 Review Article
Review Article
What Is the Potential Role of Exercise in Modulating the Gut Microbiota for Improving Bone Metabolism? A Mini-Review
Jie Li1, Rui Wang2; Jianda Kong1*
1School of Sports Science, Qufu Normal University, China
2Langfang traditional Chinese medicine hospital, China
Jianda Kong, School of Sports Science, Qufu Normal University, China.
Received Date:January 30, 2024; Published Date:February 06, 2024
Background: Recent research has shown that gut microbiota (GM) plays a significant role in bone health and metabolism. Exercise is considered
an important strategy in regulating GM and has positive effects on bone metabolism.
Objective: This review aims to explore how exercise improves bone health and metabolism through the regulation of GM and summarize the
contributions and existing issues in this field.
Conclusion: Through reviewing existing literature, we found that exercise can promote osteoblast differentiation, inhibit osteoblast apoptosis,
and improve osteoporosis (OP) by regulating GM. Additionally, exercise also regulates the abundance and diversity of GM, influences the generation
of metabolites produced by GM, and affects the nutritional metabolism of bone cells through these metabolites. However, the understanding of the
potential role of exercise in regulating GM to improve bone metabolism is still limited. Future research should further explore the regulatory effects
of different types and intensities of exercise on GM and conduct more human studies to validate the existing findings.
Keywords:Exercise; Gut Microbiota; Bone Metabolism; Osteoblast Differentiation; Osteoporosis
Introduction
Osteoporosis (OP) is a condition associated with excessive bone loss and fragility, significantly impacting an individual’s quality of life and health status. The health and stability of bones are regulated by the differentiation of bone cells and bone metabolism. However, recent research has indicated that the gut microbiota (GM) also plays a critical role in maintaining bone health and metabolism [1]. The GM is a community of microorganisms residing in the human intestinal tract, exerting a profound influence on human metabolism and immune function [2]. As an increasing body of evidence reveals the regulatory role of exercise on the GM, attention has turned to how exercise can enhance bone health and metabolism through the modulation of the GM.
Numerous studies have demonstrated that exercise can have a positive impact on bone metabolism through the regulation of the GM. Exercise has been found to play a crucial role in promoting osteoblast differentiation and activity, inhibiting osteoblast apoptosis, and improving OP [1-3]. Furthermore, exercise can modulate the abundance and diversity of the GM, influence the production of GM metabolites, and regulate bone cell nutritional metabolism through these metabolites [4-6]. However, the current understanding of the potential role of exercise in modulating the GM to improve bone metabolism remains limited. Further research is needed to delve into how different types of exercise affect the composition and function of the GM and how exercise regulates the association between the GM, endocrine function, and bone cell differentiation. Understanding the interaction between exercise and the GM and their impact on bone metabolism can lead to the development of novel strategies for the prevention and treatment of OP.
In summary, this review aims to explore the potential role of exercise in modulating the GM to improve bone metabolism. We will review existing literature, summarize the mechanisms through which exercise regulates the GM, and how this regulation affects bone cell differentiation, apoptosis, and nutritional metabolism. Additionally, we will discuss the current gaps in knowledge and propose future research directions to further elucidate the significance of the interaction between exercise and the GM on bone health.
Exercise regulates the GM to promote osteoblast differentiation
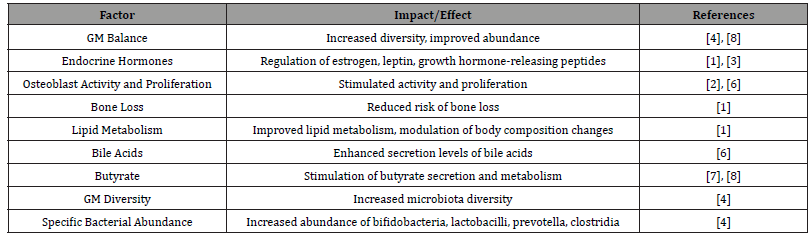
The role of exercise in regulating osteoblast differentiation has been investigated, with studies indicating its impact on GM balance, modulation of endocrine hormone levels, and stimulation of osteoblast activity and proliferation [1]. Japanese researchers conducted a 6-month trial involving a combined intervention of isoflavones and walking in postmenopausal women, revealing that the synergistic effects of isoflavones and walking positively influence lipid metabolism and body composition changes, effectively stimulating osteoblast activity and improving bone loss in postmenopausal Japanese women [1]. Furthermore, exerciseinduced leptin production can promote osteoblast proliferation [2]. Research suggests that aerobic exercise can enhance insulin sensitivity, regulate leptin levels, and reduce local and systemic endotoxin levels, thereby ameliorating gut dysbiosis and osteoarthritis [3].
