 Research Article
Research Article
Recent Chemical Candidates for Tackling Antibiotics- Resistance Crisis Caused by Metallo-β-Lactamases
Yoonsik Park*
Department of Biological Sciences, Konkuk University, 120 Neungdong-ro, Gwangjin-gu, Seoul 05029, Korea
Yoonsik Park, Department of Biological Sciences, Konkuk University, 120 Neungdong-ro, Gwangjin-gu, Seoul 05029, Korea
Received Date: September 09, 2021; Published Date: September 22, 2021
Abstract
Antibiotic resistance had been one of the major risks of the healthcare system since early 2000 [1]. After the early 1980s, the number of new antibiotics developed and approved is kept on decreasing while the number of antibiotic resistant strains are on increase due to overuse of antibiotics and international human and animal mobility [2]. When the number of clinically available antibiotics is limited by antibiotic resistance, co-administrating conventional antibiotics with compounds that reverse resistance is an effective approach [3]. In this regard, many β-lactamase inhibitors (BLIs), which reverse resistance against one of the most widely used β-lactam antibiotics [4], are developed. However, until now there is no effective inhibitor for metallo-β-lactamases (MBLs), which have a distinct mechanism and structure from serine-β-lactamases (SBLs). This is a problem when there is a global prevalence of MBL producing Enterobacteriaceae and P. aeruginosa strains [5]. In this mini-review article, we will look into some of the most recent (2019-2021) researches regarding MBL inhibitor development to facilitate researches and help find new research objectives. Compounds were sorted into categories according to the criteria of main mechanism of action. Compound’s characteristics were summarized with emphasis on the mechanism of action, β-lactam (carbapenem) potentiating activity, and measured/expected cytotoxicity.
Keywords:Metallo-β-lactamase, MBL, β-lactamase, β-lactamase Inhibitor, BLI, Antibiotics
Abbreviations: Metallo-β-Lactamase, BLI – Β-Lactamase Inhibitor, NDM – New delhi MBL, VIM - Verona integron MBL, IMP – Imipenemase, SBL – Serine-β-Lactamase, PBP – Penicillin Binding Protein, OM – Outer membrane, CRE - Carbapenem-Resistant Enterobacteriaceae, SPM - São Paulo MBL, GIM – German Imipenemase, CphA – Carbapenem hydrolyzing and first (A) from Aeromonas hydrophila, L1 – Labile enzyme, OXA - Active on oxacillin, B1, B2, B3 – β-lactamase of Ambler Class B subclass 1, 2, 3, MPC – Minimum Potentiating Concentration, MIC – Minimum Inhibitory Concentration, MBC – Minimum Bactericidal Concentration, PK, PD – Pharmaco-Kinetics/Dynamics, AMA - Aspergillomarasmine A, PMPCs – 6-Phosphonomethylpyridine-2-carboxylates, DM- N-[2-(dansylamino)ethyl] maleimide, CAMP – Cyclic Antimicrobial Peptide, SLAY - Surface Localization Antimicrobial display, MTT assay – Tetrazolium dye assay. Used to measure cytotoxicity in hRBC in mentioned article, BC – Red blood cell, MMP - matrix metalloproteinases, SICLOPPS - split intein mediated circular ligation of peptides and proteins, PEP4 - Peptidomimetic 4, MCR - mobilized colistin resistance, (Meropenem susceptibility) Breakpoint – By CLSI definition, MIC bigger than 4 mg/L for Enterobacteriaceae, and 8 mg/L for P. aeruginosa strains. By EUCAST definition, MIC bigger than 8 mg/L for both strains
Introduction
Carbapenem is a derivative of beta-lactam antibiotics which is favored for their broad-spectrum inhibition capability. The reason why the trend of increasing carbapenem resistance is so daunting is that carbapenem is treated to the patient who has contracted the strains resistant to other types of beta-lactam antibiotics [6]. One of the most commonly encountered carbapenemase producing strains is Enterobacteriaceae. Enterobacteriaceae is the common cause of most community and hospital-acquired infection cases [7]. Research documenting enzymes that Carbapenem-Resistant Enterobacteriaceae (CRE) produces had shown that the most widely discovered carbapenemase worldwide was New Delhi MBL-1 (NDM-1), an enzyme from MBL subclass 1 [8]. Metallo-β-lactamase (MBL) is the subclass of carbapenemase that differed from the serine- β-lactamase (SBL) which uses serine as catalytic residue. Unlike SBL, there is no clinically available inhibitor to MBLs [9]. Combined with the fact that recently elucidated mechanism of bacteria utilizing conjugative plasmids for spreading antibiotic resistance across species [6], metallo-β-lactamase poses a real threat to the modern healthcare system. The difficulties of developing effective MBL inhibitors center around the fact that MBL has structurally different subclasses, having zinc ion as the catalytic site which troubles designing suicidal inhibitor and has rather flexible active site loop which makes it difficult to ensuring tight binding of inhibitors. This article’s purpose is to document recent attempts to overcome the difficulties and facilitates the development of clinically available MBL inhibitors.
