 Mini Review
Mini Review
The Basic Principles of Biomedical and Medical Science Study Designs
Alphonsus Ogbonna Ogbuabor*, Edwin Obiorah Ohotu and Luke Jideofor Ugwu
Department of Medical Laboratory Sciences, Faculty of Basic Medical Sciences, College of Health Sciences, Enugu State University of Science and Technology, Nigeria
Alphonsus Ogbonna Ogbuabor, Department of Medical Laboratory Sciences, Faculty of Basic Medical Sciences, College of Health Sciences, Enugu State University of Science and Technology, Nigeria.
Received Date: February 24, 2023; Published Date: March 27, 2023
Abstract
In biomedical and medical research studies, useful conclusions can only be inferred from data derived using a valid study design. Therefore, the selection of an appropriate study design is pertinent in providing unbiased, error free and scientific evaluation of the hypothesis. The present review is intended to serve as a guide to our teaming beginners in Biomedical and Medical Science research.
Keywords: Research; Beginners; Medicine
Introduction
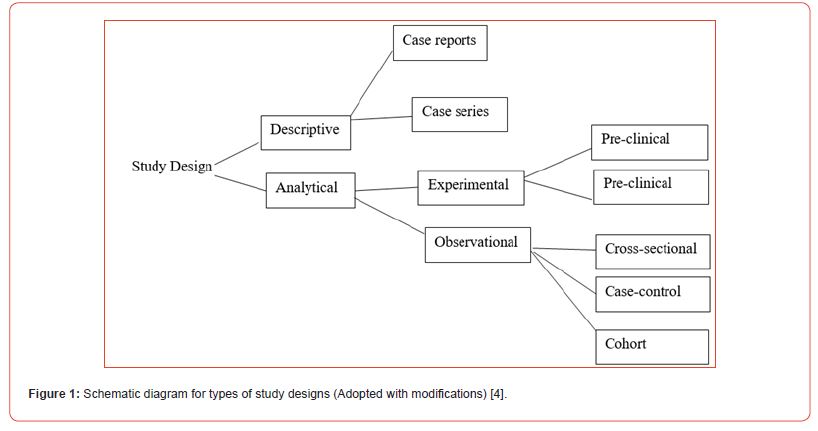
Study design is the set of evidence-based procedures that provide the framework for conducting research. They could be generally classified into two broad groups, namely descriptive and analytical studies [1-3].
Descriptive studies
These are studies which describe specific characteristics in a population of interest. The most common forms are (a) case reports; in which we discuss our experience with a particular patient’s presentations and management. (b) case series; in which we discuss our experience with several patients with similar presentations and their management as a group [4].
Analytical studies
These are sub-classified into two groups, namely experimental studies and observational studies. Experimental studies are studies we conduct with interventions using randomization or blinding. Randomization refers to the process of assigning subjects randomly to either the treatment or control groups to equally distribute the demographic and clinical variables in the study subjects. Blinding on the other hand, is a process that prevents subjects and the research team from having prior knowledge about the interventions in the subjects [4-6].
A good example of experimental study design are pre-clinical trials (experimental studies involving animals) and clinical trials (experimental studies involving humans). The primary advantage of experimental studies over observational studies is that it provides stronger evidence of an association and potential causality between outcome and predictor variables through randomization or blinding [4]. On the other hand, observational studies are studies that we conduct without intervention. The subtypes of observational studies include. (a) Cross sectional studies; in which case, the researcher takes a specific sample at a specific time without any follow-up. This allows researchers to determine the frequency of disease (prevalence) or the frequency of a risk factor. For example, if we want to determine the frequency of lung cancer in a population, we can conduct a cross-sectional study whereby we take a sample from the population and calculate the numbers of individual who were diagnosed positive for lung cancer in the population. (b) Cohort study; in which two samples from the study population are compared by the researcher. One sample has a risk factor while the other lacks the risk factor. Those with the risk factor are compared to those without the risk factor. For example, to find out the relative risk for developing lung cancer among smokers, we took a sample including smokers and non-smokers. Then we calculate the number of individuals with lung cancer among both. Cohort studies may take a prospective approach developed we follow the subjects in the future to know who developed the disease) or retrospective approach (where we look to the past using some data such as hospital medical records to know who developed the disease) [6]. (c) Case control studies; in which case the researcher identifies the odds of having a risk factor or an exposure if an individual has a specific disease (odds ratio). We conduct this type of study by comparing two groups. (i) one group with the disease (cases) and (ii) another group without the disease (control). For example, if we want to identify the odds of being a smoker among hypertensive lung cancer patients compared to healthy individuals. To achieve this, we chose a group of patients diagnosed with lung cancer and another group of healthy individuals that serves as control. We then study their smoking history to find out if there is a correlation [7- 10] (Figure 1).
Conclusion
Identifying the most appropriate study design is critical for generating the best evidence to support or refute a hypothesis. A well-designed study will clearly identify a range of variables including the subjects, the population, the intervention of interest and the outcomes to be investigated. Understanding the various study designs is important not only in devising a new study but also for critically reviewing published studies.
Acknowledgement
None.
Conflicts of Interest
No conflict of interest.
References
- Ambika G Chidambaram, Maureen Josephson (2019) Clinical research study designs: the essentials. Pediatr Investig 3(4): 245-252.
- Hyun Ja Lim, Raymond G Hoffmann (2007) Study design: the basics. Methods Mol Bio 404: 1-17.
- Majid U (2018) Research fundamentals: Study design, population and sample size. Undergraduate Res Natl Clin SciTechnol; 2(1): 1-7.
- David A Grimes, Kenneth F Schulz (2002) An overview of clinical research: the lay of the land. Lancet 359(9300): 57-61.
- Venkataramana Kandi (2022) Research process, study variables, statistical validations and sampling methods in public health related research: an update. Am J Biomed Res 10(1): 1-8.
- Bernd Röhrig Jean Baptist du Prel, Maria Blettner (2009) Study design in medical research. Dtsch Arztebl Int 106(11): 184-189.
- Soto A, Cvetkovic-Vega A (2020) Case-control studies. Rev Faculty Hum Med 20(1): 138-143.
- Sutton Tyrrel K (1991) Assessing bias in case-control studies, proper selection of cases and controls. Stroke 22(7): 938-942.
- Sergi Fàbregues, Michael D Fetters (2019) Fundamentals of case study research in family medicine and Community health. Fam Med Comm Health 7(2): e000074.
- Barlow WE, Ichikawa L, Rosner d, Izumi S (1999) Analysis of case-cohort designs. J Clin Epidmiol 52(12): 1165-1172.
-
Alphonsus Ogbonna Ogbuabor*, Edwin Obiorah Ohotu and Luke Jideofor Ugwu. The Basic Principles of Biomedical and Medical Science Study Designs. Acad J Health Sci & Res. 1(1): 2023. AJHSR.MS.ID.000501.
Research, Beginners, Medicine, Hypothesis, Blinding, Lung cancer, Clinical trials
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






