 Research Article
Research Article
Invasive Cancer of the Colon with Gastric Metastasis
Antonio Gligorievski*
University Clinic of Surgery, The Monastery of Saint Naum, Macedonia
Antonio Gligorievski, University Clinic of Surgery, The Monastery of Saint Naum, Macedonia.
Received Date: February 26, 2021; Published Date: March 15, 2021
Introduction
The stomach is a very rare location of metastatic deposits. We present a case of a gastric metastatic deposit from primary colon cancer. Computed tomography (CT) indicated primary colon cancer, but also revealed the presence of cancer in the region of the gastric greater curvature and gastric antrum. With the progression of primary cancer and the creation of an extraluminal tumor mass, the process progresses in the manner that the primary transversal colon tumor merges into the metastatic deposit located in the stomach. The patient underwent subtotal gastrectomy and D2 lymphadenectomy, as well as partial resection of the transversal colon, thus the tumor mass of the stomach and the one in the transversal colon being removed in a single act. Pathohistological analysis of the tumor mass and immunohistochemical tests revealed a primary neoplastic infiltrative process in the colon metastasizing into the stomach.
Introduction
Bowel cancer is the fourth most common cause of cancer death in the world, and the second most common cause of cancer death in developed countries [1]. The colorectal cancer mortality rate is lowest in Central Africa and Southeast Asia and highest in Central and Eastern Europe [1]. Mortality from colon cancer is age-related. Mortality rates increase with age (1). The prevalence of right bowel cancer increases with increasing patient age [1]. Most colon adenocarcinomas (80%) occur in existing adenomatous polyps, and progression from a polyploidy adenoma to cancer occurs over several years. Most cancers are asymptomatic until the disease is advanced. The patient may notice blood in the stool, but occult bleeding is more common. Ferro deficiency anemia occurs due to blood loss. The initial diagnosis is usually made by colonoscopy or double-contrast barium enema examination. However, with the increasing use of computed tomography (CT), the initial diagnosis of suspected colon cancer can also be made based on CT findings. Radiographs of the double-contrast barium enema examination usually show ring lesions that are irregular and lead to narrowing of the lumen of the colon. The spread of colon cancer most commonly occurs by the hematogenous route, by the lymphatic route, directly by local invasion of adjacent organs and the peritoneum. Colorectal cancer most often metastasis to the liver, lungs, and peritoneum, but it is also possible for cancer to spread locally, as well as to recur in another part of the colon (metachronous metastases). Rare cases of metastases from colorectal cancer of the spleen, thyroid, esophagus, stomach, urinary tract, and abdominal wall have also been reported in the literature [2]. Stomach metastases from colorectal cancer are extremely rare, 3-4 cases [3-5] have been reported in the literature. We present a case of metastatic gastric cancer, which originates from cancer of the transverse part of the colon.
Presentation of Case
In February 2016, a 63-year-old man was admitted to our institution due to anemia and suspected bleeding from the digestive tract. The patient complains of difficulty walking and fatigue does not complain of pain in the abdomen but notices that the stool is dark for more than two weeks. The physical Citation: Antonio Gligorievski. Invasive Cancer of the Colon with Gastric Metastasis. Acad J Gastroenterol & Hepatol. 2(4): 2021. AJGH. MS.ID.000544. DOI: 10.33552/AJGH.2021.02.000544 Page 2 of 6 examination, except for pallor and slight weight loss, revealed no other changes. Preoperative laboratory examination showed Hgb levels. of 79 mmol/L, Er. of 2.64 x 1012/L, Le. of 2.64 x 109/L, HCT of 0.234/L. All other biochemical and hematological tests are normal. Laboratory tests help detect bleeding from the digestive tract. Previously, the patient was treated with anti-ulcer therapy, but after the therapy, the clinical picture did not improve, and the patient left the hospital at his request and come to our clinic for further diagnostic examination and treatment. The patient underwent gastroscopy and an ulcerated infiltration of the gastric wall in the anterior part by a large curvature was detected. A clinical diagnosis of gastric cancer was made and the patient was referred for a further radiological examination of the abdomen. The patient had undergone a CT scan of the abdomen, after intravenous administration of contrast agents. The examination was performed on a GE Bright Speed MDCT computed tomography device with 16 rows of detectors. The CT scan was performed at a tube voltage of 120KV. The rotation speed is 0.8 sec. The pitch factor is 1.375: 1. The examination was performed in an Auto mA setting. The crosssectional thickness of the native series was 1.5 mm, and that of the post-contrast series was 1.25 mm. The Noise index is 13. The maximum wide field of view (FOV) was used. The reconstruction matrix was 512 x 512 pixels. Non-ionic, low-osmolar contrast is administered intravenously in a total dose of 100 mL, the rate of contrast application is 3 mL/sec, and the delay time after contrast application is 25 sec. After the end of the examination, an analysis of the obtained scans was made.