Main body
Cyclic Boronate
The early attempt to develop cyclic boronate derivative inhibitors made vaborbactam (RPX7009). Unlike the bicyclic boronates listed below, it was monocyclic and has only moderately inhibited MBLs [10]. However, it did provide a great starting point for looking for unconventional, non-acylating inhibitors [11]. This type of inhibitor work through binding to MBL active site and prevent substrate entry into the active site. In some cases, it follows conformation change by reaction between cyclic boronate and enzyme bound hydroxide. Though this type of β-lactamase inhibitor (BLI) also inhibits SBLs (Ambler class A, C, and D), this article will mainly discuss inhibition capacity against MBLs [12].
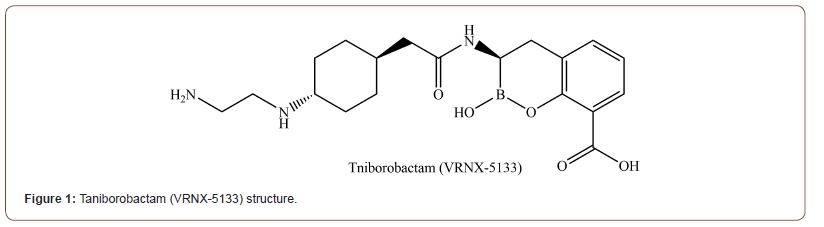
Taniborobactam [12]
Taniborobactam (VRNX-5133) (Figure 1) has a distinct mechanism to SBL and MBL enzymes. While it inhibits SBL by forming a reversible covalent bond with the boronate chain, it also inhibits MBL by working as a competitive inhibitor. Unlike currently clinically available vaborbactam, taniborobactam can inhibit clinically important MBLs (SPM-1, GIM-1, NDM-1/5/7, and VIM-1/2/4) as well as SBLs. It reacts with the hydroxide anion coordinated by 2 zinc ions of MBL B1 to forms tetrahedral geminal diol formation which mimics the structure of transient tetrahedral intermediate in β-lactam breakdown reaction [13]. It inhibits NDM-1 and VIM-2 utilizing conserved Asn233, 120, and Glu149 residues as well as the interaction between zinc and boronate. However, Taniborobactam still has limits. Though it effectively inhibits most clinically important B1 MBLs, it cannot inhibit clinically important IMP-1, B2, B3 MBLs (CphA and L1). Notably, it is rendered bicyclic as well as tricyclic in crystal structure bound to NDM-1 (PDB ID: 6RMF) [14]. Tricyclic conformation seems to be the result of sp2 oxygen of the acylamido group reacting with boronate. Its role in binding affinity and target preference is not known.
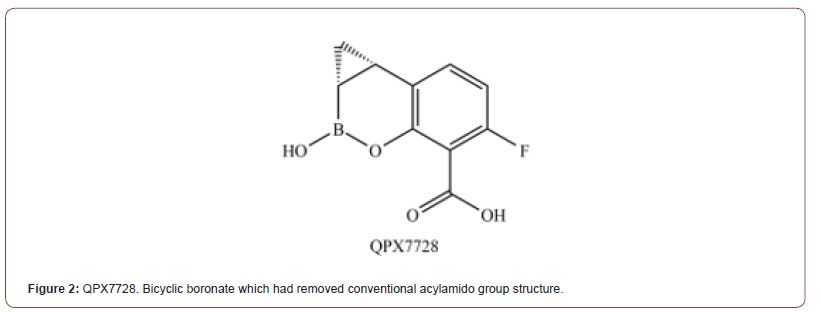
QPX7728 [15]
Although many structures had been tested while developing promising candidates and clinically available BLI, little had been done in respect to substituents on aromatic rings. Hecker and 16 others had experimented on various substitutions in the article about QPX7728 (Compound 35) (Figure 2). They’ve delved into the effect of substituting largely unmodified regions on the aromatic ring. The most notable founding of thiss experiment was the potency of the BLI which has thioester group instead of acylamido group. Thioester group had shown overall better minimum potentiating concentration (MPC) when used with other carbapenems against MBLs variants compared to the Taniborobactam. Including the B2, B3, and IMP-1 (B1) which Taniborobactam failed to inhibit efficiently. The other notable improvements from the previous cyclic boronates were the reduced oligomerization inside the aqueous solution and reduced efflux rate by host efflux pump (MexAB-OprM of P.aeruginosa). However, even though it had not inhibited human metalloproteases, protective groups and unconventional substituents can have unexpected effects on pharmacokinetics as shown in the case of compound 17B, which had been suspended due to the long-living metabolite in the system.