QUEIPO-ORTUÑO et al. found that different dietary combinations with exercise can increase GM diversity, enhance the abundance of specific bacteria such as bifidobacteria, lactobacilli, prevotella, and clostridia, raise peripheral serum leptin levels, lower growth hormone-releasing peptide levels, thus improving GM balance, regulating host metabolism, and satiety [4]. These studies demonstrate that exercise can modulate the GM’s regulation of hormone secretion levels, including estrogen, leptin, and growth hormone-releasing peptides, to promote osteoblast differentiation. Additionally, bile acids and butyrate are important regulatory factors for promoting osteoblast differentiation. MEISSNER et al.’s research showed that voluntary exercise for 12 weeks can upregulate bile acid secretion levels. Exercise can also promote butyrate secretion, stimulating osteoblast differentiation. In comparison to sedentary mice, 8 weeks of running exercise can enrich the metabolism of propionate and butyrate in mice [6]. ALLEN et al. [7] conducted a 6-week aerobic exercise intervention with sedentary adults of different body types and found that aerobic exercise can increase the relative abundance of butyrate-producing bacteria and butyrate levels. Six weeks of endurance exercise can enhance the relative abundance of colon bacteria, Lachnospiraceae, Akkermansia, and Methanobacteriaceae in sedentary and overweight women, stimulating butyrate secretion [8]. These studies indicate that exercise can promote the enrichment of bile acids and butyrate, the metabolic products of GM, thus promoting osteoblast differentiation. In summary, walking contributes to the regulation of GM’s endocrine effects on osteoblast differentiation. However, current research on how exercise modulates GM and the levels of metabolic products affecting endocrine function and osteoblast differentiation remains somewhat limited. Further research is needed to explore this emerging field, especially in the context of cross-comparisons of different types of exercise in regulating GM for the promotion of osteoblast differentiation. We have created (Table 1) to describe the relationship between exercise, gut microbiota regulation, and osteoblast differentiation.
Table 1:Relationship between Exercise, Gut Microbiota Regulation, and Osteoblast Differentiation.
Exercise regulates the GM to suppress apoptosis of bone cells
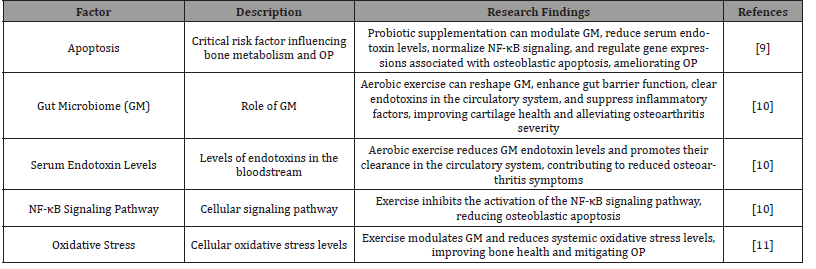
Apoptosis of bone cells constitutes a critical risk factor influencing bone metabolism and precipitating OP [9]. Inhibiting osteoblastic apoptosis holds significant implications for preventing bone loss. Research conducted by LEE et al. demonstrates that supplementation with fermented dairy products produced by probiotics can modulate the GM, reduce serum endotoxin levels, normalize the nuclear factor-κB (NF-κB) signaling pathway, and regulate gene expressions associated with osteoblastic apoptosis, thereby ameliorating OP [9]. This study underscores the potential of probiotics in modulating the stability of GM, reducing serum endotoxins and oxidative stress levels, suppressing the NF-κB signaling pathway, and ultimately inhibiting osteoblastic apoptosis.