In the upper part of the abdomen, there is a soft tissue mass that infiltrates the stomach, but 80% of the mass is outside the stomach and as we move to the lower part of the abdomen it can be seen that the mass infiltrates the middle part of the transverse colon. A thick and infiltrated wall with a visible ulcer can be seen on the front wall of the stomach by the large curve in the lower part of the corpus and towards the antrum. The TU mass spreads antropyloric as well as to the posterior wall of the stomach. Pediatrically has seen borderline enlarged lymph nodes (Figure 1).
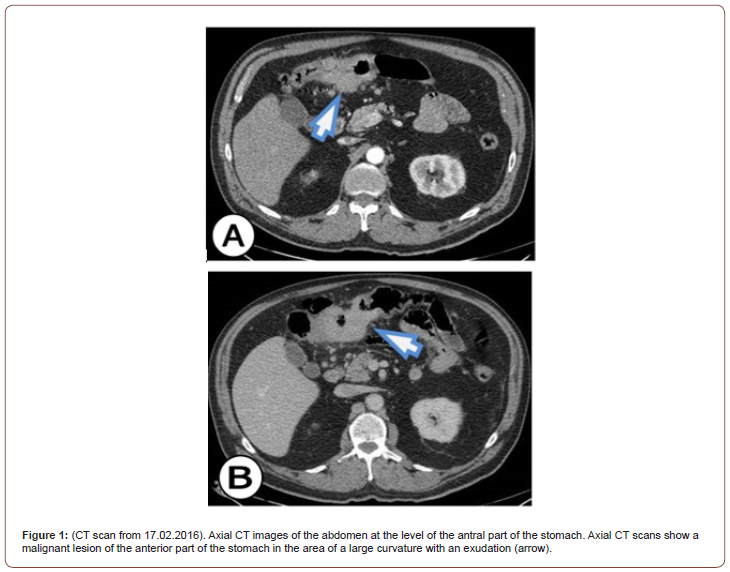
About 80% of the TU mass is located between the transverse colon and the stomach. The TU mass also infiltrates the wall of the transverse colon where endoluminal masses can be seen. About 90 - 100 mm from the length of the transverse colon was with an infiltrated wall, a narrowed lumen, and visible endoluminal TU masses. In the vicinity of the transverse colon, borderline enlarged lymph nodes and a reaction of the surrounding adipose tissue are seen. On the post-contrast series, the tumor showed completely and almost homogeneous contrast enhancement and increases HU. After the analysis of the CT scans, the dilemma remains, whether it is a primary tumor that originates from the stomach and then occupies and infiltrates the transverse colon, or it is a primary tumor of the transverse colon that occupies and infiltrates the stomach. It can also be a gastrointestinal stromal tumor (GIST) that affects and infiltrates the transverse colon and the distal part of the stomach. Our initial conclusion was that it was a GIST that infiltrates the transverse part of the colon and the distal part of the stomach.
Partial resection of the stomach was performed and a TU mass (segment 18 cm long by the great curvature and 11 cm by the small curvature) was removed. TU mass was 20 mm thick and ulcerated. Partial resection Billroth II and a terminal-lateral anastomosis with the jejunum was performed. Also, resection of the transverse colon was performed, TU mass (segment in the length of 14 cm) was removed, and terminal-terminal anastomosis was performed. The TUf mass was ulcerated and 9 cm in length occupies the entire circulatory space of the colon wall. An omentectomy was also performed (on greater omentum 39 x 18 x 2 cm and on lesser omentum 7 x 4.5 x 1 cm). An oval TU mass with a diameter of 90 mm, which is in a form of a block tumor with the stomach and the transverse colon was removed (Figure 2).
