 Research Article
Research Article
Postoperative Lipolysis, Intraperitoneal and Subcutaneously during Epidural Analgesia
Daniel Jansson1, Tamara Yacoub Youssef2 and Kjell Jansson2*
1University of Medicine Gdansk, Poland
2Department of Surgery, University hospital of Örebro, Örebro University, Sweden
Kjell Jansson, Department of Surgery, University hospital of Örebro, Örebro University, Sweden.
Received Date: November 08, 2019; Published Date: November 15, 2019
Abstract
Objective: The introduction of epidural anesthesia in the first postoperative days has shown in previous studies not only better pain relief but also lower postoperative metabolism and inflammation in these patients. It has certainly not been possible to establish the mechanisms behind this.
Methods: 14 patients with epidural anesthesia were compared with 6 patients without epidural anesthesia after major gastrointestinal surgery. Metabolic microdialysis measurements were used subcutaneously and intraperitoneally for comparison. The assays were glucose, pyruvate, lactate and glycerol.
Results: The results showed significantly lower glycerol levels in patients with post-operative pain relief with epidural catheters both intraperitoneally and subcutaneously. No differences could be detected in analyzes of glucose, pyruvate and lactate, but differences in glycerol could be detected. Intraperitoneally with epidural catheter median glycerol value was 97.5 vs 152 μmol / L for patients without epidural catheter. Subcutaneous median was 245 μmol / L in patients with epidural catheter vs 336 in patients without. Higher glycerol values were observed subcutaneously compared to intraperitoneal.
Conclusion: Probably the higher glycerol levels seen subcutaneously are due to greater fat content in subcutaneous tissue. We interpret the lower glycerol levels seen in postoperative pain relief in patients with epidural catheters, both subcutaneously and intraperitoneally as a sign that the hormone-induced initiation of lipolysis is controlled by neurogenic mechanisms of the autonomic nervous system.
Introduction
The intraperitoneal fat metabolism increases in the initial postoperative period. This is probably due to changes in carbohydrate metabolism, increased insulin resistance and an increased energy demand makes a switch from aerob to anaerob utilization, it will be a lack of glucose when lactate is produced in the anaerob situation. Which means that the energy lost from carbohydrate metabolism needs to be covered and this by a larger combustion of amino acids and free fatty acids. Triglyceride complexes consisting of a glycerol molecule bonded with 3 free fatty acids (FFA) there after the complex becomes cleaved by the enzyme hormone sensitive lipase to the glycerol molecule which is converted to glucose and the 3 FFAs enter via the cell’s mitochondria in Kreb’s cycle and Coenzyme A for energy production in the form of ATP [1]. In modern postoperative treatment, ERAS is currently used in many clinics worldwide, the concept being based on increased energy utilization through aerobic cell metabolism and thereby reduced glycolysis, proteolysis and lipolysis. As part of it, post-operative epidural anesthesia is used with local anesthesia, as a component, which has shown less lipolysis in studies [2]. Intraperitoneal microdialysis has been introduced as a method for early diagnosis of surgical complications. An increased lactate / pyruvate ratio, increased lactate and decreased glycerol are findings noted before a surgical complication has become manifest. Decreasing glycerol levels in surgical complications have been interpreted as an increased energy requirement and increased consumption, although increased inflammation cannot be excluded as part of this phenomenon [3-7]. Epidural anesthesia studies have been conducted to demonstrate lower levels of glycerol subcutaneously and in plasma in patients who had postoperative epidural anesthesia with local anesthesia as a constituent of the epidural catheter. It has been interpreted as reduced postoperative lipolysis in epidural anesthesia [8-11]. This study was improved of the local ethical committee. None of the authors had any disclosures and the study was financially supported by Nyckelfonden Örebro Sweden.