Additionally, exercise can mediate the reduction of endotoxins in the GM, the expression of Toll-like receptor 4/nuclear factor- κB inflammatory signaling pathways, and the decrease in internal oxidative stress, thereby restraining osteoblastic apoptosis. Studies reveal that an eight-week running regimen can reshape the GM, enhance gut barrier function, augment the clearance of endotoxins in the circulatory system, suppress inflammatory factor expression, increase cartilage thickness, ameliorate cartilage degeneration, and alleviate the severity of osteoarthritis [10]. Consequently, aerobic exercise can regulate the diversity and abundance of the GM, improve gut barrier function, enhance the clearance of endotoxins in the circulatory system, and inhibit the activation of the NF-κB signaling pathway, thereby inhibiting osteoblastic apoptosis.
Furthermore, exercise-mediated GM can reduce systemic oxidative stress levels [11]. Current evidence substantiates that exercise can modulate the GM, and these microbial communities can impact oxidative stress within bones [11]. Simultaneously, the GM can regulate oxidative stress in bones to ameliorate OP [12]. In summary, exercise not only reduces serum endotoxin and oxidative stress levels and inhibits osteoblastic apoptosis by modulating the GM, but also improves gut barrier function, enhances endotoxin clearance, and suppresses the activation of the NF-κB signaling pathway. Consequently, exercise exerts a positive impact on preventing OP. (Table 2) shows factors Affecting Osteoporosis and Their Implications.
Table 2:Factors Affecting Osteoporosis and Their Implications.
Exercise modulation of gut flora inhibits osteoclast differentiation
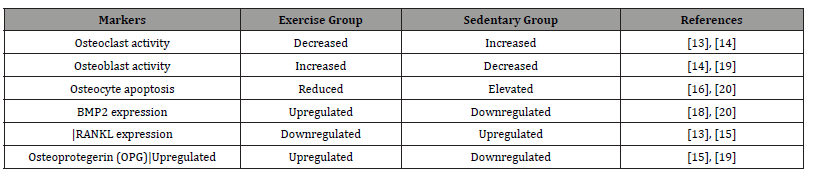
Exercise plays a critical role in regulating the GM in inhibiting osteoclast differentiation. Inhibiting the proliferation and differentiation of osteoclasts is an effective method to reduce bone absorption and prevent OP, with the balance of GM playing a pivotal role in this process [13]. Studies have indicated that the ratio of Firmicutes to Bacteroidetes is a significant factor influencing osteoclast differentiation [13]. A study conducted by McCabe et al. [14] established an obese mouse model, revealing that after 14 weeks of voluntary wheel running, the ratio of Firmicutes to Bacteroidetes decreased, the abundance of GM from the Lactobacillaceae family increased, bone mass increased, skeletal mechanical properties improved, and bone loss decreased. This study suggests that through walking exercise, we can modulate microbial composition, reduce the Firmicutes to Bacteroidetes ratio, and thus prevent bone loss caused by a high-fat diet.
Furthermore, exercise can also regulate the stability of the GM, elevate levels of short-chain fatty acids, and modulate the immune system, thereby inhibiting osteoclast differentiation. Maintaining the balance between regulatory T cells (Tregs) and Th17 cells is crucial for maintaining bone homeostasis. Research has demonstrated that Treg cells can directly inhibit osteoclast differentiation, whereas Th17 cells stimulate osteoclast differentiation by inducing local inflammation [15,16]. Researchers have also found that different types of exercise have varying effects. Chen et al.’s study found that strength training helps enhance the stability of the GM, strengthen the intestinal barrier function, mediate neuroimmune regulation, suppress Th17 cell differentiation, and increase the number of Treg cells, thereby alleviating the severity of experimental autoimmune encephalomyelitis and lymphocyte infiltration [17]. Additionally, short-chain fatty acids can regulate Treg cell differentiation, promote the secretion of interleukin-4, interleukin-10, and transforming growth factor β1, thus inhibiting osteoclast formation [18]. Endurance exercise also plays a significant role by improving the stability of the GM, promoting the secretion of short-chain fatty acids, and thereby suppressing inflammation and immune cell chemotaxis [19]. Moreover, a study found a noticeable difference in the GM between professional rugby players and sedentary individuals, highlighting the role of rugby training in regulating GM metabolites, specifically short-chain fatty acids [20].