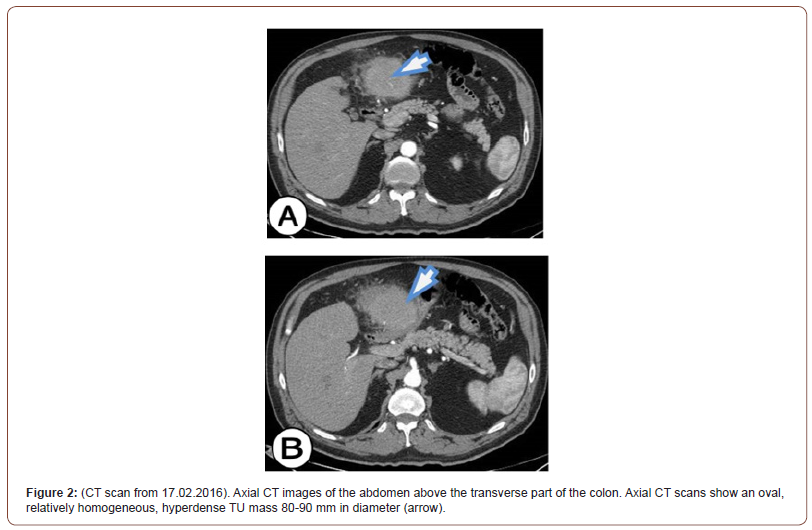
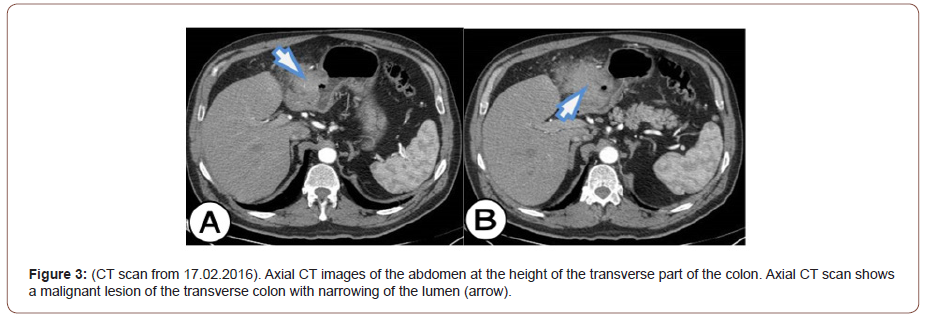
After the surgery, the patient’s son handed us a CD of an examination performed at his father about 7 months ago (on 22.07.2015) when he visited a doctor for the first time due to gait problems. The patient underwent CT angiography, which did not show the significant disease of the arteries of the lower extremities. Seven months after the examination, we performed an analysis of the examination, and the focus of our examination was the shown part of the abdomen on the CT angiography. What we have noticed is an infiltration of the wall of the transverse colon with visible endoluminal TU masses, extraluminal TU masses were not visible. There was a pronounced infiltration of the transverse colon in the length of 92 mm, and the anterior-posterior diameter was 46 mm. Infiltration of the gastric wall into the antropyloric part with volume reduction was also seen. The maximum thickness of the infiltration of the anterior wall of the stomach in the antropyloric region was 14 mm. No evidence of a TU mass connects the stomach and colon as it was predominantly noticeable on the CT examination performed after 7 months (on 17.02.2016). With the analysis of CT angiography and the comparison with the CT examination of the abdomen, our opinion is that this is a primary, invasive, malignant neoplasm of the transverse colon that metastasized to the stomach, and in a short time expanded into the omentum and formed block tumor that associates primary colon cancer with metastatic gastric deposit. This opinion stems from the fact that the TU of the colon is much larger in volume than the TU of the stomach, but we cannot rule out with certainty that it is the synchronous occurrence of adenocarcinoma of the colon and adenocarcinoma of the stomach (Figure 3).
After the pathohistological analysis of the removed abdominal TU mass and the performed immunohistochemically examinations with CK20 which is positive for the tumor population and CK7 which is negative for the tumor population, a final pathohistological diagnosis is made for primary cancer of the colon with metastasis. According to the morphological appearance and immunohistochemically profile, it is suitable for adenocarcinoma of the colon with a direct spread in the omentum and stomach with the following characteristics according to AJCC 2010 - pTNM - pT4b, pN0, pMx, pL0, G2, NG2 Stage IIC. Dukes B. MAC B3.