Patients and Methods
Twenty patients were included in the study, 14 patients with epidural anesthesia, 9 men and 5 women, median age 71 years (range 51-86). 6 patients without epidural anesthesia, 1 male and 5 women, median age 68 years (range 50-89). Epidural anesthesia was applied preoperatively between the intervertebral spaces Th VII-VIII and L I-II. The samples were taken and analyzed immediately every second hour with microdialysis equipment during the first two postoperative days. The samples analyzed were glucose, pyruvate, lactate and glycerol. Microdialysis: The microdialysis catheter is a 0.9 mm thin concentric dual lumen plastic tube with a 30 mm semipermeable tubular membrane (cut at 20,000 Daltons) at its distal end. An M-dialysis 62-gastrointestinal catheter with 210 mm axis and 30 mm membrane was used in the abdominal cavity. Physiological perfusion fluid T1 (M-dialysis AB, Stockholm, Sweden) was pumped at a rate of 0.3 μl / min from an M-dialysis 106 microdialysis pump (M-dialysis AB, Stockholm, Sweden) through the outer tube of the catheter and flowed under the membrane where the exchange between the intraperitoneal fluid and the perfusion fluid occurred. At the tip, the liquid entered the tube through a small hole, flowing backwards and finally collected in a microvial. The perfusate equilibrates with molecules in the intraperitoneal fluid. Thus, microdialysis monitors substances from the blood as well as substances derived from cell metabolism. A microvial with the microdialysis sample takes 7 minutes to be analyzed for glucose, pyruvate, lactate and glycerol. The lactate / pyruvate ratio was calculated in the analyzer (M-dialys AB, Stockholm, Sweden). Statistical analysis: Samples took every second hour, the median value of each patient and analysis for 48 hours were compared.
Results
Intraperitoneal analysis
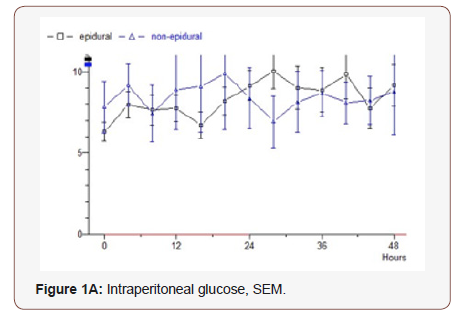
Glucose: The epidural group has an initial postoperative value of 6 mmol / L which rises to 9 during the study. Median value 7.24. Non epidural group starts at 7.8 following the epidural group in parallel and the final value is 9.2. Median value 8.75 (p = 0.232) (Figure 1A).
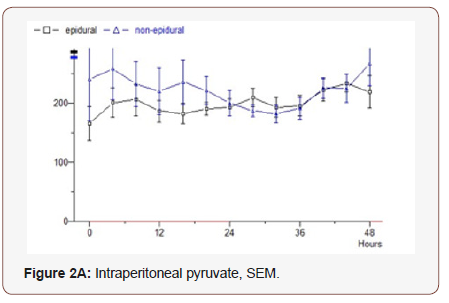
Pyruvate: In the epidural group, the first analysis 165 mmol / L is a slight even increase during the study to 220. Median value 197. Non epidural group starts at 240, which drops during the first 30 hours, and then increases to the starting level. Median value 180 (p = 0.710) (Figure 2A).
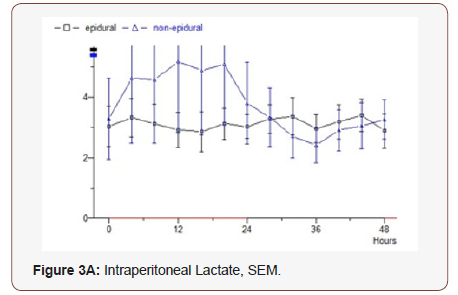
Lactate: In the epidural group, throughout the study, small variations with values around 3 mmol / L are observed. Median 2.58. In the non epidural group, a starting value of 3 is seen as the epidural group, but it increases to 5 after 12 hours, then drops rapidly to 3 and then follows the epidural group’s values during the remaining study period. Median 1.88 (p = 0.837) (Figure 3A).