Metal Chelators
As MBLs activity requires coordinated zinc ions, the idea of using chelators as MBL inhibitors had arisen at the early phase of MBL inhibitor design. Metal chelators inhibit MBL by removing zinc from the active site or through sequestration of zinc ions released from MBL [16]. EDTA was considered as a candidate [17] of this class but consider not suitable for clinical use for its high toxicity [18]. Other metal chelating agents like dipicolinic acid (DPA) are also under clinical survey for further refinement [19]. However, using metal chelator had met with medical challenges as it has various effects on human metalloproteins like carbonic anhydrase, angiotensin- converting enzyme (ACE), matrix metalloproteinase (MMP), and other proteins with zinc coordinating motif [20]. Hence reducing side effects on human metalloenzymes while having enough chelation activity to deactivate MBL is required.
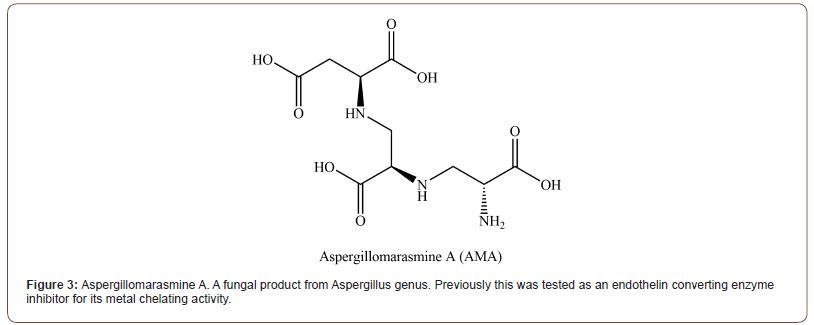
Aspergillomarasmine A (AMA)[21]
AMA (Figure 3) is a natural fungal product from Aspergillus genus [22]. Its chemical isolation was conducted back in 1965, but its capacity as an MBL inhibitor had been discovered in recent years [23,24]. It is still not clear whether AMA makes direct interaction with the enzyme. However, the experiment result indicates that AMA works in a mechanism similar to L-captopril. AMA inhibits NDM-1 in an indirect way which sequesters spontaneously dissociated Zn2+ ions from binding sites with lower affinity (Zinc binding site with Asp 124, Cys 208, and His 250), instead of directly sequestering ions from the enzyme active site. About half the molar equivalent of Zn2+ was release even at the point of total enzyme activity loss. It seems it utilizes the transient state of zinc binding site 2 (ZBS2) [25]. This is backed up by the biotin-maleimide and N-[2-(dansylamino)ethyl] maleimide (DM) assay, which reacts with exposed cysteine at the zinc binding side 2 of NDM-1. It has shown a similar Kd (0.20.04 nM) to zinc ion with EGTA (0.6 nM), a wide range chelator. Even though AMA has a relatively low affinity amongst chelators, AMA has narrow selectivity toward Zn, Ni, and Co divalent ions. This allowed 8 μg/ml of AMA to inhibit NDM-1 in the presence of other metal ions and restores Meropenem sensitivity (2μg/ ml) in vitro. Ki of AMA in zinc depleted medium against NDM-1 was 11 nM, and 7 nM against VIM-2 [23]. However, AMA’s activity as BLI is varied upon the concentration of zinc in medium, enzyme stability, and the affinity of the enzyme toward Zn2+. 10μM of ZnSO4 did reverse 90% of NDM-1 inhibition and while IC50 against NDM-1 was between 8 μg/ml to 16 μg/ml, more stable and tightly bound IMP-7 shown little change up to 64 μg/ml of AMA. The narrow selectivity of AMA confers relatively low toxicity against animals (Intravenous LD50 = 160 mg/kg in mice) when compared to EDTA (28.5 mg/kg), a strong zinc chelator (Kd = 10−9 M). However, as this experiment was related to the inhibition of endothelin converting enzyme, which plays an important role in bET-l-induced responses such as sudden death, hypertension, and hemoconcentration [18]. Hence it has a limit to compare on par with other BLIs.
Peptide/Peptidomimetic
After depleting most of the natural sources for search over the potent leading molecule, research moved into a more novel pool of substances to find potent antimicrobial leading molecules [26]. In 1994, β-lactamase inhibitor protein (BLIP) had been reported [27]. And in 2013, BLIP-II had been reported [28]. While these proteins’ inhibition activity is mainly against SBLs, it had precedented the use of peptides as a β-lactamase inhibitor. The biggest pro of this approach is the vast size of possible combinations of amino acids. While the biggest con is the limited mode of administration, acquiring enough stability of peptide until it reaches the target molecule, and acquiring enough selectivity [29].
