rep 11(1): 13793.
- Ira Sierwald, Mike T John, Oliver Schierz, Christian Hirsch, Darius Sagheri, et al. (2015) Association of temporomandibular disorder pain with awake and sleep bruxism in adults. J. Orofac. Orthop. Fortschritte Kieferorthopadie OrganOfQicial J. Dtsch. Ges. Kieferorthopadie 76(4): 305-317.
- A Dinsdale, Z Liang, L Thomas, J Treleaven (2021) Is jaw muscle activity impaired in adults with persistent temporomandibular disorders? A systematic review and meta-analysis. J. Oral Rehabil 48(4): 487-516.
- D Pietropaoli, E Ortu, M Giannoni, R Cattaneo, A Mummolo, et al. (2019) Alterations in Surface Electromyography Are Associated with Subjective Masticatory Muscle Pain. Pain Res. Manag: 6256179.
- Tatiana B Silva, Fernanda R Ortiz, Lucas M Maracci, Gabriela B P Silva, Rafaela S Salbego, et al. (2022) Association among headache, temporomandibular disorder, and awake bruxism: A cross- sectional study. Headache 62(6): 748-754.
- Fabíola Jardim Barbon, Yuri M Costa, Clarissa Delpizo Castagno, Ana Paula Perroni, Wellington Luiz de Oliveira da Rosa, et al. Sleep-related factors and orofacial pain symptoms associated with rhythmic masticatory muscle activity frequency scored by polysomnography recordings: A case-control study. Sleep Med 101: 461-467.
- R Jensen (1999) Pathophysiological mechanisms of tension-type headache: a review of epidemiological and experimental studies. Cephalalgia Int J Headache19(6): 602-621.
- Jéssica Conti Réus, Helena Polmann, Beatriz Dulcineia Mendes Souza, Carlos Flores-Mir, Paulo Cesar Trevisol Bittencourt, et al. (2021) Association Between Primary Headache and Bruxism: An Updated Systematic Review. J Oral Facial Pain Headache 35(2): 129-138.
- S Yang, FG Exposto, S Mahmoodi, P Svensson (2022) Mechanical sensitivity changes in pericranial muscles after local anesthesia and experimentally induced pain in the temporalis tendon: Implications for headache and facial pain. Cephalalgia Int J Headache 42(11-12): 1127-1137.
- Kazuhiko Hara, Takahiro Shinozaki, Akiko Okada Ogawa, Yumiko Matsukawa, Ko Dezawa, et al. (2016) Headache attributed to temporomandibular disorders and masticatory myofascial pain. J Oral Sci 58(2): 195-204.
- F Agha-Hosseini, N Sheykhbahaei, I Mirzaii-Dizgah, F Fatehi (2017) The efcicacy of oral habit modicication on headache. J. Korean Assoc. Oral Maxillofac. Surg 43(6): 401-406.
- V Donnarumma, A Michelotti, R Cimino, S Vollaro, I Ciofci (2022) Short-term Effects of a First-Line Treatment Including Counseling and Self-Management Strategies on Chronic TMD Muscle Pain and Awake Bruxism in Women. J. Oral Facial Pain Headache 36(1): 36-48.
- T Sun, DYR Chong, B Shao, Z Liu (2023) A deep dive into the static force transmission of the human masticatory system and its biomechanical effects on the temporomandibular joint. Comput. Methods Programs Biomed 230: 107336.
- L Baad-Hansen, F Jadidi, E Castrillon, PB Thomsen, P Svensson (2007) Effect of a nociceptive trigeminal inhibitory splint on electromyographic activity in jaw closing muscles during sleep. J Oral Rehabil 34(2): 105-111.
- AM Blumenfeld, JP Boyd (2022) Adjunctive treatment of chronic migraine using an oral dental device: overview and results of a randomized placebo-controlled crossover study. BMC Neurol 22(1): 72.
- A Silveira, IC Gadotti, S Armijo-Olivo, DA Biasotto-Gonzalez, D Magee (2015) Jaw Dysfunction Is Associated with Neck Disability and Muscle Tenderness in Subjects with and without Chronic Temporomandibular Disorders. BioMed Res Int: 512792.
- AD Sanchla, S Shrivastav, L Bharti, R Kamble (2022) Comparative Evaluation and Correlation of Pain Pattern in Neck Musculature Observed in Mild, Moderate, and Severe Temporomandibular Joint Disorder Cases as Compared to Non-temporomandibular Joint Disorder Cases. Cureus 14(10): e30099.