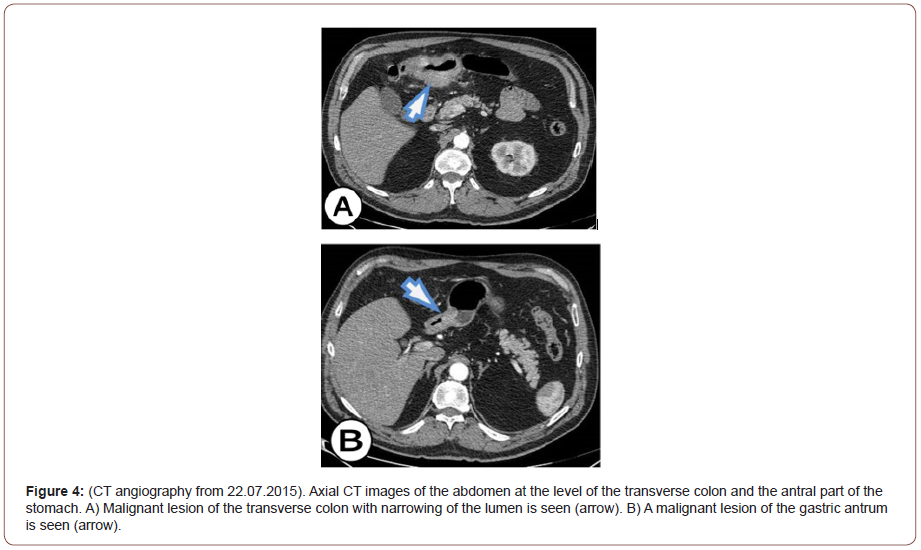
In the presented case, an extremely fast progression of the malignant process is noticed, which is only 7 months leads to the creation of a block tumor between the antrum of the stomach and the transverse colon. After a CT scan of the abdomen, our initial diagnosis was GIST. But after the analysis of CT angiography made 7 months ago, we think that it is the primary cancer of the colon with a metastatic deposit in the stomach. However, the possibility remains that these are synchronous adenocarcinomas of the colon and adenocarcinoma of the stomach, which is extremely rare. The final resolution of the dilemma is made possible by the pathohistological analysis of the removed abdominal TU mass and immunohistochemically examinations performed with CK20 and CK7. Immunohistochemically studies have shown that CK20 is positive for the tumor population, CK7 is negative for the tumor population corresponding to primary colon cancer, and TU in the stomach is a metastatic deposit. If CK20 and CK7 are positive for the tumor population or CK20 is negative for the tumor population and CK7 is positive for the tumor population, then it may be primary gastric cancer (Figure 4).
In the available literature, we found a case of adenocarcinoma of the colon that metastasized to the stomach. Paulo Moacir de Oliveira Campoli, et al. [6] in their paper cite a case of adenocarcinoma of the colon that metastasized to the stomach. The presence of gastric metastases is a rare condition, and major studies published in the literature are based on endoscopic findings, autopsies, and surgical specimens, or a combination of these three methods. The most commonly described primary cancers that metastasize to the stomach are lung cancer, breast cancer, malignant melanoma, and esophageal cancer [6-8]. Metastasis of malignant processes is very rare in the digestive tract, however, research and autopsy findings indicate a much higher incidence. After all, gastric metastases have been described for certain tumors, such as melanoma, breast cancer, and lung cancer.