NDM-1 inhibiting peptides discovered with SLAY assay [30]
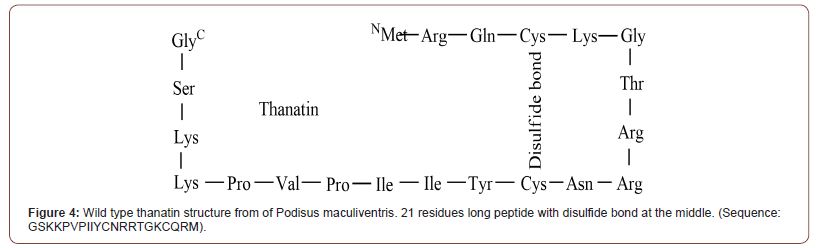
Misha I. Kazi and 12 others from university of Texas used Surface Localization Antimicrobial display (SLAY) to search 1700 random decapeptide sequences for antimicrobial activity. Among the 9 most potentiating peptide adjuvants, P5 (sequence: LRCLMLKFPI) had shown the highest Minimum Bactericidal Concentration (MBC, determined by 99.90% of the killing of initial inoculums) on tested NDM-1 producing strains with 4 mg/L of meropenem. MBC goes as low as 4 μM to 8 μM depending on the strain. Ki value was lowest on P6 (0.11 μM, sequence : CLRPSIISRA). Enzyme kinetic assay suggests among the 4 most meropenem-potentiating peptides, 3 had shown competitive inhibition whiles 1 had shown non-competitive inhibition. Interestingly, except for P4 (sequence: ASVTWLLYAM), all 8 peptide has positive charge like cationic antimicrobial peptide (CAMP), but when treated against NDM-producing E.coli, peptide had limited effect on the increase of cell membrane permeability. This indicates that these peptides have a different mechanism of action from the CAMPs, which embed into bacterial membrane and form pores to have bactericidal effect [31]. Cytotoxicity and hemolytic effect of the peptides were tested with MTT assay on human RBC. Among the 4 most potentiating agents, none had shown notable toxicity except for P7 (sequence: WRYQWTILFI), which had shown dose-dependent cytotoxicity against HEK293 at 256 μM. Thanatin (Figure 4) is a pathogen-inducible single-disulfide- bond-containing β-hairpin AMP which was first isolated from the insect Podisus maculiventris [33]. Even though our focus is on BLI capacity, thanatin has its own bactericidal capacity. And in sub- MIC dosage, thanatin acts as an adjuvant by displacing zinc ions from the NDM-1 active site by interacting with protein directly. The antimicrobial activity is caused by the removal of divalent cations which bridge the negatively charged phosphate group of lipopolysaccharides (LPS), the staple substance of gram-negative bacterial outer membrane (OM). And it seems there is an alternate mode of action in the above MIC dose by inhibiting LptA and LptD which limits LPS transportation in turn [34,35]. In sub-MIC dose, thanatin can work as BLI through direct interaction with MBL. It is not clear which residue that thanatin interacts with, peptides with a similar mode of action did interact with Trp93 and Asp124 in the case of PEP4 [36] and pterostilbene [37]. Though the article lacks structural data, inductively coupled plasma-mass spectrometry (ICP-MS) confirmed the release of the zinc ions from NDM-1 in vitro setup when treated with thanatin. This was supported with microscale thermophoresis (MST) experiment results. Kd value of apo NDM- 1 to thanatin (0.71 ± 0.06 μM) was approximately 10 times lower than Kd of NDM-1 to zinc divalent ions (7.36 ± 0.45 μM). When its cytotoxicity was tested against human and mice cell lines (human umbilical vein endothelial cells, pulmonary alveolar epithelial cells, and mouse neuron cells) at 200 μM, cytotoxicity was far much lower compared to the colistin. Considering that MIC of thanatin was about 0.4 to 0.32 μM, much lower toxicity is expected in therapeutic circumstances.