In summary, different types of exercise can elevate the secretion of short-chain fatty acids, regulate the Th17: Treg cell ratio, and consequently inhibit osteoclast differentiation, preventing bone loss. Thus, moderate exercise is of great significance in regulating GM and inhibiting osteoclast differentiation. Various exercise modalities mediate the regulation of GM through different mechanisms to achieve this goal. (Table 3) shows comparison of bone cell differentiation and apoptosis markers between exercise and sedentary groups.
Table 3:Comparison of bone cell differentiation and apoptosis markers between exercise and sedentary groups.
Exercise Regulation of GM to Regulate Bone Cell Nutrient Metabolism
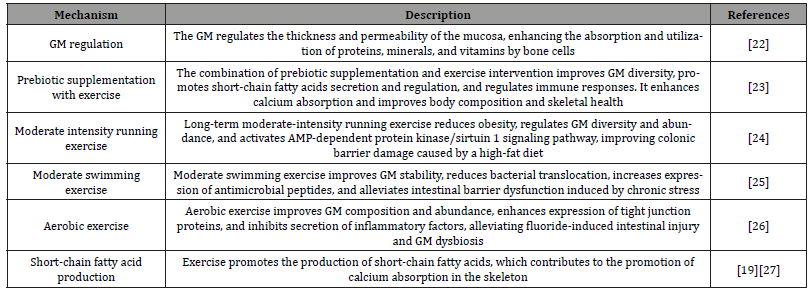
It is crucial to alter the GM to regulate the nutritional metabolism of bone cells, as a deficiency in calcium and vitamin D can lead to poor skeletal development [21]. Exercise plays an important role in this process, as it helps regulate the GM, enhance gut barrier function, and promote the absorption of nutrients by bone cells. Studies have found that the GM can regulate the thickness and permeability of the mucosa, improve gut barrier function, and consequently enhance the absorption and utilization of proteins, minerals, and vitamins, which contributes to the improvement of bone cell nutritional metabolism [22].
A randomized controlled trial on humans revealed that supplementation with prebiotics combined with exercise intervention helps improve the diversity of the GM, promote the secretion and regulation of short-chain fatty acids, and regulate immune responses. This intervention can also lower the pH value of the gut, thereby enhancing calcium absorption and ultimately improving body composition and skeletal health [23]. Furthermore, long-term moderate-intensity running exercise significantly reduces obesity in mice, regulates the diversity and abundance of the GM, and effectively activates the AMP-dependent protein kinase/sirtuin 1 signaling pathway, thus improving colonic barrier damage caused by a high-fat diet [24]. Moderate swimming exercise also has a positive impact on the stability of the GM, reducing bacterial translocation, increasing the expression of antimicrobial peptides, and alleviating intestinal barrier dysfunction induced by chronic stress [25]. Aerobic exercise improves the composition and abundance of GM, enhances the expression of tight junction proteins, and inhibits the secretion of inflammatory factors, thereby alleviating fluoride-induced intestinal injury and GM dysbiosis in mice [26].
Furthermore, the secretion of short-chain fatty acids contributes to the promotion of calcium absorption in the skeleton [87], and exercise promotes the production of short-chain fatty acids [19] [27]. This series of mutually reinforcing processes helps maintain skeletal health by regulating the GM and improving the nutritional metabolism of bone cells, ensuring sufficient absorption of calcium and vitamin D, and effectively preventing skeletal problems. Table 4 shows Mechanisms of exercise-regulated bone cell nutrient metabolism.
Table 4:Mechanisms of exercise-regulated bone cell nutrient metabolism.
Conclusions and Future Directions
Previous Contributions and Outstanding Issues in the Field
In previous research, many scholars have explored the regulatory effects of exercise on GM and bone metabolism. The findings of these studies have revealed the positive impact of exercise on promoting osteoblast differentiation, inhibiting osteoblast apoptosis, and regulating osteoblast nutritional metabolism. Furthermore, early studies have also indicated that alterations in GM homeostasis can affect the balance between bone resorption and bone formation. However, these studies still face certain limitations, such as sample restrictions, inconsistent study designs, and varied responses to different types and intensities of exercise. Therefore, further research is needed to validate the findings from previous studies and address these issues.
Unique Characteristics of the Author’s Review
This review differs from previous studies in several aspects. Firstly, it focuses on the regulatory effects of exercise on GM, with an emphasis on elucidating how exercise influences bone metabolism through the modulation of GM. Secondly, this review incorporates the latest research developments and compares the effects of different types of exercise on GM and osteoblasts. Additionally, this review proposes future research directions and recommendations to further advance the field.