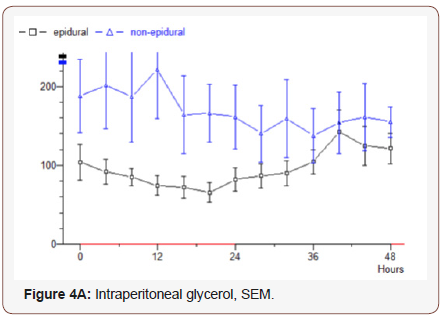
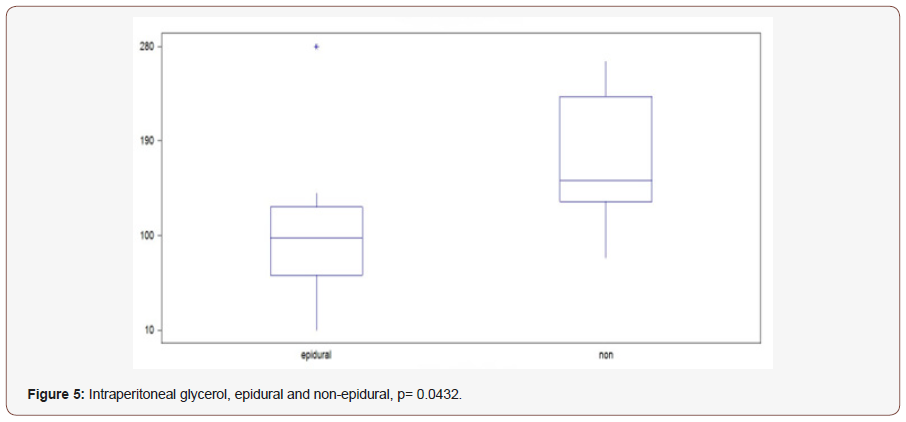
Glycerol: Epidural group starts at 105 μmol / L, decreasing values observed for 20 hours to 67, then rising values during the study to 120. Median value 97.5. In the non-epidural group, the start value 190 which increases slightly for 12 hours to 220, after which lowered values are recorded to the final value 155. Median value 152 (p = 0.0432) (Figure 4A).
Subcutaneous assays
Glucose: In the epidural group, rising values from 5.1 mmol / L to 8.4 over 24 hours are observed, then values decreased to 7.3 at the end of the study. Median value 6.74. In the non-epidural group, the starting value is 5.6, which rises for 16 hours to 10.5, then drops to 9.2 after 48 hours. Median value 7.14 (p = 0.794) (Figure 1B).
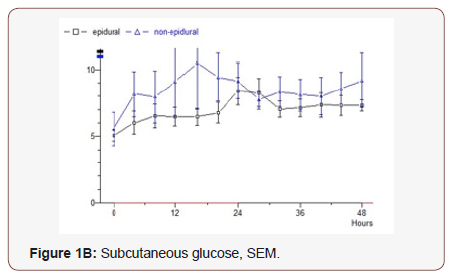
Pyruvate:The epidural group’s initial value is 136 and slowly continuously rising values to 188 mmol / L during the study. Median value 147. In the non epidural group, the first value is 218 and constant levels are seen where the final value is 226. Median value 152 (p = 0.792) (Figure 2B).
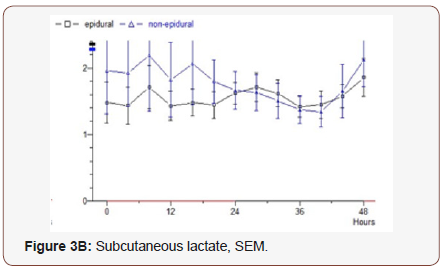
Lactate: The epidural group starts at 1.5 mmol / L, very slightly increasing values during the study to 1.9. Median 1.23. Non epidural group, starting on 2.0 in the next almost constant values and the final value is 2.1. Median 1.36 (p = 0.826) (Figure 3B).
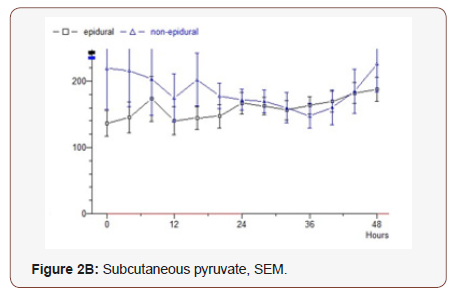
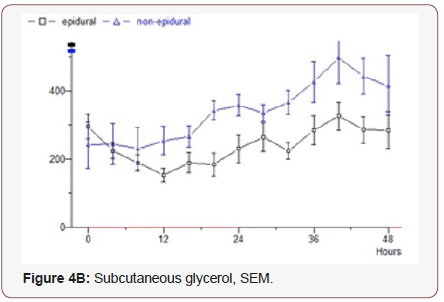
Glycerol: The epidural group starts at 294 μmol / L, drops for 12 hours to 154, after which an increase to 274 is seen at the end of the study. Median value 245. The non-epidural group starts at 241 and rises to 496 after 40 hours, after which decreasing values are seen and the final value is 414. Median value 336 (p = 0.0124) (Figure 4B).