Overall, more than 96% of cases of signet ring cell carcinoma occur in the stomach, with the remaining cases occurring in the colon, rectum, gallbladder, pancreas, bladder, and lungs. The incidence of signet ring cell type cancer in the colon is 0.1 to 2.4%, and clinical features include an advanced stage at diagnosis, large tumor mass, proximal location, young patient, susceptibility to lymph vascular invasion, and peritoneal scattering [9-11]. Synchronous (at the same time) and metachronous (at different time intervals) gastrointestinal (GIT) cancers are extremely rare. In 3-5% of cases of colorectal cancer, synchronous and metachronous development of two or more primary adenocarcinomas occurs [12]. There are only a few such cases that have been published in the literature [13]. Zubair Ahmad, et al. [14] reported a case of a 41-year-old patient with metachronous carcinoma of the right colon and gastric cancer, stating that they had not encountered such a case in the previously published literature. Synchronous or metachronous adenocarcinoma of the colon and adenocarcinoma of the stomach is extremely rare. The patient was diagnosed with both adenocarcinomas of the colon and adenocarcinoma of the stomach. On the immunohistochemically profile gastric adenocarcinoma in this patient was CK7 positive and CK20 negative; while adenocarcinoma of the colon was CK7 negative and CK20 positive. CK7/CK20 expression for gastric cancer and colon cancer is significantly different. About 70% of cases of gastric cancer are CK7 positive, and only 20% are CK20 positive. On the other hand, 95% of colorectal cancer cases are CK7 negative and CK20 positive. Negative CK7, and positive CK20 profile, in a large percentage, are in favor of colon cancer, while CK7 positive and CK20 negative profile is in favor of metastasis [15,16]. In the literature, we find several papers that describe several metachronous and synchronous cancers of the gastrointestinal tract. Iioka, et al. [2] report a triple case of metachronous carcinoma of the sigmoid colon, stomach, and esophagus. Oncel, et al. [8] report a case of a patient with metachronous carcinoma of the stomach, colon, and thyroid. Although patients with multiple cancers are uncommon, they are of particular importance because of the possibility of developing a second or third carcinoma in patients who have already been treated for primary cancer. Mukai, et al. [17] describe a case of a patient who develops triple metachronous cancer of the esophagus, colon, and kidney several years after treatment. Tamura, et al. [13] show a case of triple synchronous cancer of the stomach, colon, and gallbladder in a 70-year-old man. Pricop, et al. [5] report a case of metachronous primary cancer of the colon and stomach in a 69-year-old woman. They also stressed the importance of the possibility of developing metachronous cancer in patients treated for a primary malignant tumor. Another case of synchronous carcinoma of the transverse colon and early gastric cancer has been reported by Nakata, et al. [18]. Parag Brahmbhatta, et al. [19] report a case of recurrent colon adenocarcinoma as duodenal metastasis in a 54-year-old woman who underwent surgery for caecum adenocarcinoma two years ago.
Acknowledgement
None.
Conflict of Interest
No conflict of interest.
References
- Mohammad Rostami-Nejad, Thea Haldane, David Aldulaimi, Seyed Moayed Alavian, Mohammad Reza Zali, et al. (2013) The role of celiac disease in severity of liver disorders and effect of a gluten free diet on diseases improvement. Hepat Mon 13(10): e11893.
- Linnea Äärelä, Samuli Nurminen, Laura Kivelä, Heini Huhtala, Markku Mäki, et al. (2016) Prevalence and associated factors of abnormal liver values in children with celiac disease. Dig Liver Dis 48(9): 1023-1029.
- G Novacek, W Miehsler, F Wrba, P Ferenci, E Penner, et al. (1999) Prevalence and clinical importance of hypertransaminasaemia in coeliac disease. Eur J Gastroenterol Hepatol 11(3): 283-288.
- Lawson A, West J, Aithal GP, RFA Logan, et al. (2005) Autoimmune cholestatic liver disease in people with coeliac disease: a population-based study of their association. Aliment Pharmacol Ther 21(4): 401-405.
- MT Bardella, L Valenti, C Pagliari, M Peracchi, M Farè, et al. (2004) Searching for coeliac disease in patients with non-alcoholic fatty liver disease. Dig Liver Dis 36(5): 333-336.
- Rubio-Tapia A, Murray JA (2007) The liver in celiac disease. Hepatology 46(5): 1650-1658.
- Holmes GKT, Muirhead A (2018) Mortality in coeliac disease: a population-based cohort study from a single centre in Southern Derbyshire, UK. BMJ Open Gastroenterol 5(1): e000201.
- Abdulbaqi Al-Toma, Umberto Volta, Renata Auricchio, Gemma Castillejo, David S Sanders, et al. (2019) European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United European Gastroenterology J 7(5): 583-613.
- Alberto Rubio-Tapia, Joseph A Murray (2007) The Liver and Celiac Disease. Hepatology 46(5): 1650-1658.
-
Antonio Gligorievski. Invasive Cancer of the Colon with Gastric Metastasis. Acad J Gastroenterol & Hepatol. 2(4): 2021. AJGH. MS.ID.000544.
-
Computed tomography, Colon cancer, Gastric antrum, Spleen, Thyroid, Esophagus, Stomach, Urinary Tract, Abdominal wall, Gastric cancer, Angiography, Liver, Lungs, Peritoneum
-

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.