- AIS de Oliveira-Souza, JK de O Ferro, MMMB Barros, DA de Oliveira (2020) Cervical musculoskeletal disorders in patients with temporomandibular dysfunction: A systematic review and meta-analysis. J. Bodyw. Mov. Ther 24(4): 84-101.
- J Stedý, M Rocabado, LE Olate, M Vlna, R Ztižka (2022) Neural Basis of Etiopathogenesis and Treatment of Cervicogenic Orofacial Pain. Med. Kaunas Lith 58(10): 1324.
- Qi Pan, Yunfeng Wang, Ruimin Tian, Qianwen Wen, Guangcheng Qin, et al. (2022) Sphingosine-1 phosphate receptor 1 contributes to central sensitization in recurrent nitroglycerin- induced chronic migraine model. J. Headache Pain 23(1): 25.
- YM Costa, PCR Conti, FAC de Faria, LR Bonjardim (2017) Temporomandibular disorders and painful comorbidities: clinical association and underlying mechanisms. Oral Surg. Oral Med. Oral Pathol. Oral Radiol 123(3): 288-297.
- NV Latysheva, AS Platonova, EG Filatova (2019) Temporomandibular disorder and cervicalgia: pathophysiology underlying the comorbidity with chronic migraine. Zh. Nevrol. Psikhiatr. Im. S. S. Korsakova 119(1): 17-22.
- Bryce F Kunkle, Venkatraman Kothandaraman, Jonathan B Goodloe, Emily J Curry, Richard J Friedman, et al. (2021) Orthopaedic Application of Cryotherapy: A Comprehensive Review of the History, Basic Science, Methods, and Clinical Effectiveness. JBJS Rev 9(1): e20.00016.
- JM Wilson, I Haliwa, J Lee, NJ Shook (2023) The role of dispositional mindfulness in the fear-avoidance model of pain. PLOS ONE 18(1): e0280740.
- Amira E Joseph, Rajat N Moman, Ross A Barman, Donald J Kleppel, Nathan D Eberhart, et al. (2022) Effects of Slow Deep Breathing on Acute Clinical Pain in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Evid. -Based Integr. Med 27: 2515690X221078006.
- T Shimodaira, S Mikoshiba, T Taguchi (2019) Nonsteroidal anti-inclammatory drugs and acetaminophen ameliorate muscular mechanical hyperalgesia developed after lengthening contractions via cyclooxygenase-2 independent mechanisms in rats. PloS One 14(11): e0224809.
- C Yamaguchi, D Yamamoto, Y Fujimaru, T Asano, A Takaoka (2021) Acetaminophen Exerts an Analgesic Effect on Muscular Hyperalgesia in Repeated Cold-Stressed Rats through the Enhancement of the Descending Pain Inhibitory System Involving Spinal 5-HT3 and Noradrenergic α2 Receptors. Biol. Pharm. Bull 44(8): 1067-1074.
- Alvarez DJ, Rockwell PG (2002) Trigger points: A comprehensive review of their physiology and management. Pain Physician 5(3): 239-248.
- Schmid AB, Elliott MA (2011) The effect of trigger point injections on myofascial pain syndrome: a systematic review. Clinical Journal of Pain 27(6): 547-557.
- Gatchel RJ (2013) The impact of myofascial pain on the daily functioning of patients with chronic pain: A pilot study. Pain Physician. 16(2): E193-E202.
- Huisman M, Schots M (2012) Botulinum toxin A in the treatment of myofascial pain syndromes: Preliminary results from a clinical study. Journal of Pain Research 5: 549-555.
- Tepper SJ, Dahlof CG (2009) Botulinum toxin in the treatment of chronic migraines and myofascial pain. Headache: The Journal of Head and Face Pain 49(1): 166-175.
- Gatchel RJ (2013) The role of botulinum toxin in treating myofascial pain syndromes: A review. Archives of Physical Medicine and Rehabilitation 94(8): 1535-1548.
-
Bradley Eli, Vincent Fricton, Linda Sangalli, Dilip Dudhat and James Fricton*. Football Sport Injuries and the Temporomandibular Complex. Aca J Spo Sci & Med. 2(4): 2024. AJSSM.MS.ID.000542.
-
Sport Injuries, Temporomandibular Complex, Pain Cycle, TMJ Complex, Trauma, Helmeted Sports, Myofascial Pain
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