Discussion
Metabolism in the intraperitoneal space has previously been investigated and shown to be a generator in shock and organ failure development. Its importance arises from hypoxia probably based on the small intestine triggers an inflammatory reaction and spreads further through the body’s cascade system [12, 13]. In gastrointestinal surgery, the importance of the intraperitoneal cavity with increasing lactate and lactate / pyruvate ratio has been demonstrated, as evidence of hypoxia / ischemia early in the course of patients who later develop anastomotic leakage3 [14-17]. Measurements of intraperitoneal glycerol have been performed in studies of this patient group and have shown decreasing levels in patients who later develop anastomosis leakage and a possible explanation for this was an increased consumption [18, 19].
Glycerol release has been used in previous studies as a marker for detecting lipolysis in studies of the effects of epidural anesthesia on lipolysis in the postoperative course [9]. Subcutaneous and plasma analyzes of glycerol have shown higher levels in patients who do not have postoperative epidural catheters following gastrointestinal surgery [8]. Studies have also shown that local anesthetics as part of the epidural catheter have the ability to reduce lipolysis but pure morphine in the epidural catheter lacks this ability [8-10]. The results suggest that the autonomic nervous system mediates hormone release of lipolysis-initiating hormones [8]. The intraperitoneal metabolism in previous studies has been shown to be higher than the subcutaneous and one has assessed the higher subcutaneous lipolysis to be an expression for releasing glycerol and FFA and covering the need for the higher intraperitoneal energy requirement postoperatively [15, 20, 21].
The subcutaneous microdialysis catheter was located under the right of the fifth thoracic dermatome. We cannot correlate the results to the level that epidural anesthesia has taken but the insert level is between Th VII-VIII and L I-II. The results shown in this study show no significant differences between the groups of the three metabolites, glucose, pyruvate and lactate, neither intraperitoneally nor subcutaneously. However, glycerol differed significantly between groups, both intraperitoneally and subcutaneously. Postoperative epiduralkateters with bipuvacain and morphine decrease in this study the intraperitoneal lipolysis as well as the subcutaneous corresponding LV dermatometry, but in this study, a secondary intraperitoneal metabolism with less risk of intestinal hypoxia cannot be demonstrated. The results also suggest that lipolysis is mediated not only subcutaneously but also intraperitoneally through the autonomic nervous system. Routine combination of Morphine and Bipuvacaine is used in the epidural catheters in major gastrointestinal surgery at our clinic and has been done in this study as well. The patient group without epidural declined epidural catheter or that an epidural catheter could not be given. Limitations in this study are the small size of the study and that groups were not randomized (Figure 5).
Acknowledgment
None.
Conflict of Interest
No conflict of interest.
References
- Angin Y, Beauloye C, Horman S, Bertrand L (2016) Regulation of Carbohydrate Metabolism, Lipid Metabolism, and Protein Metabolism by AMPK. Experientia supplementum 107: 23-43.
- Ljungqvist O, Scott M, Fearon KC (2017) Enhanced Recovery After Surgery: A Review. JAMA surgery 152(3): 292-298.
- Jansson K, Ungerstedt J, Jonsson T, Redler B, Andersson M, et al. (2003) Human intraperitoneal microdialysis: increased lactate/pyruvate ratio suggests early visceral ischaemia. A pilot study. Scand J Gastroenterol 38(9): 1007-1011.
- Horer TM, Norgren L, Jansson K (2011) Intraperitoneal glycerol levels and lactate/pyruvate ratio: early markers of postoperative complications. Scand J Gastroenterol 46(7-8): 913-919.
- Ellebaek MB, Daams F, Jansson K, Matthiessen P, Cosse C, et al. (2018) Peritoneal microdialysis as a tool for detecting anastomotic leakage in patients after left-side colon and rectal resection. A systematic review. Scand J Gastroenterol 53(12): 1625-1632.