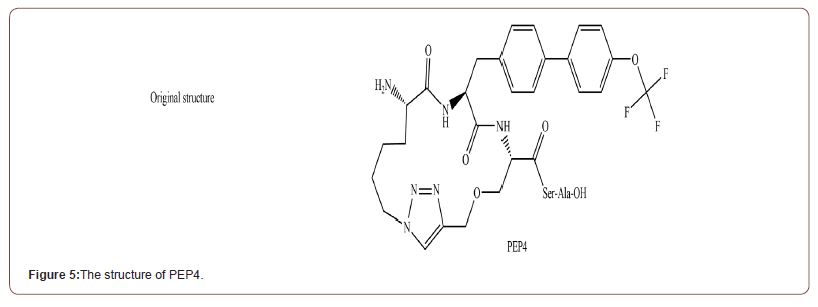
Peptidomimetic 4 (PEP4) [36]
PEP4 (Figure 5) is the cyclic analogue of Pentapeptide (Ile-Met- Ile-Ser-Phe) which had been known to inhibit matrix metalloproteinases (MMP), which is structurally similar to NDMs [38]. It has high membrane permeability and high stability in serum due to the cyclization [39]. PEP4 alone did not show any bactericidal effect and its effect as an adjuvant was restricted to the strains producing MBL, supporting direct inhibition of MBL. The mechanism of action is still being studied, but the Molecular dynamic (MD) simulation based on molecular docking suggests it works via Van der Waals interaction between the Trp93, His122, Gln 123, and Asp 124 residues at the active site of NDM-5 with -10.8 Kcal/mol binding ener- gy. In in vivo experiment, the meropenem/PEP4 treatment (both 16 mg/kg) increased the survival rate of NDM-producing E.coli infected mice from 0% to 70% at 10 days post-infection. When tested in vitro setup, PEP4 had shown IC50 of 5.926 μg/ml against NDM-5. As the original pentapeptide is known to inhibit MMP, which have various role in human body, cytotoxicity assay was conducted against Vero, CHO and HEK293T mammalian cell lines. The result showed a low level of cytotoxicity at the high concentration (10% at the 128 μg/ml).
Other Type of Compound
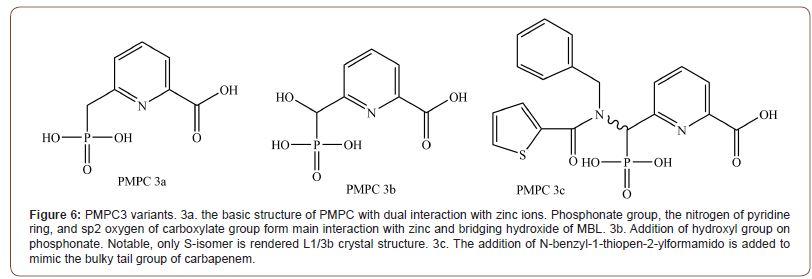
6-Phosphonomethylpyridine-2-carboxylates (PMPCs) [40]
Even though phosphonate was observed bound to zinc ions in many MBL crystal structures, the utility of phosphonate as a zinc binding group had not been explored thoroughly. Hence Hinchliffe and his team have designed PMPCs to explore the potential of phosphonate as a zinc binding group. PMPC (Figure 6) is consisting of 3 structures, the phosphonate group, pyridine, and carboxylate group. It interacts with B1 and B3 MBLs in a different manner. Against B1, it interacts without displacing any component in the active site. Carboxylate and nitrogen from the pyridine ring interact with Zn2, while phosphonate reacts with bound hydroxide (PDB ID: 5EV6). In B3, However, PMPCs displaced both Zn2 and hydroxide ion, with phosphonate group and pyridine nitrogen and carboxylate making direct interaction with Ser 221, 223 (PDB ID: 1SML). But this starkly different mode of binding is different from what the enzyme kinetic equation suggested. It might be due to the much higher concentration of inhibitors in the crystallization situation. Notably, 3b bound to the L1 was all S-isomer, indicating that adding hydroxyl group in the R-isomer form is highly not recommendable. Even though enzyme kinetics generally indicated it follows competitive inhibition, the inhibition rate was differed by the preincubation time. As the replotted graph of [I] and Kobs followed linear graph, stepwise dehydration of active site seems to be the cause. Amongst PMPC variants, PMPC 3, Which has a carboxylate group at the C2 and phosphonomethyl group at the C6 of pyridine ring had shown the best potential. Especially 3c, which has a hydrophobic group on the phosphonomethyl group had shown the best potentials against tested B1 MBLs (NDM-1, IMP-1). IC50 range of 0.306~2.91 μM and Ki range of 0.034~2.91 μM. PMPC3 variant was also effective against tested B3 MBL (L1, observed IC50 1.48~2.05 μM), but additional hydrophilic/phobic group on the phosphonomethyl group had not shown much difference. However, unlike the in vitro experiment, when tested against clinically isolated strains, PMPC 3a (the one without additional group on phosphonomethyl group) had shown the best potentiating effect as PMPC 3b and 3c failed to reduce E. coli UWB75 and Pseudomonas spp.’s meropenem MIC below 8 mg/L even with 128 mg/L of inhibitor concentration. PMPC was affected only slightly by the host efflux pump. The potentiating effect was differed only by 2-fold in resistance nodulation division (RND) efflux pump overexpression strain and knockout strain. No noticeable cytotoxicity was observed until the concentration required to lower the meropenem MIC below the breakpoint in tested cell lines (Human epithelial Caco-2, HEPG2, and rat H4IIE).