Limitations of the Review
However, this review also has certain limitations. Firstly, due to the relative novelty of the research field, the current body of research is still limited, and our understanding of the mechanisms underlying the regulatory effects of exercise on GM and bone metabolism remains limited. Secondly, this review mainly focuses on animal experiments and a few human studies, thus requiring further human studies to validate the specific impacts of exercise on the human body.
Significance of the Review
The significance of this review lies in highlighting the interaction between exercise and GM and their impact on bone metabolism. Understanding the mechanisms of this interaction can provide deeper insights into the regulation of bone health and metabolism, as well as offer new strategies for the prevention and treatment of OP.
Recommendations for Future Research
Given the relatively limited research on the interaction between exercise and GM, future studies can be further explored in the following aspects: Firstly, further exploration should be conducted on the regulatory effects of different types and intensities of exercise on GM, as well as the differential responses to exercise among different populations. Secondly, more human studies are needed to validate the findings from existing animal experiments and gain a deeper understanding of the impacts of exercise on human bone metabolism. Moreover, larger-scale prospective studies can be conducted to evaluate the potential benefits of long-term exercise on GM balance and skeletal health.
Funding
No funding sources were used for this study.
Authors’ contributions
JL, RW and JK conducted literature searches, while JK wrote and revised the review.
Acknowledgement
We would like to thank all those who provided assistance throughout the project.
Conflict of interest
No conflict of interest.
References
- Jian Wu, Jun Oka, Mitsuru Higuchi, Izumi Tabata, Toshiya Toda, et al. (2006) Cooperative effects of isoflavones and exercise on bone and lipid metabolism in postmenopausal Japanese women: a randomized placebo-controlled trial. Metabolism 55(4): 423-433.
- J Cornish, K E Callon, U Bava, C Lin, D Naot, et al. (2002) Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J Endocrinol 175(2): 405-415.
- Rios JL, Bomhof MR, Reimer RA, Hart DA, Collins KH, et al. (2019) Protective effect of prebiotic and exercise intervention on knee health in a rat model of diet-induced obesity. Sci Rep 9(1): 3893.
- MI Queipo-Ortuño, LM Seoane, M Murri, M Pardo, JM Gomez-Zumaquero, et al. (2013) Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS One 8(5): e65465.
- Meissner M, Lombardo E, Havinga R, Tietge UJ, Kuipers F, et al. (2011) Voluntary wheel running increases bile acid as well as cholesterol excretion and decreases atherosclerosis in hypercholesterolemic mice. Atherosclerosis 218(2): 323-329.
- Chen See JR, Amos D, Wright J, Lamendella R, Santanam N, et al. (2022) Synergistic effects of exercise and catalase overexpression on gut microbiome. Environ Microbiol 24(9): 4220-4235.
- Jacob M Allen, Lucy J Mailing, Grace M Niemiro, Rachel Moore, Marc D Cook, et al. (2018) Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med Sci Sports Exerc 50(4): 747-757.
- Eveliina Munukka, Juha P Ahtiainen, Pere Puigbó, Sirpa Jalkanen, Katja Pahkala, et al. (2018) Six-Week Endurance Exercise Alters Gut Metagenome That Is Not Reflected in Systemic Metabolism in Over-weight Women. Front Microbiol 9: 2323.
- Chul SL, Byoung KK, IO Lee, NH Park, SH Kim, et al. (2020) Prevention of bone loss by using Lactobacillus-fermented milk products in a rat model of glucocorticoid-induced secondary osteoporosis. Int Dairy J 109: 104788.
- Kefeng Li, Anli Liu, Wenhao Zong, Lulu Dai, Yang Liu, et al. (2021) Moderate exercise ameliorates osteoarthritis by reducing lipopolysaccharides from gut microbiota in mice. Saudi J Biol Sci 28(1): 40-49.
- Orla O'Sullivan, Owen Cronin, Siobhan F Clarke, Eileen F Murphy, Micheal G Molloy, et al. (2015) Exercise and the microbiota. Gut Microbes 6(2): 131-136.