- Ungerstedt U (1991) Microdialysis--principles and applications for studies in animals and man. J Intern Med 230(4):365-373.
- Matthiessen P, Strand I, Jansson K, Tornquist C, Andersson M, et al. (2007) Is early detection of anastomotic leakage possible by intraperitoneal microdialysis and intraperitoneal cytokines after anterior resection of the rectum for cancer. Dis Colon Rectum 50(11): 1918-1927.
- Ederoth P, Flisberg P, Ungerstedt U, Nordstrom CH, Lundberg J, et al. (2005) Local metabolic changes in subcutaneous adipose tissue during intravenous and epidural analgesia. Acta Anaesthesiol Scand 46(5): 585-591.
- Lattermann R, Carli F, Schricker T (2002) Epidural blockade suppresses lipolysis during major abdominal surgery. Reg Anesth Pain Med 27(5): 469-475.
- Carli F, Lattermann R, Schricker T (2002) Epidural analgesia and postoperative lipid metabolism: stable isotope studies during a fasted/fed state. Reg Anesth Pain Med 27(2): 132-138.
- Chambrier C, Bouletreau P (1992) [Epidural anesthesia and metabolic response to surgical stress]. Ann Fr Anesth Reanim 11(6): 636-643.
- Landow L, Andersen LW (1994) Splanchnic ischaemia and its role in multiple organ failure. Acta Anaesthesiol Scand 38(7): 626-639.
- Fiddian-Green RG (1998) Splanchnic ischaemia and multiple organ failure in the critically ill. Ann R Coll Surg Engl 70(3): 128-134.
- Jansson M, Strand I, Jansson K (2006) Intraperitoneal microdialysis: Postoperative monitoring of splanchnic ischemia by measurements of the lactate pyruvate ratio. Crit Care Med 34(10): 2695-2697.
- Jansson K, Strand I, Redler B, Magnuson A, Ungerstedt U, et al. (2004) Results of intraperitoneal microdialysis depend on the location of the catheter. Scand J Clin Lab Invest 64(1): 63-70.
- Jansson K, Wickbom M, Skoog P, Nilsson K, Hörer T, et al. (2017) Intraperitoneal microdialysis-after hemicolectomy and rectal resections as a method for early postoperative complications detection. Internal Medicine Review 4(7): 1-15.
- Ellebaek M, Qvist N, Fristrup C, Mortensen MB (2014) Mediastinal microdialysis in the diagnosis of early anastomotic leakage after resection for cancer of the esophagus and gastroesophageal junction. Am J Surg 208(3): 397-405.
- Horer T, Norgren L, Jansson K (2010) Complications but not obesity or diabetes mellitus have impact on the intraperitoneal lactate/pyruvate ratio measured by microdialysis. Scand J Gastroenterol 45(1): 115-121.
- Horer TM, Skoog P, Norgren L, Magnuson A, Berggren L, et al. (2013) Intra-peritoneal microdialysis and intra-abdominal pressure after endovascular repair of ruptured aortic aneurysms. Eur J Vasc Endovasc Surg 45(6): 596-606.
- Jansson K, Redler B, Truedsson L, Magnuson A, Ungerstedt U, et al. (2014) Postoperative on-line monitoring with intraperitoneal microdialysis is a sensitive clinical method for measuring increased anaerobic metabolism that correlates to the cytokine response. Scand J Gastroenterol 39(5): 434-439.
- Jansson K, Jansson M, Andersson M, Magnuson A, Ungerstedt U, et al. (2005) Normal values and differences between intraperitoneal and subcutaneous microdialysis in patients after non-complicated gastrointestinal surgery. Scand J Clin Lab Invest 65(4): 273-281.
-
Daniel Jansson, Tamara Yacoub Youssef, Kjell Jansson. Postoperative Lipolysis, Intraperitoneal and Subcutaneously during Epidural Analgesia. Acad J Gastroenterol & Hepatol. 1(4): 2019. AJGH.MS.ID.000518.
-
Gastrointestinal surgery, Anesthesia, Glucose, Pyruvate, Lactate Glycerol, Mitochondria, Hypoxia, Ischemia, Microanalysis, Morphine decrease
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