PEP4 (Figure 5) is the cyclic analogue of Pentapeptide (Ile-Met- Ile-Ser-Phe) which had been known to inhibit matrix metalloproteinases (MMP), which is structurally similar to NDMs [38]. It has high membrane permeability and high stability in serum due to the cyclization [39]. PEP4 alone did not show any bactericidal effect and its effect as an adjuvant was restricted to the strains producing MBL, supporting direct inhibition of MBL. The mechanism of action is still being studied, but the Molecular dynamic (MD) simulation based on molecular docking suggests it works via Van der Waals interaction between the Trp93, His122, Gln 123, and Asp 124 residues at the active site of NDM-5 with -10.8 Kcal/mol binding energy. In in vivo experiment, the meropenem/PEP4 treatment (both 16 mg/kg) increased the survival rate of NDM-producing E.coli infected mice from 0% to 70% at 10 days post-infection. When tested in vitro setup, PEP4 had shown IC50 of 5.926 μg/ml against NDM-5. As the original pentapeptide is known to inhibit MMP, which have var ious role in human body, cytotoxicity assay was conducted against Vero, CHO and HEK293T mammalian cell lines. The result showed a low level of cytotoxicity at the high concentration (10% at the 128 μg/ml).
Conclusion
Importance of Zinc binding site 2 in NDM-1 and VIM-1
The loss of zinc observed from thanatin treated NDM-1 had shown half of the zinc loss per molecule whereas the treatment resulted in almost total inactivation of the enzyme [32]. It might indicate that thanatin treatment affects mainly the weaker binding site of 2 zinc binding site of NDM-1. As this type of impartial effect (either by indirect sequestration or direct displacement) on Zn2 coordination had been observed repeatedly, [16,23,40] focusing on Zinc binding site 2 (ZBS2) might be a valid approach. However, I have to admit that there’s rapid mutation ongoing on ZBS2, resulting in higher thermostability and zinc binding affinity of ZBS2 [41], and there is a conflicting report about AMA’s mode of action, reporting 1.8 molar ratio of zinc loss per molecule [23].
Inhibition characteristics of cyclic boronates against MBL
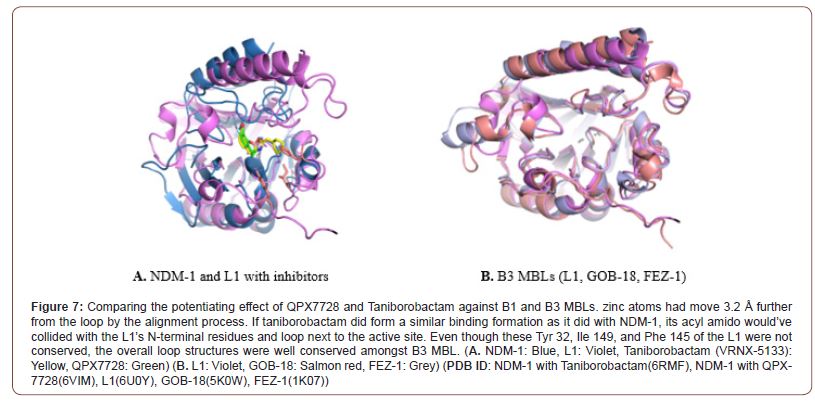
QPX 7728 had shown a better potentiating effect compared to the taniborobactam against the B3 MBLs. Whereas showing a similar level of effect against B1 MBLs [42]. It seems like the substitution of the acylamido group with a much smaller structure have played important role in binding to the 2 different active sites without steric hindrance. As Structural alignment shows acylamido group of taniborobactam cannot fit into the binding site of the B3 MBLs which have a different loop structures around the active site. (Figure 7) And even though most of the attention is gathered around the bicyclic boronate candidates, monocyclic Vaborbactam had shown better performance against the fugitive targets like B2, B3, and IMP-1 variant compared to the Taniborobactam [10]. Leaving the possibility of a binary solution for further refinement in structure.
General side effects by molecular types
Quite a lot of the zinc binding MBL inhibitor candidates were also tested for ACE and MMP inhibitors, including captopril [43], AMA [18], and other metal chelators. Other research indicates that carboxypeptidase and carbonic anhydrase (mammalian metalloenzymes) [44-46], was also inhibited by MBL inhibitor candidates. Cyclic boronate inhibitors did not show particular toxicity in recent reviews, but clinically approved vaborbactam phase I review indicates a mild level of lethargy [47]. Peptide’s off-target bindings are hard to be mentioned in general terms due to the wide range of structure variety, but cationic peptides can have hemolytic side effects by the same pathway as CAMPs.