- Ding K, Hua F, Ding W (2020) Gut Microbiome and Osteoporosis. Aging Dis 11(2): 438-447.
- Yuan Y, Yang J, Zhuge A, Li L, Ni S, et al. (2022) Gut microbiota modulates osteoclast glutathione synthesis and mitochondrial biogenesis in mice subjected to ovariectomy. Cell Prolif 55(3): e13194.
- LR McCabe, R Irwin, A Tekalur, C Evans, JD Schepper, et al. (2019) Exercise prevents high fat diet-induced bone loss, marrow adiposity and dysbiosis in male mice. Bone 118: 20-31.
- Zhu L, Hua F, Ding W, Ding K, Zhang Y, et al. (2020) The correlation between the Th17/Treg cell balance and bone health. Immun Ageing 17: 30.
- FL Yuan, Xia Li, WG Lu, RS Xu, YQ Zhao, et al. (2010) Regulatory T cells as a potent target for controlling bone loss. Biochem Biophys Res Commun 402(2): 173-176.
- H Chen, L Shen, Y Liu, X Ma, L Long, et al. (2021) Strength Exercise Confers Protection in Central Nervous System Autoimmunity by Altering the Gut Microbiota. Front Immunol 12: 628629.
- YW Zhang, MM Cao, Y Juan Li, RL Zhang, MT Wu, et al. (2022) Fecal microbiota transplantation as a promising treatment option for osteoporosis. J Bone Miner Metab 40(6): 874-889.
- Huang WC, Tung CL, Yang YSH, Lin IH, Ng XE, et al. (2022) Endurance exercise ameliorates Western diet-induced atherosclerosis through modulation of microbiota and its metabolites. Sci Rep 12(1): 3612.
- W Barton, NC Penney, O Cronin, IG Perez, MG Molloy, et al. (2018) The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut 67(4): 625-633.
- Uday S, Högler W (2017) Nutritional Rickets and Osteomalacia in the Twenty-first Century: Revised Concepts, Public Health, and Prevention Strategies. Curr Osteoporos Rep 15(4): 293-302.
- Charlotte AA, Kurtis FB, Henry MG, Saima FR, Jacqueline EM, et al. (2021) Impact of diet and the bacterial microbiome on the mucous barrier and immune disorders. Allergy 76(3): 714-734.
- Ilesanmi-Oyelere BL, Roy NC, Kruger MC (2021) Modulation of Bone and Joint Biomarkers, Gut Microbiota, and Inflammation Status by Synbiotic Supplementation and Weight-Bearing Exercise: Human Study Protocol for a Randomized Controlled Trial. JMIR Res Protoc 10(10): e30131.
- Wang J, Zhang Q, Xia J, Sun H (2022) Moderate Treadmill Exercise Modulates Gut Microbiota and Improves Intestinal Barrier in High-Fat-Diet-Induced Obese Mice via the AMPK/CDX2 Signaling Pathway. Diabetes Metab Syndr Obes 15: 209-223.
- Luo B, Xiang D, Nieman DC, Chen P (2014) The effects of moderate exercise on chronic stress-induced intestinal barrier dysfunction and antimicrobial defense. Brain Behav Immun 39: 99-106.
- Rong Fu, Ruiyan Niu, Fangye Zhao, Jixiang Wang, Qiqi Cao, et al. (2022) Exercise alleviated intestinal damage and microbial disturbances in mice exposed to fluoride. Chemosphere 288(Pt 3): 132658.
-
Jie Li, Rui Wang; Jianda Kong*. What Is the Potential Role of Exercise in Modulating the Gut Microbiota for Improving Bone Metabolism? A Mini-Review. Aca J Spo Sci & Med. 1(5): 2024. AJSSM.MS.ID.000521.
-
Microbiota, Osteoporosis, Exercise, Bone Metabolism, Health Status, Apoptosis, Osteoblast Differentiation
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
- Abstract
- Introduction
- Materials and Methods
- The Basic Tools of Scientific Inquiry
- Literature Review
- Theoretical Framework
- Research Design
- Findings and Discussion
- Conclusion and Recommendations
- Based on the findings, the following recommendations are considered:
- Declaration Statements
- Funding
- Data Availability Statement (DAS)
- Compliance with Ethical Standards
- Acknowledgement
- Conflict of Interest
- References