Acknowledgment
Thanks for encouraging me to publish article and filling me in with editorial practice. (Mr. Hong SH)
Conflict of interest
No conflict of interest.
References
- Bax R (2001) Surveillance of antimicrobial resistance--what, how and whither? Clin Microbiol Infect. 7(6): 316-325.
- Ventola C L (2015) The antibiotic resistance crisis: part 1: causes and threats. P T. 40(4): 277-283.
- Venter H (2019) Reversing resistance to counter antimicrobial resistance in the World Health Organisation's critical priority of most dangerous pathogens. Biosci Rep 39(4).
- Bush, K. and P.A. Bradford (2016) beta-Lactams and beta-Lactamase Inhibitors: An Overview. Cold Spring Harb Perspect Med. 6(8).
- Kazmierczak K M (2016) Multiyear, Multinational Survey of the Incidence and Global Distribution of Metallo-beta-Lactamase-Producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 60(2): 1067-78.
- T J B de Man (2021) Multispecies Outbreak of Verona Integron-Encoded Metallo-ss-Lactamase-Producing Multidrug Resistant Bacteria Driven by a Promiscuous Incompatibility Group A/C2 Plasmid. Clin Infect Dis 72(3): 414-420.
- Ben-Ami R (2009) A multinational survey of risk factors for infection with extended-spectrum beta-lactamase-producing enterobacteriaceae in nonhospitalized patients. Clin Infect Dis 49(5): 682-690.
- Logan L K and R A Weinstein (2017) The Epidemiology of Carbapenem-Resistant Enterobacteriaceae: The Impact and Evolution of a Global Menace. J Infect Dis. 215(suppl_1): S28-S36.
- Paterson D L (2006) Resistance in gram-negative bacteria: enterobacteriaceae. Am J Med. 119(6 Suppl 1): p. S20-S8; S62-S70.
- Langley, G.W (2019) Profiling interactions of vaborbactam with metallo-beta-lactamases. Bioorg Med Chem Lett 29(15): 1981-1984.
- Krajnc A (2019) Will morphing boron-based inhibitors beat the beta-lactamases? Curr Opin Chem Biol 50: 101-110.
- Hamrick J C (2020) VNRX-5133 (Taniborbactam), a Broad-Spectrum Inhibitor of Serine- and Metallo-beta-Lactamases, Restores Activity of Cefepime in Enterobacterales and Pseudomonas aeruginosa. Antimicrob Agents Chemother 64(3).
- Page M L. A Badarau (2008) The mechanisms of catalysis by metallo beta-lactamases. Bioinorg Chem Appl.
- Krajnc A (2019) Bicyclic Boronate VNRX-5133 Inhibits Metallo- and Serine-beta-Lactamases. J Med Chem 62(18): 8544-8556.
- Hecker S J (2020) Discovery of Cyclic Boronic Acid QPX7728, an Ultrabroad-Spectrum Inhibitor of Serine and Metallo-beta-lactamases. J Med Chem 63(14): 7491-7507.
- Wade N (2021) Mechanistic Investigations of Metallo-beta-lactamase Inhibitors: Strong Zinc Binding Is Not Required for Potent Enzyme Inhibition*. ChemMedChem 16(10): 1651-1659.
- Chen P (2012) 2-Substituted 4,5-dihydrothiazole-4-carboxylic acids are novel inhibitors of metallo-beta-lactamases. Bioorg Med Chem Lett 22(19): 6229-32.
- Matsuura A (1993) Pharmacological profiles of aspergillomarasmines as endothelin converting enzyme inhibitors. Jpn J Pharmacol. 63(2): 187-193.
- Chen A Y (2017) Dipicolinic Acid Derivatives as Inhibitors of New Delhi Metallo-beta-lactamase-1. J Med Chem 60(17): 7267-7283.
- Somboro A M (2018) Diversity and Proliferation of Metallo-beta-Lactamases: a Clarion Call for Clinically Effective Metallo-beta-Lactamase Inhibitors. Appl Environ Microbiol 84(18).
- Sychantha D (2021) Aspergillomarasmine A inhibits metallo-beta-lactamases by selectively sequestering Zn(2). J Biol Chem 297(2): 100918.
- Zhang J (2017) Synthesis and biological evaluation of Aspergillomarasmine A derivatives as novel NDM-1 inhibitor to overcome antibiotics resistance. Bioorg Med Chem 25(19): 5133-5141.
- King A M (2014) Aspergillomarasmine A overcomes metallo-beta-lactamase antibiotic resistance. Nature 510(7506): 503-506.
- Von Nussbaum F, G Schiffer (2014) Aspergillomarasmine A, an inhibitor of bacterial metallo-beta-lactamases conferring blaNDM and blaVIM resistance. Angew Chem Int Ed Engl. 53(44): 11696-11698.
- Thomas P W (2011) Characterization of purified New Delhi metallo-beta-lactamase-1. Biochemistry 50(46): 10102-10113.
- Mahlapuu M (2016) Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front Cell Infect Microbiol.
- Strynadka N C (1994) Structural and kinetic characterization of a beta-lactamase-inhibitor protein. Nature 368(6472): 657-60.
- Brown N G (2013) Identification of the beta-lactamase inhibitor protein-II (BLIP-II) interface residues essential for binding affinity and specificity for class A beta-lactamases. J Biol Chem 288(24): 17156-17166.
- Muttenthaler M (2021) Trends in peptide drug discovery. Nat Rev Drug Discov 20(4): 309-325.
- Kazi M I (2020) Discovery and characterization of New Delhi metallo-beta-lactamase-1 inhibitor peptides that potentiate meropenem-dependent killing of carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 75(10): 2843-2851.
- Falanga A (2017) Cyclic Peptides as Novel Therapeutic Microbicides: Engineering of Human Defensin Mimetics. Molecules 22(7).
- Ma B (2019) The antimicrobial peptide thanatin disrupts the bacterial outer membrane and inactivates the NDM-1 metallo-beta-lactamase. Nat Commun 10(1): 3517.
- DashnR , S Bhattacharjya (2021) Thanatin: An Emerging Host Defense Antimicrobial Peptide with Multiple Modes of Action. Int J Mol Sci 22(4): 1522.
- Vetterli S U(2018) Thanatin targets the intermembrane protein complex required for lipopolysaccharide transport in Escherichia coli. Sci Adv 4(11): eaau2634.
- Moura E (2020) Thanatin Impairs Lipopolysaccharide Transport Complex Assembly by Targeting LptC-LptA Interaction and Decreasing LptA Stability. Front Microbiol.
- Liu Y (2019) Repurposing Peptidomimetic as Potential Inhibitor of New Delhi Metallo-beta-lactamases in Gram-Negative Bacteria. ACS Infect Dis 5(12): 2061-2066.
- Liu S (2019) Pterostilbene restores carbapenem susceptibility in New Delhi metallo-beta-lactamase-producing isolates by inhibiting the activity of New Delhi metallo-beta-lactamases. Br J Pharmacol 176(23): 4548-4557.
- Singh D (2015) Multifaceted role of matrix metalloproteinases (MMPs). Front Mol Biosci.
- Joo S H (2012) Cyclic peptides as therapeutic agents and biochemical tools. Biomol Ther (Seoul) 20(1): 19-26.
- Hinchliffe P (2018) Structural and Kinetic Studies of the Potent Inhibition of Metallo-beta-lactamases by 6-Phosphonomethylpyridine-2-carboxylates. Biochemistry 57(12): 1880-1892.
- Cheng Z (2018) Evolution of New Delhi metallo-beta-lactamase (NDM) in the clinic: Effects of NDM mutations on stability, zinc affinity, and mono-zinc activity. J Biol Chem 293(32): 12606-12618.
- Parkova A (2020) Broad Spectrum beta-Lactamase Inhibition by a Thioether Substituted Bicyclic Boronate. ACS Infect Dis 6(6): 1398-1404.
- Brem J (2016) Structural Basis of Metallo-beta-Lactamase Inhibition by Captopril Stereoisomers. Antimicrob Agents Chemother 60(1): 142-150.
- Docquier J.D (2010) High-resolution crystal structure of the subclass B3 metallo-beta-lactamase BJP-1: rational basis for substrate specificity and interaction with sulfonamides. Antimicrob Agents Chemother 54(10): 4343-4351.
- Roll D M (2010) Inhibition of metallo-beta-lactamases by pyridine monothiocarboxylic acid analogs. J Antibiot (Tokyo) 63(5): 255-257.
- Wang X (2015) Discovery of novel new Delhi metallo-beta-lactamases-1 inhibitors by multistep virtual screening. PLoS One.
- Griffith D C (2016) Phase 1 Study of the Safety, Tolerability, and Pharmacokinetics of the beta-Lactamase Inhibitor Vaborbactam (RPX7009) in Healthy Adult Subjects. Antimicrob Agents Chemother 60(10): 6326-32.
-
Yoonsik Park. Recent Chemical Candidates for Tackling Antibiotics-Resistance Crisis Caused by Metallo-β-Lactamases. Acad J Microbiol & Immun. 1(2): 2020. AJMI.MS.ID.000506.
-
Antibiotics, Carbapenem, Boronate, Phosphonomethyl, Thanatin
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






